Abstract
The death receptor apoptosis pathway is intimately connected with the mitochondrial apoptosis pathway. Bid is a BH3-only pro-death Bcl-2 family protein and is the major molecule linking the two pathways. Bid-mediated mitochondrial activation occurs early and is responsible for the prompt progress of tumor necrosis factor alpha (TNF-α)-induced apoptosis. However, in both cultured cells and animal models of TNF-α-induced injury, later-phase Bid-independent mitochondrial activation could be demonstrated. Consequently, bid-deficient mice are still susceptible to endotoxin-induced liver injury and mortality. Notably, embryonic hepatocyte apoptosis and lethality caused by TNF-α in the absence of p65relA cannot be rescued by the simultaneous deletion of bid. Further studies indicate that multiple mechanisms including reactive oxygen species, JNK, and permeability transition are critically involved in Bid-independent mitochondrial activation. Inhibition of these events suppresses TNF-α-induced mitochondrial activation and apoptosis in bid-deficient cells. These findings thus indicate that there are at least two sets of mechanisms of mitochondrial activation upon TNF-α stimulation. While the Bid-mediated mechanism is rapid and potent, the Bid-independent mechanism progresses gradually and involves multiple players. The critical involvement of Bid-independent mitochondrial activation in TNF-α-induced apoptosis demands the intervention of TNF-α-mediated tissue injury via multiple avenues.
Tumor necrosis factor alpha (TNF-α) is a pleiotropic cytokine that can induce both cell death and cell proliferation. TNF-α-induced cell death is normally blocked by the simultaneously activated NF-κB pathway (27). Gene products regulated by NF-κB can suppress caspase activation, mitochondrial activation, reactive oxygen species (ROS) accumulation, and JNK activation (5, 10, 12, 27, 34, 40, 43, 45, 51). Deletion of key molecules that are responsible for the activity of NF-κB, such as IκB kinase β (30, 32, 50), IκB kinase γ (42), and p65RelA (1, 2), leads to overwhelming activation of the death pathway. Mice deficient in these molecules display significant apoptosis in the liver, die during embryonic development (2, 30, 32, 42, 50), and can only be rescued by the simultaneous deletion of TNF-α (15) or TNF receptor 1 (30). Under experimental conditions, TNF-α-toxicity is often manifested when NF-κB activity is blocked by either an undegradable I-κBα mutant (I-κBαsr, S32AS36A) (23) or by a general transcription inhibitor such as actinomycin or the liver-specific agent d-galactosamine (GalN) (61). Transcription inhibition may mimic certain pathological conditions in which the NF-κB pathway becomes defective and TNF-α-induced tissue injury develops.
TNF-α can induce cell death via multiple mechanisms (14, 54). TNF-α binds to TNF receptor 1, which recruits the adaptor molecule TRADD and FADD to activate a caspase cascade from upstream initiation caspase 8 or caspase 10 to the downstream effector caspase 3 (54). The TNF-α-induced caspase cascade can be blocked by multiple inhibitor-of-apoptosis proteins (IAPs) such as cIAP1, cIAP2, and XIAP (10, 29, 55) or FLIP (5, 24), which are all promoted by NF-κB activation. Mitochondrial participation is necessary to overcome these inhibitory mechanisms. Mitochondrial release of cytochrome c leads to activation of caspase 9, which bypasses caspase 8 function, and release of Smac leads to the deactivation of IAPs and allows caspase activation (10, 16, 29). Furthermore, mitochondrion-originated ROS may promote the degradation of FLIP and thus caspase 8 activation (5, 13).
Among the many death-inducing mechanisms triggered by TNF-α, the role of Bid, a BH3-only pro-death Bcl-2 family protein, is of particular note. Bid is the main molecular linker connecting the death receptor pathway and the mitochondrial pathway. Bid can activate mitochondria via direct interaction with the multidomain pro-death molecule Bax or Bak (11, 13, 58, 63, 64) or via the cathepsin B and caspase 2 pathway (20). bid-deficient mice and cells manifest defective mitochondrial activation, reduced ROS generation, weakened FLIP degradation, limited lysosome permeability, and increased resistance to TNF-α-induced apoptosis and tissue injury (13, 20, 63, 64).
Bid can be activated by caspase 8 (19, 64) or JNK (10) following TNF-α stimulation, both of which could be suppressed by the NF-κB pathway (5, 10, 12, 27, 34, 40, 43, 45, 51). In addition, NF-κB-mediated mechanisms could inhibit mitochondrial apoptotic events via the upregulation of Bcl-xL or MnSOD (27). Thus, one might wonder whether Bid is the ultimate mediator of the NF-κB-suppressible death mechanisms that act on mitochondria. However, bid-deficient mice still suffered from TNF-α-induced liver injury and mortality in an endotoxin model in which NF-κB activation is blocked by GalN (64). It was not known whether this indicates that the mitochondrial pathway or the Bid molecule is dispensable in TNF-α-mediated apoptosis and whether this is related to the way NF-κB is inhibited.
