Abstract
Colicin K exhibited pronounced inhibitory activity against uropathogenic Escherichia coli (UPEC) strains. Low prevalence of colicin K production and a relatively high prevalence of ColE1-like plasmids were determined among 215 UPEC strains from Slovenia. Sequencing of the colicin K-encoding pColK-K235 revealed a mosaic structure and the presence of the insertion sequence IS2.
An increasing health problem is the appearance and spread of antimicrobial resistance. Due to the global concern about antibiotic resistance, novel approaches, one being the use of bacteriocins, are being considered (10, 11). Colicins, bacteriocins produced by and active against Escherichia coli strains, are receiving renewed attention as an alternative means of preventing various E. coli-associated infections, for example, diarrhea, including serotype O157:H7 (15, 22, 28, 29, 30), and postweaning diarrhea and edema disease in swine (33). Colicins were recently shown to prevent colonization of urinary catheters (34). To date, no quantitative analysis of the inhibitory activity of colicins against uropathogenic E. coli (UPEC) has been performed.
Colicin K is a pore-forming colicin encoded on small ColE1-like plasmids (23). A cluster of three genes codes for the production and release of colicin: cka for colicin activity, cki for immunity, and ckl for lysis (23). The synthesis of colicin K is induced primarily by an increase in the alarmone ppGpp due to nutrient depletion (18, 19). Previous investigations found that approximately 30 to 50% of natural E. coli isolates produce colicins (27). Such a high prevalence of colicin production is in itself indicative of an ecological significance. Additionally, a recent study provided evidence that colicins E1 and E2 have an in vivo antagonistic role in promoting microbial diversity within E. coli populations in the mammalian colon (17).
In this study, we investigated the inhibitory activity of purified colicin K against 215 UPEC strains from Slovenia as well as the prevalence of colicin K production and ColE1-like plasmids among the studied strains. The nucleotide sequence of plasmid pColK-K235 encoding the investigated colicin K was determined and analyzed.
To gain insight into the ecological role of colicin K and to assess its antimicrobial efficacy, we initially determined the MIC of purified colicin K against 215 UPEC strains isolated from humans with urinary tract infections in 2001 and 2002 at the Institute of Microbiology and Immunology, Ljubljana, Slovenia (26).
Isolation of the strains was performed according to standard laboratory protocols, and UPEC isolates were from a bacterial urine monoculture of >105 CFU per ml. To elicit infections, extraintestinal pathogenic E. coli strains, including UPEC, possess virulence factors. Therefore, the investigated UPEC strains were PCR screened for virulence factor sequences as described previously (14, 20). As antimicrobial resistance is an increasing health problem, testing of susceptibility to antimicrobial agents was performed as described previously (26).
To isolate colicin K, the cka activity gene was amplified using PCR with primers ColK1 and ColK2 (Table 1). The PCR product was digested with restriction enzymes XhoI and MluI and cloned (ligated) into the expression vector pET8c (25), producing plasmid pMR1. Colicin K was expressed in E. coli strain BL21 (DE3) and large-scale expression was performed as previously described (2). The colicin K-containing fractions, as determined by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis, were dialyzed against 5 mM phosphate buffer and stored at −20°C. Protein purity was checked by sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and the protein concentration was assayed using a bicinchoninic acid protein assay kit (Pierce).
