Until the 1990s, it was thought that, with the exception of cyanobacteria, bacteria had no polyunsaturated fatty acids (PUFAs). This was probably because the bacterial species whose physiology, biochemistry, and molecular biology had been well studied until that time were mesophilic species such as Escherichia coli, which have no PUFAs. It has since been found that n-3 long-chain PUFAs such as eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are preferentially distributed in psychrophilic bacteria that inhabit relatively unusual environments including low-temperature deep-sea environments and the intestines of sea fish (25, 31, 32). Although several PUFA-producing bacterial strains had been reported until the 1970s (15), the discovery of DHA and EPA in bacteria from deep-sea water and sediments and detailed analysis of them by DeLong and Yayanos in 1986 (2) resulted in the initiation of research into PUFA-producing bacteria.
It is interesting that the PUFAs detected in those bacteria were mostly EPA or DHA and not C18 PUFAs such as linoleic and linolenic acids, which are most common in animals, plants, fungi, and cyanobacteria. However, although EPA- and DHA-producing bacteria were discovered in the 1980s, they have not been given much attention. This can easily be imagined, as microorganisms (including eukaryotes such as microalgae) with PUFAs such as EPA and DHA are widespread in marine environments (24, 25, 28). It was also thought that bacterial as well as eukaryotic EPA and DHA were biosynthesized by a combination of elongation and oxygen-dependent desaturation of existing fatty acids. Therefore, the successful cloning of genes involved in the biosynthesis of EPA from Shewanella sp. strain SCRC-2738, isolated from marine fish intestines in 1996 (33), was very important and could be regarded as the first stage of research into such genes. This EPA-producing strain is presently identified as Shewanella pneumatophori SCRC-2738 (4). It was very surprising that the deduced proteins encoded by the EPA biosynthesis genes (pfa genes) (see below) included no conserved sequences from fatty acid desaturase genes (7, 33), although data on fatty acid desaturases were very limited in the early 1990s (11). Five deduced proteins involved in EPA biosynthesis had domains that were conserved in fatty acid synthetase and/or polyketide synthases (PKSs) (7). In addition, evidence has been presented to show that bacterial PUFAs can be synthesized under anaerobic conditions (7).
After the discovery of the EPA biosynthetic pfa genes, their homologues were cloned from various marine bacteria. The pfa genes of Moritella marina MP-1 were the first genes to be cloned from DHA-producing bacteria (27). Recent genome sequencing of various organisms, including bacteria, demonstrated that pfa genes are distributed abundantly in members of the bacterial genera Shewanella (29) and Colwellia (6) and that these strains have been isolated mainly from marine sources. There are some variations in the structures of pfa gene clusters, although they have a basic structure that is common among all types of clusters (see below). There is no doubt that all bacterial EPA and DHA are synthesized by the PKS system (7, 16).
Although the recombinant production of EPA in E. coli was achieved in 1996 using pfa genes from S. pneumatophori SCRC-2738 (33), no reports of the heterologous synthesis of DHA were found in the literature. This was because the pfa gene cluster in M. marina MP-1 lacked a gene corresponding to the pfaE gene of S. pneumatophori SCRC-2738. Recently, however, the pfaE gene was cloned from M. marina MP-1 (17), and recombinant DHA was produced (19). This might be called the second stage of research. Here, we review the bacterial genes responsible for the biosynthesis of EPA and DHA, covering such aspects as gene cloning, characterization of the structure of the genes and their domain structures, and recombinant production of EPA and DHA.
GENES RESPONSIBLE FOR THE BIOSYNTHESIS OF EPA AND DHA
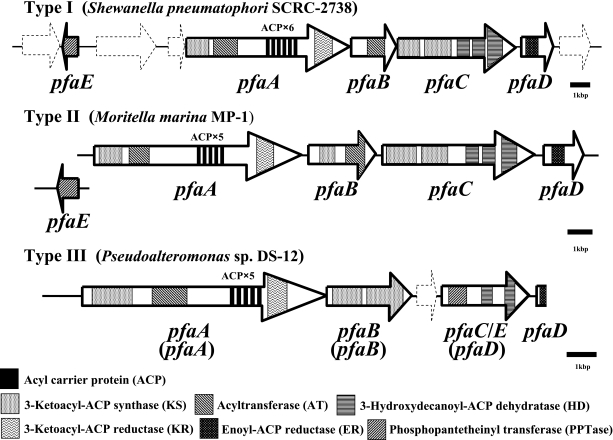
The genes responsible for the biosynthesis of EPA were first cloned as a cosmid carrying a DNA fragment of approximately 38 kbp (33). This fragment carried at least 18 open reading frames (ORFs), of which a cluster of only five was necessary for the biosynthesis of EPA. These ORFs are now named pfaA, pfaB, pfaC, pfaD, and pfaE (13, 17) (Fig. 1). The clustered genes were designated the “EPA biosynthesis gene cluster.” When various E. coli strains were transformed with the EPA biosynthesis gene cluster, they normally produced EPA at 1% to 5% of total fatty acids (20, 33).
FIG. 1.
Organization of genes responsible for bacterial EPA and DHA biosynthesis and domain structures of individual genes. The organization of gene clusters is divided into three types. Type I, which is represented by Shewanella pneumatophori SCRC-2738, is a gene cluster including all five pfa genes in a similar vicinity. Type II consists of a cluster of the four genes pfaABCD, with pfaE separate from the other genes. This type of cluster is represented by Moriella marina MP-1. The relative direction of pfaE has not been determined for this bacterium. In type III, pfaE is integrated into pfaC/E, and the cluster is considered to consist of four genes. In this review, the remnant sequence downstream of the pfaC/E gene was regarded as a partial sequence of pfaD. The original nomenclature (Dai and Zhang, unpublished) (GenBank accession no. ABF00130) is shown in parentheses. The third type of cluster has been reported for Pseudoalteromonas sp. strain DS-12 only.
Genes homologous to the pfa genes have been cloned from various EPA- and DHA-producing bacteria. Allen and Bartlett (1) previously reported the finding of a cluster of EPA biosynthesis genes in Photobacterium profundum SS9. However, this cluster did not include pfaE, which encodes phosphopantetheinyl transferase (PPTase). A partial sequence corresponding to pfaA was cloned from EPA-producing Shewanella sp. strain GA-22 (3). Clustered genes homologous to those from S. pneumatophori SCRC-2738 were cloned from the DHA-producing deep-sea bacterial species M. marina MP-1 (27). This gene cluster also included only pfaABCD and lacked pfaE. Therefore, attempts at the recombinant production of DHA in E. coli or in other host organisms were unsuccessful until quite recently. The pfa genes from M. marina MP-1 constitute the only DHA biosynthesis gene cluster currently cloned.
Genome sequencing of various bacteria either known or expected to produce EPA or DHA demonstrates the abundance and wide distribution of such genes. Interestingly, bacteria that have genes homologous to pfa genes are found mostly in marine sources. In the genome of EPA-producing Shewanella oneidensis MR-1 (29), a cluster of pfaABCDE genes was found. Colwellia psychrerythraea 34H (6), which is expected (but not confirmed) to produce DHA, has a similar cluster of genes. All of the aforementioned pfa gene clusters contained pfaE as an ORF encoding a single protein either within the cluster or outside the cluster. The former and latter structures of pfa gene clusters are designated type I and type II pfa genes, respectively, in this review (Fig. 1). However, the structure of the type I pfa genes is unlikely to be essentially different from that of the type II pfa genes. This is known because even the gene cluster from S. pneumatophori SCRC-2738, which contains all five genes, has the sequential arrangement of pfa genes broken by the presence of two ORFs unrelated to EPA biosynthesis (Fig. 1). Unlike these structures, Pseudoalteromonas sp. strain DS-12 has a unique pfa gene cluster consisting of four ORFs (M. Dai and P. Zhang, unpublished data) (GenBank accession no. ABF00130). In this strain, PfaE is integrated into PfaC/E, and pfaB and pfaC/E genes are separated by one unrelated ORF (Fig. 1). This is designated the type III pfa gene. Gene sequences homologous to those of the pfa genes have also been found in eukaryotic marine microalgae producing EPA and DHA (7). Table 1 summarizes known bacterial genes that are homologous to the EPA biosynthesis genes pfaABCDE of S. pneumatophori SCRC-2738.
TABLE 1.