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant property(ies) | Source or reference |
|---|---|---|
| Strains | ||
| DH5α | thi-1 recA hsdR17 lac | BRL Life Technologies |
| MC4100 | araD139 Δ(argF-lac) U169 rpsL150 relA flbB5301 ptsF25 deoC1 | R. Hengge-Aronis |
| BL21 (DE3) | High-level expression using T7 RNA | G. Anderluh |
| polymerase-based expression systems | ||
| AB1133 | Sensitive to all colicins | B. Bachman |
| UL98 | hlyA cnf1 usp iroN iucD fyuA fimH traT | 26 |
| Apr Tcr Nalr Cmr Kmr Str | ||
| UL114 | K1 iucD iroN fyuA usp traT Apr Cipr | 26 |
| UL31 | hly cnf1 papC papG sfa/foc iucD iroN usp Cmr Nalr | 26 |
| UL173 | K5 iroN iucD fimH traT Apr Sxtr Cipr | 26 |
| Plasmids | ||
| pColK-K235 | Wild-type plasmid | 18 |
| pUC19 | Apr cloning vector | 36 |
| pBR322 | Apr Tcr cloning vector | 3 |
| pET8c | Apr expression vector | 25 |
An estimation of the lowest concentration of colicin K preventing the appearance of turbidity (MIC) was performed by spotting 5 μl of various concentrations (0.1, 1, 10, and 100 μg/ml) of Cka diluted in 10 mM Tris, pH 8, onto LB plates overlaid with soft agar harboring the individual investigated UPEC strains. Following an overnight incubation at 37°C, the plates were examined for colicin sensitivity (clear zones) of the overlaid strains.
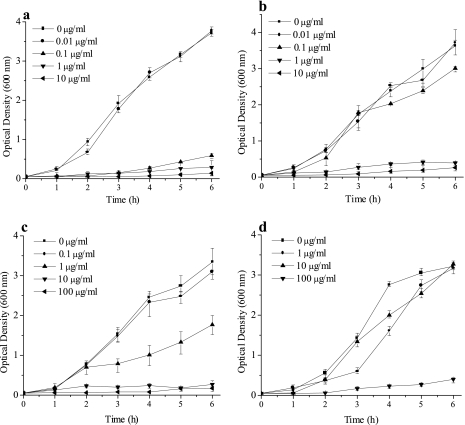
The obtained results showed that, altogether, 68% of the tested UPEC strains were susceptible and 32% were resistant to colicin K (Fig. 1). However, among the sensitive strains, various levels of susceptibility were observed. Thus, 18% of the strains were inhibited by 0.1 μg/ml, 3% by 1 μg/ml, 16% by 10 μg/ml, and 31% by 100 μg/ml of colicin K. The basis of such differences in susceptibility could be due to the different numbers of receptors per cell (32) or the shielding of colicin receptors by the O-antigenic chains of lipopolysaccharide (6, 35). Further, resistance to colicins is known to be due to the absence of functional receptors and resistant isolates appear spontaneously. Site-specific mutations in the FepA colicin receptor have been shown to affect susceptibility for colicins B and D (7).
FIG. 1.
Effect of colicin K on UPEC strains UL31 (a), UL173 (b), UL114 (c), and UL98 (d) (MIC as detected on plates containing 0.1, 1, 10, and 100 μg/ml, respectively). The experiments were repeated three times, and the means ± standard errors of the means (error bars) are shown. Only one of the two investigated strains from each sensitivity group is depicted; however, the effects of colicin K on both strains were comparable.
From each susceptibility group (corresponding to concentrations of 0.1, 1, 10, and 100 μg/ml), two strains harboring virulence factor genes (Table 1) were chosen to follow the inhibitory activity of colicin K. The inhibitory activity of Cka was quantified in liquid medium essentially as described previously (33), and activity against one strain from each group is presented in Fig. 1. Briefly, overnight cultures of the tested UPEC strains were used to inoculate prewarmed LB to an approximate optical density at 600 nm (OD600) of 0.05, followed by aliquoting 5 ml into tubes with various colicin concentrations. A total of 10 mM Tris, pH 8, was used to prepare constant volumes of added colicin (200 μl). Subsequently, the tubes harboring the tested strains and colicin were incubated with shaking at 37°C. To follow colicin activity against the tested strains, the OD600 was determined hourly. On the basis of growth inhibition as determined by OD600 values, it is evident that colicin K exhibited pronounced inhibitory activity against the tested UPEC strains.