List of known bacterial pfa genesa
| Organism | Productb |
pfa genes in the cluster
|
No. of ACP repeats in PfaA | Domain(s) in PfaB | HD domains in PfaC or PfaC/E |
pfaE (PfaE)
|
Recombinant synthesis of the product | Reference or source | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Typec | Cloning | Groupd | Cloning | |||||||
| S. pneumatophori SCRC-2738 | EPA | I | Yes | 6 | AT | FabA-FabA-FabA | I | Yes | Yes | 20 |
| S. marinintestina IK-1 | EPA | I | Yes | 6 | AT | FabA-FabA-FabA | I | Yes | Yes | Unpublishede |
| Shewanella sp. strain SC2A | EPA | —f | No | — | — | — | I | Yes | No | 1 |
| S. oneidensis MR-1 | EPA | I | No | 4 | AT | FabA-FabAg | I | No | No | 29 |
| Shewanella sp. strain GA-22h | EPAi | — | No | — | — | — | I | — | — | 3 |
| P. profundum SS9 | EPA | II | Yes | 6 | AT | FabA-FabAg | II | No | No | 1, 30 |
| Pseudoalteromonas sp. strain DS-12 | (EPA) | III | Yes | 5 | KS, KS | FabA-FabAj | Ik | Yes | No | GenBank accession no. ABF00130 |
| M. marina MP-1 | DHA | II | Yes | 5 | KS, AT | FabA-FabZ/FabA-FabA | I | Yes | Yes | 17, 18 |
| C. psychrerythreae 34H | (DHA) | I | No | 6 | KS, AT | FabA-FabAg | I | No | No | 6 |
Nucleic acid and deduced amino acid sequences were retrieved from databases (DDBJ/GenBank/EMBL) (http://www.ddbj.nig.ac.jp/Welcome-j.html). The name of each domain in individual pfa genes is described in the legend of Fig. 1.
In cases of (EPA) and (DHA), the production of EPA or DHA is expected but not confirmed.
Type I, II, and III pfa genes are defined in the text.
Group I and II PPTases are defined in the text and reference 18.
Unpublished result by N. Morita, Y. Yano, S. Ohgiya, and H. Okuyama.
—, no information is available.
Unannotated 900- to 1,000-bp sequences are present between the two FabA-like sequences.
Only a 1,624-bp partial pfaA sequence has been deposited (GenBank accession no. AJ563807).
Arachidonic acid (20:4n-6) and linoleic acid (18:2n-6) were also detected.
Unannotated 360- to 450-bp sequences are present between the two FabA-like sequences.
pfaE is included in pfaC/E.
STRUCTURE OF INDIVIDUAL pfa GENES
It is evident that only five pfaABCDE genes are generally necessary for the biosynthesis of EPA and DHA. Although the basic structures of all pfa genes for EPA and DHA biosynthesis are very similar, the domain structures of some of the individual genes are slightly different (Fig. 1 and Table 1). In the type I and type II pfa gene clusters, pfaA is tentatively thought to encode a multifunction protein that includes domains for 3-ketoacyl synthase (KS), malonyl coenzyme A:acyl carrier protein (ACP) acyltransferase, normally five or six ACP repeats, and 3-ketoacyl-ACP reductase (KR). The pfaC gene encodes a protein with two KS repeats and two or three 3-hydroxydecanoyl-ACP dehydratases (HD). The second KS domain in PfaC of S. pneumatophori SCRC-2738, P. profundum SS9, and M. marina MP-1 was considered to be a chain length factor (1, 17, 19). In this study, however, both domains remained undifferentiated, as information about their precise function was not available. Previously, the number of HD domains in PfaC was recognized to be two (1, 7, 17); however, a very recent database search demonstrated that PfaC includes three sequential domains of HD based on S. pneumatophori SCRC-2738, Shewanella marinintestina IK-1, and M. marina MP-1 only (Fig. 1 and Table 1). Interestingly, in PfaC for EPA, all three HD domains were homologous to FabA, with a high degree of similarity, while PfaC for DHA had two HD domains similar to that of FabA and one domain, in the center, similar to that of FabZ/FabA (Table 1). Genes pfaB and pfaD encode proteins with KS and acyltransferase domains and an enoyl reductase domain, respectively (7, 20). A KS domain is included only in PfaB for DHA derived from M. marina MP-1 (27) and C. psychrerythraea 34H (6). However, the KS domain of PfaB in M. marina MP-1 lacked an active-site sequence (1). The domain structure of the pfa genes of Pseudoalteromonas sp. strain DS-12 was quite different from that of the same genes in the other bacterial strains. PfaB was found to have two KS domains, and PfaC/E (which was registered as a product of the pfaD gene in the database) (Dai and Zhang, unpublished) has one PPTase domain and two HD domains (Fig. 1). At the downstream end of the pfaC/E gene was a 483-bp remnant DNA region (this was regarded as the pfaD gene in this review), which included a partial sequence similar to that of enoyl reductase.
Compared with PfaABCD, the domain structures of PfaE are well characterized. pfaE (PPTase gene) for the biosynthesis of EPA or DHA can be regarded as a member of a large gene family of Sfp-type PPTases based on the sizes of the deduced protein molecules and their domain structures (17). Based on their domain structures, Orikasa et al. (17, 18) divided all Sfp-type PPTases into two groups: PPTases responsible mainly for the biosynthesis of EPA or DHA (group I), characterized by P0, P1a, and P1b domains, and those responsible mainly for the synthesis of polyketides and nonribosomal peptides (group II), characterized by the domains 1A, P1a′, and P1b′, which correspond to the P0, P1a, and P1b domains of group I (18). Although the P2 and P3 domains are commonly conserved in the two groups, there is a higher degree of similarity in these domains within groups than between groups (18). The pfaE gene complementing the pfaABCD genes of P. profundum SS9 has not been cloned. However, genome sequencing of this bacterium (30) provided a candidate sequence (GenBank accession no. CAG23685) from the group II Sfp-type PPTase (PfaE). On the other hand, the Sfp-type PPTase of Bacillus subtilis, Sfp itself, which is involved in the biosynthesis of surfactin (a nonribosomal peptide) (12), belongs to group I (18).
FUNCTIONAL COMPATIBILITY OF INDIVIDUAL pfa GENES
The compatibility of pfa genes involved in the biosynthesis of PUFAs has been investigated mostly using the pfa genes from the EPA-producing S. pneumatophori strain SCRC-2738 and DHA-producing M. marina strain MP-1. The pfaE gene (pETSTV::pfaE) from M. marina MP-1 complemented the pDHA3 vector carrying pfaABCD genes from DHA-producing M. marina MP-1 (19) as well as the pEPAΔ1,2,3 vector carrying pfaABCD genes from EPA-producing S. pneumatophori SCRC-2738 (17). To examine the compatibility of pfaE from EPA-producing S. pneumatophori SCRC-2738 with pDHA3 from M. marina MP-1, an E. coli DH5α transformant that utilized pEPAΔ5 carrying pfaBCDE genes from S. pneumatophori SCRC-2738 and pDHA3 was produced (18). Both EPA and DHA were produced in this combination, suggesting that the pfaE gene in pEPAΔ 5 is involved in producing DHA (18); that is, the PPTase (PfaE) from S. pneumatophori SCRC-2738 was able to recognize the ACP repeats (substrate) integrated into the pfaA gene product of M. marina MP-1, and the pfaA product of the DHA biosynthesis gene cluster played a role in EPA biosynthesis. Orikasa et al. (18, 19) obtained pDHA2 in the course of preparing pDHA3. Although pDHA2 carried pfaABCD genes from M. marina MP-1, the combined expression of pDHA2 and pfaE produced neither EPA nor DHA, as the pfaA in pDHA2 had one fatal base pair replacement, inactivating the gene cluster. However, when pDHA2 was coexpressed with any of the three types of deletion clones of the pEPA clusters from S. pneumatophori SCRC-2738 (that is, the clones carrying pfaA, pfaC, pfaD, and pfaE; pfaA, pfaB, pfaD, and pfaE; or pfaA, pfaB, pfaC, and pfaE) in E. coli, all transformants produced both EPA and DHA. In contrast, neither EPA nor DHA was produced by a combination of pDHA2 and a deletion clone carrying pfaB, pfaC, pfaD, and pfaE (Y. Orikasa, A. Yamada, A. Yu, K. Watanabe, and H. Okuyama, unpublished data). All these results suggest that not only pfaE and pfaA but also pfaB, pfaC, and pfaD are functionally compatible in the biosynthesis of EPA and DHA. The recombinant production of EPA and trace levels of DHA in E. coli was confirmed by the use of pfa genes (pIK814) of S. marinintestina IK-1 and by a combination of these genes with pDHA2 from M. marina MP-1 (N. Morita, Y. Yano, S. Ohgiya, and H. Okuyama, unpublished data).
According to Allen and Bartlett (1), the pfaABCD gene cluster in P. profundum SS9 did not complement PPTase genes from Shewanella sp. strain SC2A and B. subtilis, both of which are classed into group I, in the production of EPA. No sequences similar to those PPTases, other than the deduced sequence reported under GenBank accession no. CAG23685, have been found in the genome (i.e., in either chromosome 1 or 2) of P. profundum SS9 (30). It would be interesting to examine the compatibility of the PPTase gene from P. profundum SS9 with the pfaABCD genes from this strain and those from other EPA- or DHA-producing bacteria such as S. pneumatophori SCRC-2738 and M. marina MP-1. The functional compatibility of each pfa gene is summarized in Table 2.