An analysis of the nucleotide sequences of colicin-encoding plasmids is essential to elucidate their role in bacterial populations and to gain a full understanding of their evolutionary histories. In spite of the high prevalence of colicin production among natural E. coli populations, to date, the complete nucleotide sequences of only a small number of colicin-encoding plasmids have been studied (8, 13, 21, 31). We therefore determined and analyzed the nucleotide sequence of the colicin K-encoding pColK-K235 (24). Nucleotide sequence data were obtained by a combination of subcloning of restriction fragments and primer walking with specific primers (Table 2). Sequencing reactions were performed using the Thermo Sequenase Cy5 dye terminator cycle sequencing kit and the ALFexpress II DNA sequencer (both from Amersham Biosciences). The nucleotide sequence was searched for potential open reading frames by GeneMark (4).
TABLE 2.
Oligonucleotide primers used in this study
| Oligonucleotide primer | Nucleotide sequencea | Positionb |
|---|---|---|
| ColK1 | TCACTCGAGCATGGCTAAAGAAC | 2527-2555 |
| TAAGTGGATATGGACC | ||
| ColK2 | CCACGCGTTAAATCCCTAACAA | 4172-4143 |
| CTCATTAAGTTTGCTC | ||
| cka1 | CAGAGGTCGCTGAACATGAAAC | 3263-3284 |
| cka2 | CTCAGCTAAACGCTTCTCTGCTT | 3336-3314 |
| P1 | TAACCGGGAAGGGGCGAGAGTTCT | 6268-6291 |
| P2 | GAATGAACTGGACGCCGACG | 5737-5718 |
| P3 | TTCTGAACTGACGGGGATCGC | 4591-4611 |
| P4 | TGAAACACGCCAGTTCGCCC | 1899-1918 |
| P5 | GCTTCCAGGGCCGCTCTTCCT | 7154-7134 |
| PK1 | GGCGTATTTTCCCCGGTTTC | 673-692 |
| PK2 | TGGTGCCAGCCAGTCTGCTC | 594-575 |
| PK3 | GGGCGAACTGGCGTGTTTCA | 1918-1899 |
| PK4 | ACACCCTTGTTGCTGCCGGA | 2234-2215 |
| PK5 | TCCGGCAGCAACAAGGGTGT | 2215-2234 |
| PK6 | AGCTGCGTGGCCTTTTCCGG | 5216-5235 |
| PK7 | TCCGCCTTTCTCCCTTTGGG | 5054-5035 |
| PK8 | TACAGCGCCCCTTTCAGCCT | 6881-6862 |
| PK9 | AGGAAGAGCGGCCCGGAAGC | 7134-7154 |
| PK10 | AGGCTGAAAGGGGCGCTGTA | 6862-6881 |
| PK11 | CCCCTTCACGGATTTGCAGC | 7531-7550 |
| mob1 | GGCAGTGGTCCGGTTGAT | 6230-6248 |
| mob2 | TGCAGCCCGTAATGGTGA | 6630-6612 |
| rna1 | AGGATCTTCTTGAGATCCTT | 4632-4652 |
| rm2 | TATCCACAGAATCAGGGG | 5291-5273 |
| rom1 | AAGCGGGCCATGTTAAGG | 5774-5792 |
| rom2 | AATCAACCGGACCACTGC | 6248-6230 |
Nucleotides in boldface correspond to introduced XhoI and MluI restriction sites.
Numbers indicate positions on plasmid pColK-K235.
DNA sequencing revealed that the total length of plasmid pColK-K235 is 8,318 bp. The average GC content of the colicin K gene cluster was found to be 37.3%, while that of the remaining plasmid sequences was 52.9%, indicating that pColK-K235 has a mosaic structure.
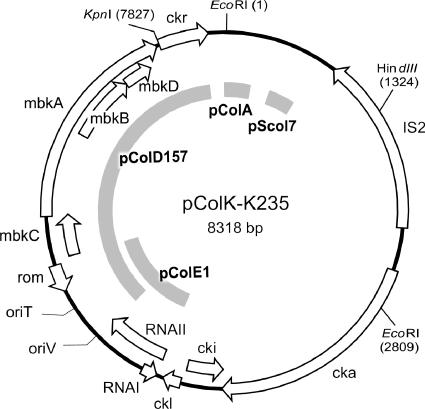
Using BLAST (1), the positions of sequences identical to the genes mob, ckr, and rom of a number of other colicin-encoding plasmids were found. The positions of oriV, RNA I, and RNA II were determined by alignment with the sequences of plasmids pColE1 (5), pWQ799 (16), and pNTP1 (12). The position of oriT was likewise deduced by alignment with pColE1. The genes and functions of pColK-K235 are illustrated in Fig. 2.