TABLE 2.
Recombinant production of EPA and/or DHA using pfa genes from Shewanella pneumatophori SCRC-2738, Moritella marina MP-1, and Photobacterium profundum SS9a
| Source and combination of pfa genes of S. pneumatophori SCRC-2738 | Source and combination of pfa genes
|
Production of EPA and/or DHA | Reference | |
|---|---|---|---|---|
| M. marina MP-1 | P. profundum SS9 | |||
| pfaABCDEb | EPA | 20 | ||
| pfaABCDc plus pfaEd | EPA | 20 | ||
| pfaABCDc | pfaEe | EPA | 17 | |
| pfaABCDf plus pfaEg | DHA | 19 | ||
| pfaBCDEh | pfaABCDf | EPA and DHA | 18 | |
| pfaEd | pfaA*BCDi | None | 17 | |
| pfaA*BCDi plus pfaEg | None | 17 | ||
| pfaABCDj plus pfaEk | None | 1 | ||
This table is modified from data reported previously in reference 18.
pfa genes are harbored in pEPAΔ1.
pfa genes are harbored in pEPAΔ1,2,3.
The pfaE gene is harbored in pSTV::pfaE.
The pfaE gene is harbored in pETSTV::pfaE.
pfa genes are harbored in pDHA3.
The pfaE gene is harbored in pET21::pfaE.
pfa genes are harbored pEPAΔ5.
pfa genes are harbored in pDHA2, where pfaA* is inactive.
pfa genes are harbored in pFOS8E1.
PPTase genes from Bacillus subtilis and Shewanella sp. strain SC2A were used.
FUTURE PERSPECTIVES
The EPA and DHA biosynthesis gene clusters were initially cloned with the aim of expressing them in various host organisms such as cyanobacteria, yeast, and plants (27, 33) for the purpose of producing commercially important materials. However, only low levels of EPA were produced by the recombination of the genes in cyanobacteria (26, 34). The recombinant production of DHA in E. coli has been reported quite recently (18). Although fish oils are the most important source of EPA and DHA, the contamination of fish due to pollution, as well as unstable fish catches, has created a need for alternative ways to provide those PUFAs (21). The use of the PKS system to produce EPA or DHA in heterologous host organisms has some benefits, such as the need for lesser amounts of reducing equivalents such as NADPH (19, 23) and the simplicity that the PUFAs have in consisting solely of EPA or DHA. Since bacterial PKS systems involved in the production of EPA and DHA are generally less active at moderate temperatures (19, 20), their genetic modification and selection in host organisms should be considered.
The PKS systems would provide a useful tool for investigating the physiological roles of EPA and DHA and their biosynthetic mechanisms. EPA levels in recombinants can be changed at random using various vectors carrying the pfa gene(s) from S. pneumatophori SCRC-2738 (20). The antioxidative function of EPA was first observed using such a recombinant in E. coli (13, 14, 15). Similar systems could be constructed for DHA by isolating individual pfa genes involved in DHA production. A biosynthetic mechanism has been proposed for EPA (and probably DHA) production, which is similar to the anaerobic pathway of unsaturated fatty acid biosynthesis (7, 16). However, no direct evidence is available to support this proposed mechanism. To detect intermediates in the biosynthesis of EPA or DHA, PKS recombinant systems could be used. The heterologous production of DHA in E. coli that had been transformed with pfa genes from the marine M. marina MP-1 was more active at lowered growth temperatures (19). The finding coincided with the finding that this bacterium is psychrophilic and that it inherently formed more DHA at low temperatures (2). However, the effects of salinity of culture media and hydrostatic conditions on the recombinant production of PUFAs have not been elucidated. The transcriptional regulation of pfaABCD genes has been studied by targeting the pfaABCD genes of P. profundum SS9 (1). More information would be produced by using recombinant systems carrying various combinations of pfa genes. Normal concentrations (10 to 100 μM) of cerulenin, an inhibitor of de novo biosynthesis of fatty acids, enhanced the synthesis of EPA and DHA in P. profundum SS9, S. marinintestina IK-1, and M. marina MP-1 (1, 8). It has been demonstrated that M. marina MP-1 has a fatty acid biosynthetic (fab) gene cluster that takes part in the de novo synthesis of fatty acids with moderate chain lengths up to C18 (9, 10). The relationship between the PKS system and de novo fatty acid biosynthesis could be investigated using the PKS recombinant systems.
Menzella et al. (5) previously proposed combinatorial polyketide biosynthesis by the design and rearrangement of modular PKS genes. Type I PKS genes of 3 to 6 kbp are similar to the pfa genes. Some pfa genes, such as pfaA and pfaC, that are structurally similar to PKS genes might be used for the production of novel and commercially beneficial polyketides. If five or six repeats of ACP domains operate as a cluster to enhance the biosynthesis of PUFAs, as is the case in the biosynthesis of the polyketide antibiotic mupirocin in Pseudomonas fluorescens, in which two tandem repeats of carrier proteins are involved (22), the corresponding DNA region would become a useful tool for the enhanced production of various types of polyketides.
We were unable to identify the factor(s) that determines the final product in PKS systems that produce PUFAs. It is speculated that some cooperative interactions between domains of different Pfa proteins, rather than the activity of any single Pfa protein, might be involved in directing the final product in the system. To find an answer to this question would be the third stage in this research.
ADDENDUM IN PROOF
A recent study (A. Hauvermale, J. Kuner, B. Rosenzweig, D. Guerra, S. Diltz, and J. M. Metz, Lipids 41:739-347, 2006) reports that PPTase genes from B. subtilis and Nostoc sp. strain PCC7120 complemented pfa genes from Schizochytrium sp. in the E. coli recombinant system. In this review, those two PPTases can be categorized to group I Sfp-type PPTase, which is involved in the biosynthesis of EPA or DHA.
Acknowledgments
All pfa genes from Shewanella pneumatophori SCRC-2738 used in this study were provided by Sagami Chemical Research Center, Japan.
This work summarized here was financially supported, in part, by the National Institute of Polar Research.
Footnotes
Published ahead of print on 22 November 2006.
REFERENCES
- 1.Allen, E. E., and D. H. Bartlett. 2002. Structure and regulation of the omega-3 polyunsaturated fatty acid synthase genes from the deep-sea bacterium Photobacterium profundum strain SS9. Microbiology 148:1903-1913. [DOI] [PubMed] [Google Scholar]
- 2.DeLong, E. F., and A. A. Yayanos. 1986. Biochemical function and ecological significance of novel bacterial lipids in deep-sea procaryotes. Appl. Environ. Microbiol. 51:730-737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gentile, G., V. Bonasera, C. Amico, L. Giuliano, and M. M. Yakimov. 2003. Shewanella sp. GA-22, a psychrophilic hydrocarbonoclastic Antarctic bacterium producing polyunsaturated fatty acids. J. Appl. Microbiol. 95:1124-1133. [DOI] [PubMed] [Google Scholar]
- 4.Hirota, K., Y. Nodasaka, Y. Orikasa, H. Okuyama, and I. Yumoto. 2005. Shewanella pneumatophori sp. nov., eicosapentanoic-acid-producing marine bacterium isolated from pacific mackerel (Pneumatophorus japonicus) intestine. Int. J. Syst. Evol. Microbiol. 55:2355-2359. [DOI] [PubMed] [Google Scholar]
- 5.Menzella, H. G., R. Reid, J. R. Carney, S. S. Chandran, S. J. Reisinger, K. G. Patel, D. A. Hopwood, and D. V. Santi. 2005. Combinatorial polyketide biosynthesis by de novo design and rearrangement of modular polyketide synthase genes. Nat. Biotechnol. 23:1171-1176. [DOI] [PubMed] [Google Scholar]
- 6.Methé, B. A., K. F. Nelson, J. W. Deming, B. Momen, E. Melamud, X. Zhang, J. Moult, R. Madupu, W. C. Nelson, R. J. Dodson, L. M. Brinkac, S. C. Daugherty, A. S. Durkin, R. T. DeBoy, J. F. Kolonay, S. A. Sullivan, L. Zhou, T. M. Davidsen, M. Wu, A. L. Huston, M. Lewis, B. Weaver, J. F. Weidman, H. Khouri, T. R. Utterback, T. V. Feldblyum, and C. M. Fraser. 2005. The psychrophilic lifestyle as revealed by the genome sequence of Colwellia psychrerythraea 34H through genomic and proteomic analyses. Proc. Natl. Acad. Sci. USA 102:10913-10918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Metz, J. G., P. Roessler, D. Facciotti, C. Levering, F. Dittrich, M. Lassner, R. Valentine, K. Lardizabal, F. Domergue, A. Yamada, K. Yazawa, V. Knauf, and J. Browse. 2001. Production of polyunsaturated fatty acids by polyketide synthases in both prokaryotes and eukaryotes. Science 293:290-293. [DOI] [PubMed] [Google Scholar]