FIG. 2.
Physical and genetic map of plasmid pColK-K235. Regions of similarity with other colicin-encoding plasmids are indicated. The arrows indicate the positions and orientations of functional genes on the basis of sequence comparisons. Restriction sites for several restriction endonucleases are indicated.
The 1,331-bp-long insertion sequence IS2 (9) was found upstream from the colicin K gene cluster. The latter has been extensively discussed elsewhere (23). The putative mobilization genes were designated mbkA, mbkB, mbkC, and mbkD according to the nomenclature (5).
Altogether, approximately 3 kbp of pColK-K235 (position numbers 5185 to 8162) is identical (97%) to pColD157 (13) in regions of the genes rom and mbk and the ckr determinant (Fig. 2). Shorter regions of similarity with other known colicin-encoding plasmids, namely, pColE1, pColA, and pScol7, are designated in Fig. 2. Our results demonstrate that pColK-K235 has a mosaic structure as different segments exhibit identity with different plasmids. Notable is the presence of the insertion sequence IS2, which can mediate recombination between homologous sequences on chromosomes or other plasmids, including conjugative plasmids.
Subsequently, the prevalence of related ColE1-like plasmids among the 89 colicinogenic UPEC isolates was examined by PCR probing for mobA-, rom-, and RNA II-specific sequences (Table 2). Our results demonstrated that 38 (43%) of the colicinogenic isolates and, thus, 18% of the altogether 215 UPEC isolates examined harbored sequences characteristic of ColE1-like plasmids.
To gain additional insight into the ecological role of colicin K, the prevalence of colicin K production among the studied UPEC strains was investigated. For this purpose, the 89 colicinogenic strains were PCR probed for cka-specific sequences (Table 2). A low prevalence of colicin K was determined as only two strains (1%) were positive by PCR for the tested sequences. Colicinogenic strains are immune to the produced colicin, and both strains were insensitive to colicin K as determined by bioassay, indicating that they indeed harbor and express the colicin K gene cluster. Our results thus demonstrate that, among the colicin K-insensitive strains, only approximately 6% exhibited immunity.
Our data show pronounced inhibitory activity of colicin K against UPEC strains. Nevertheless, for therapeutic purposes, it would be more effective to use a combination of colicins employing different receptors, translocations, and modes of action. Further, the here-described presence of IS2 and the relatively high prevalence of ColE1-like plasmids indicate that these plasmids might play a significant role as vehicles of DNA rearrangements as well as gene mobilization.
Nucleotide sequence accession number.
The obtained plasmid pColK-K235 sequence data have been deposited with the EMBL and GenBank data libraries under accession no. AY929248.
Acknowledgments
This work was supported by grant PO-0508-0487 from the Slovene Ministry of Higher Education, Science, and Technology.
We thank V. Križan-Hergouth for fruitful discussions.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Altschul, S. F., T. L. Madden, A. A. Schäffer, J. Zhang, Z. Zhang, W. Miller, and D. J. Lipman. 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25:3389-3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderluh, G., I. Gokce, and J. H. Lakey. 2003. Expression of proteins using the third domain of the Escherichia coli periplasmic-protein TolA as a fusion protein. Protein Express. Purif. 28:173-181. [DOI] [PubMed] [Google Scholar]
- 3.Bolivar, F., R. L. Rodriguez, P. J. Greene, M. C. Betlach, H. L. Heyneker, and H. W. Boyer. 1977. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene 2:95-113. [PubMed] [Google Scholar]
- 4.Borodovsky, M., and J. McIninch. 1993. Recognition of genes in DNA sequence with ambiguities. Biosystems 30:161-171. [DOI] [PubMed] [Google Scholar]
- 5.Boyd, A. C., J. A. K. Karcher, and D. J. Sherratt. 1989. Characterization of the ColE1 mobilization region and its protein products. Mol. Gen. Genet. 217:488-498. [DOI] [PubMed] [Google Scholar]
- 6.Bradley, D. E., and S. P. Howard. 1991. Colicinogeny of 0157:H7 enterohemorrhagic Escherichia coli and the shielding of colicin and phage receptors by their O-antigenic side chains. Can. J. Microbiol. 37:97-104. [DOI] [PubMed] [Google Scholar]
- 7.Cao, Z., and P. E. Klebba. 2002. Mechanisms of colicin binding and transport through outer membrane porins. Biochimie 84:399-412. [DOI] [PubMed] [Google Scholar]
- 8.Chan, P. T., H. Ohmori, J. Tomizawa, and J. Lebowitz. 1985. Nucleotide sequence and gene organization of ColE1 DNA. J. Biol. Chem. 260:8925-8935. [PubMed] [Google Scholar]
- 9.Ghosal, D., H. Sommer, and H. Saedler. 1979. Nucleotide sequence of the transposable DNA-element IS2. Nucleic Acids Res. 6:1111-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gillor, O., B. C. Kirkup, and M. A. Riley. 2004. Colicins and microcins: the next generation antimicrobials. Adv. Appl. Microbiol. 54:129-146. [DOI] [PubMed] [Google Scholar]
- 11.Gillor, O., L. M. Nigro, and M. A. Riley. 2005. Genetically engineered bacteriocins and their potential as the next generation of antimicrobials. Curr. Pharm. Dis. 11:1067-1075. [DOI] [PubMed] [Google Scholar]
- 12.Grindley, J. N., and D. Nakada. 1981. The nucleotide sequence of the replication origin of plasmid NTP1. Nucleic Acids Res. 9:4355-4366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofinger, C., H. Karch, and H. Schmidt. 1998. Structure and function of plasmid pColD157 of enterohemorrhagic Escherichia coli and its distribution among strains from patients with diarrhea and hemolytic-uremic syndrome. J. Clin. Microbiol. 36:24-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson, J. R., and A. L. Stell. 2000. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 181:261-272. [DOI] [PubMed] [Google Scholar]
- 15.Jordi, B. J. A. M., K. Boutaga, C. M. E. van Heeswijk, F. van Knapen, and L. J. A. Lipman. 2001. Sensitivity of Shiga toxin-producing Escherichia coli (STEC) strains for colicins under different experimental conditions. FEMS Microbiol. Lett. 204:329-334. [DOI] [PubMed] [Google Scholar]
- 16.Keenleyside, W. J., and C. Whitfield. 1995. Lateral transfer of rfb genes: a mobilizable ColE1-type plasmid carries the rfbO:54 (O:54 antigen biosynthesis) gene cluster from Salmonella enterica serovar Borreze. J. Bacteriol. 177:5247-5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirkup, B. C., and M. A. Riley. 2004. Antibiotic-mediated antagonism leads to a bacterial game of rock-paper-scissors in vivo. Nature 428:412-414. [DOI] [PubMed] [Google Scholar]
- 18.Kuhar, I., and D. Žgur-Bertok. 1999. Transcription regulation of the colicin K cka gene reveals induction of colicin synthesis by differential responses to environmental signals. J. Bacteriol. 181:7373-7380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuhar, I., J. van Putten, D. Žgur-Bertok, W. Gaastra, and B. Jordi. 2001. Codon-usage based regulation of colicin K synthesis by the stress alarmone ppGpp. Mol. Microbiol. 41:207-216. [DOI] [PubMed] [Google Scholar]