- 8.Morita, N., T. Nishida, M. Tanaka, Y. Yano, and H. Okuyama. 2005. Enhancement of polyunsaturated fatty acid production by cerulenin treatment in polyunsaturated fatty acid-producing bacteria. Biotechnol. Lett. 27:389-393. [DOI] [PubMed] [Google Scholar]
- 9.Morita, N., M. Tanaka, and H. Okuyama. 2000. Biosynthesis of fatty acids in the docosahexaenoic acid-producing bacterium Moritella marina strain MP-1. Biochem. Soc. Trans. 28:943-945. [PubMed] [Google Scholar]
- 10.Morita, N., A. Ueno, M. Tanaka, S. Ohgiya, H. Hoshino, K. Kawasaki, I. Yumoto, K. Ishizaki, and H. Okuyama. 1999. Cloning and sequencing of clustered genes involved in fatty acid biosynthesis from the docosahexaenoic acid-producing bacterium, Vibrio marinus strain MP-1. Biotechnol. Lett. 21:641-646. [Google Scholar]
- 11.Murata, N., and H. Wada. 1995. Acyl-lipid desaturases and their importance in the tolerance and acclimatization to cold of cyanobacteria. Biochem. J. 308:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nakano, M. M., N. Corbell, J. Besson, and P. Zuber. 1992. Isolation and characterization of sfp: a gene that functions in the production of the lipopeptide biosurfactant, surfactin, in Bacillus subtilis. Mol. Gen. Genet. 232:313-321. [DOI] [PubMed] [Google Scholar]
- 13.Nishida, T., Y. Orikasa, Y. Ito, R. Yu, A. Yamada, K. Watanabe, and H. Okuyama. 2006. Escherichia coli engineered to produce eicosapentaenoic acid becomes resistant against oxidative damages. FEBS Lett. 580:2731-2735. [DOI] [PubMed] [Google Scholar]
- 14.Nishida, T., Y. Orikasa, K. Watanabe, N. Morita, and H. Okuyama. Evaluation of the antioxidative effects of eicosapentaenoic acid by growth inhibition testing on plates using Escherichia coli transformed with pfa genes. In C. Benning and J. Ohlrogge (ed.), Advances in plant lipid research. Proceedings of the 17th International Symposium on Plant Lipids, East Lansing, Michigan, July 2006, in press. Michigan State University Press, East Lansing, MI.
- 15.Oliver, J. D., and R. R. Colwell. 1973. Extractable lipids of gram-negative marine bacteria: fatty acid composition. Int. J. Syst. Bacteriol. 114:897-908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ootaki, M., N. Morita, T. Nishida, M. Tanaka, A. Hase, Y. Yano, A. Yamada, R. Yu, K. Watanabe, and H. Okuyama. 2003. Genes and pathways involved in biosynthesis of eicosapentaenoic and docosahexaenoic acids in bacteria, p. 49-52. In N. Murata, M. Yamada, I. Nishida, H. Okuyama, J. Sekiya, and H. Wada (ed.), Advanced research on plant lipids. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 17.Orikasa, Y., T. Nishida, A. Hase, K. Watanabe, N. Morita, and H. Okuyama. 2006. A phosphopantetheinyl transferase gene essential for biosynthesis of n-3 polyunsaturated fatty acids from Moritella marina strain MP-1. FEBS Lett. 580:4423-4429. [DOI] [PubMed] [Google Scholar]
- 18.Orikasa, Y., T. Nishida, K. Watanabe, N. Morita, and H. Okuyama. Phosphopantetheinyl transferase genes essential for biosynthesis of polyunsaturated fatty acids and their domain structures and compatibility. In C. Benning and J. Ohlrogge (ed.), Advances in plant lipid research. Proceedings of the 17th International Symposium on Plant Lipids, East Lansing, Michigan, July 2006, in press. Michigan State University Press, East Lansing, MI.