- 20.Kurazono, H., M. Nakano, S. Yamamoto, O. Ogawa, K. Yuri, K. Nakata, M. Kimura, S. Makino, and G. B. Nair. 2003. Distribution of the usp gene in uropathogenic Escherichia coli isolated from companion animals and correlation with serotypes and size-variations of the pathogenicity island. Microbiol. Immunol. 47:797-802. [DOI] [PubMed] [Google Scholar]
- 21.Morlon, J., M. Chartier, M. Bidaud, and C. Lazdunski. 1988. The complete nucleotide sequence of the colicinogenic plasmid ColA. High extent of homology with ColE1. Mol. Gen. Genet. 211:231-243. [DOI] [PubMed] [Google Scholar]
- 22.Murinda, S. E., R. F. Roberts, and R. A. Wilson. 1996. Evaluation of colicins for inhibitory activity against diarrheagenic Escherichia coli strains, including serotype O157:H7. Appl. Environ. Microbiol. 62:3196-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pilsl, H., and V. Braun. 1995. Strong function-related homology between pore-forming colicins K and 5. J. Bacteriol. 177:6973-6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pugsley, A. P. 1985. Escherichia coli K12 strains for use in the identification and characteriziation of colicins. J. Gen. Microbiol. 131:369-376. [DOI] [PubMed] [Google Scholar]
- 25.Raggett, E. M., G. Brainbridge, L. J. Evans, A. Cooper, and J. H. Lakey. 1998. Discovery of critical TolA-binding residues in the bactericidal toxin colicin N: a biophysical approach. Mol. Microbiol. 28:1335-1343. [DOI] [PubMed] [Google Scholar]
- 26.Rijavec, M., M. Starčič Erjavec, J. Ambrožič Avguštin, R. Reissbrodt, A. Fruth, V. Križan-Hergouth, and D. Žgur-Bertok. 2006. High prevalence of multidrug resistance and random distribution of mobile genetic elements among uropathogenic Escherichia coli (UPEC) of the four major phylogenetic groups. Curr. Microbiol. 53:158-162. [DOI] [PubMed] [Google Scholar]
- 27.Riley, M. A., and D. M. Gordon. 1996. The ecology and evolution of bacteriocins. J. Ind. Microbiol. 17:151-158. [Google Scholar]
- 28.Schamberger, G. P., and F. Diez-Gonzalez. 2002. Selection of recently isolated colicinogenic Escherichia coli strains inhibitory to Escherichia coli O157:H7. J. Food Prot. 65:1381-1387. [DOI] [PubMed] [Google Scholar]
- 29.Schamberger, G. P., R. L. Phillips, J. L. Jacobs, and F. Diez-Gonzalez. 2004. Reduction of Escherichia coli O157:H7 populations in cattle by addition of colicin E7-producing E. coli to feed. Appl. Environ. Microbiol. 70:6053-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schamberger, G. P., and F. Diez-Gonzalez. 2004. Characterization of colicinogenic Escherichia coli strains inhibitory to enterohemorrhagic Escherichia coli. J. Food Prot. 67:486-492. [DOI] [PubMed] [Google Scholar]
- 31.Šmajs, D., and G. M. Weinstock. 2001. Genetic organization of plasmid ColJs, encoding colicin Js activity, immunity, and release genes. J. Bacteriol. 183:3949-3957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Šmarda, J., and D. Šmajs. 1998. Colicins—exocellular lethal proteins of Escherichia coli. Folia Microbiol. 43:563-582. [DOI] [PubMed] [Google Scholar]
- 33.Stahl, C. H., T. R. Callaway, L. M. Lincoln, S. M. Lonergan, and K. J. Genovese. 2004. Inhibitory activities of colicins against Escherichia coli strains responsible for postweaning diarrhea and edema disease in swine. Antimicrob. Agents Chemother. 48:3119-3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trautner B. W., R. A. Hull, and R. O. D. Arouiche. 2005. Colicins prevent colonization of urinary catheters. J. Antimicrob. Chemother. 56:413-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Walker, D., M. Rolfe, A. Thompson, G. R. Moore, R. James, J. C. D. Hinton, and C. Kleanthous. 2004. Transcriptional profiling of colicin-induced cell death of Escherichia coli MG1655 identifies potential mechanisms by which bacteriocins promote bacterial diversity. J. Bacteriol. 186:866-869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yanisch-Perron, C., J. Vieira, and J. Messing. 1985. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene 33:103-109. [DOI] [PubMed] [Google Scholar]