- 19.Orikasa, Y., T. Nishida, A. Yamada, R. Yu, K. Watanabe, A. Hase, N. Morita, and H. Okuyama. 2006. Recombinant production of docosahexaenoic acid in a polyketide biosynthesis mode in Escherichia coli. Biotechnol. Lett. 28:1841-1847. [DOI] [PubMed] [Google Scholar]
- 20.Orikasa, Y., A. Yamada, R. Yu, Y. Ito, T. Nishida, I. Yumoto, K. Watanabe, and H. Okuyama. 2004. Characterization of the eicosapentaenoic acid biosynthesis gene cluster from Shewanella sp. strain SCRC-2738. Cell. Mol. Biol. 50:625-630. [PubMed] [Google Scholar]
- 21.Qi, B., T. Fraser, S. Mugford, G. Dobson, O. Sayanova, J. Butler, J. A. Napier, A. T. Stobart, and C. M. Lazarus. 2004. Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat. Biotechnol. 22:739-745. [DOI] [PubMed] [Google Scholar]
- 22.Rahman, A. S., J. Hothersall, J. Crosby, T. J. Simpson, and C. M. Thomas. 2005. Tandemly duplicated acyl carrier proteins, which increase polyketide antibiotic production, can apparently function either in parallel or in series. J. Biol. Chem. 280:6399-6408. [DOI] [PubMed] [Google Scholar]
- 23.Ratledge, C. 2004. Fatty acid biosynthesis in microorganisms being used for single cell oil production. Biochimie 86:807-815. [DOI] [PubMed] [Google Scholar]
- 24.Russell, N. J., and D. S. Nichols. 1999. Polyunsaturated fatty acids in marine bacteria—a dogma rewritten. Microbiology 145:767-779. [DOI] [PubMed] [Google Scholar]
- 25.Singh, A., and O. P. Ward. 1997. Microbial production of docosahexaenoic acid (DHA, C22:6). Adv. Appl. Microbiol. 45:271-312. [DOI] [PubMed] [Google Scholar]
- 26.Takeyama, H., D. Takeda, K. Yazawa, A. Yamada, and T. Matsunaga. 1997. Expression of the eicosapentaenoic acid synthesis gene cluster from Shewanella sp. in a transgenic marine cyanobacterium, Synechococcus sp. Microbiology 143:2725-2731. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka, M., A. Ueno, K. Kawasaki, I. Yumoto, S. Ohgiya, T. Hoshino, K. Ishizaki, H. Okuyama, and N. Morita. 1999. Isolation of clustered genes that are notably homologous to the eicosapentaenoic acid biosynthesis gene cluster from the docosahexaenoic acid-producing bacterium Vibrio marinus strain MP-1. Biotechnol. Lett. 21:939-945. [Google Scholar]
- 28.Valentine, R. C., and D. L. Valentine. 2004. Omega-3 fatty acids in cellular membranes: a unified concept. Prog. Lipid Res. 43:383-402. [DOI] [PubMed] [Google Scholar]
- 29.Venkateswaran, K., D. P. Moser, M. E. Dollhopf, D. P. Lies, D. A. Saffarini, B. J. MacGregor, D. B. Ringelberg, D. C. White, M. Nishijima, H. Sano, J. Burghardt, E. Stackebrandt, and K. H. Nelson. 1999. Polyphasic taxonomy of the genus Shewanella and description of Shewanella oneidensis sp. nov. Int. J. Syst. Bacteriol. 2:705-724. [DOI] [PubMed] [Google Scholar]
- 30.Vezzi, A., S. Campanaro, M. D'Angelo, F. Simonato, N. Vitulo, F. M. Lauro, A. Cestaro, G. Malacrida, B. Simionati, N. Cannata, C. Romualdi, D. H. Bartlett, and G. Valle. 2005. Life at depth: Photobacterium profundum genome sequence and expression analysis. Science 307:1459-1461. [DOI] [PubMed] [Google Scholar]
- 31.Wirsen, C. O., H. W. Jannaschm, S. G. Waleham, and E. A. Canuel. 1987. Membrane lipids of a psychrophilic and barophilic deep-sea bacterium. Curr. Microbiol. 14:319-322. [Google Scholar]
- 32.Yano, Y., A. Nakayama, H. Saito, and K. Ishihara. 1994. Production of docosahexaenoic acid by marine bacteria isolated from deep sea fish. Lipids 29:527-528. [DOI] [PubMed] [Google Scholar]
- 33.Yazawa, K. 1996. Production of eicosapentaenoic acid from marine bacteria. Lipids 31:S297-S300. [DOI] [PubMed] [Google Scholar]
- 34.Yu, R., A. Yamada, K. Watanabe, K. Yazawa, H. Takeyama, T. Matsunaga, and R. Kurane. 2000. Production of eicosapentaenoic acid by a recombinant marine cyanobacterium, Synechococcus sp. Lipids 35:1061-1064. [DOI] [PubMed] [Google Scholar]