In this study, we found that the mitochondrial apoptosis pathway remains critical in TNF-α-induced apoptosis in the absence of Bid, independently of how NF-κB is suppressed. Notably, we found that deletion of bid could not rescue the liver apoptosis and embryonic lethality caused by p65relA deficiency. Bid-independent mitochondrial activation involves the participation of ROS, mitochondrial permeability transition (PT), and JNK with Bax and Bak activated. In contrast to the Bid-mediated events, which occur early in TNF-α activation, the Bid-independent mechanisms function at a later time point. The activation of mitochondria by different mechanisms induced by TNF-α ensures successful completion of the apoptosis program and demands new strategies to intervene TNF-α-induced toxicity and tissue injury.
MATERIALS AND METHODS
Animals.
Wild-type and bid-deficient mice were maintained in a C57BL/6 background as previously described (62, 64). Mice deficient in p65relA were maintained in a mixed background of C57BL/6 and 129SvJ as previously described (2). All animals received humane care. Animal procedures were conducted according to the guidelines of the National Institutes of Health and protocols approved by the Institutional Animal Care and Use Committee of the University of Pittsburgh.
Reagents.
The following antibodies were used: anti-p65RelA (Santa Cruz), anti-caspase 8 (Razq Hakem, University of Toronto), anti-caspase 9, anti-caspase 3 (Cell Signaling), anti-β-actin (Sigma), anti-cytochrome c (BD Pharmingen), anti-Smac (BD Bioscience), anti-JNK (clone 666; BD Pharmingen), anti-phosphorylated JNK and c-Jun (Cell Signaling), anti-cIAP1 (Santa Cruz), anti-cIAP2 (Santa Cruz), anti-XIAP (BD Bioscience), anti-Bcl-xL (Cell Signaling), anti-mouse Mcl-1 (Gerard P. Zambetti, St. Jude Children's Research Hospital), anti-Bax (6A7 [BD PharMingen] and N-20 [Santa Cruz]), anti-Bak (Upstate Biotechnology), anti-VDAC (Calbiochem), anti-Bad, anti-S112-phosphorylated Bad (Cell Signaling), and anti-Bid (56). All chemicals were obtained from Sigma {TNF-α, ActD, manganese III tetrakis(5,10,15,20-benzoic acid) porphyrin chloride [MnTBAP], cyclosporine A [CsA], GalN, and LPS}, except dithiobis succinimidylpropionate (DSP; Pierce), SP600125 (Calbiochem), tetramethyl rhodamine methyl ester (Molecular Probes), and z-VAD-fmk (Biomol). An adenoviral vector expressing the superrepressor of I-κBα (S32AS36A) (Ad-I-κBαsr) or human MnSOD was prepared as previously described (23, 49).
Cell culture.
Primary hepatocytes were prepared and cultured as previously described (13), by retrograde, nonrecirculating perfusion of livers with 0.05% collagenase H (Roche Molecular Biochemicals). Hepatocytes were cultured in Williams' medium E. Primary murine embryonic fibroblasts (MEFs) were isolated from embryonic day 13.5 (E13.5) fetuses as previously described (1) and cultured in Dulbecco modified Eagle medium with 10% fetal bovine serum and other standard supplements.
Lipotransfection.
Primary murine hepatocytes were first cultured in Williams' medium E plus 10% fetal bovine serum for 2 h following isolation. Cells (2 × 105) were then washed with phosphate-buffered saline twice and cultured in serum-free Williams' medium E overnight before transfection with pcDNA3-Bcl-2, pcDNA3-Bcl-xL, pcDNA3-GFP, or pcDNA3-GFP-Bax (1 to 2 μg) with Lipofectamine 2000 according to the supplier's (Invitrogen) protocol. Cells were analyzed 24 h later.
Induction of TNF-α-mediated liver injury.
Mice were treated as previously described (64). Briefly, male or female mice about 20 to 30 g in weight were intraperitoneally administered GalN (20 mg/mouse or 700 mg/kg in some cases). Thirty minutes later, they were intraperitoneally given lipopolysaccharide (LPS; Sigma L-2630, Escherichia coli 0111:B4) at 50 μg/kg. All reagents were prepared in 0.9% (vol/vol) endotoxin-free sterile saline (Sigma). Animals were sacrificed at designated time points for analysis. Survival analysis was based on the time when mice became moribund.
Analysis of apoptosis, caspase activation, and JNK activation.
Apoptosis was determined by nuclear staining with Hoechst 33342 (5 μg/ml for 10 min) as previously described (13). Caspase activation was determined by an immunoblot assay with antibodies against caspase 8, caspase 9, or caspase 3. Caspase activities were measured with 30 μg of proteins and 20 μM fluorescent substrates (Ac-DEVD-AFC, AC-IETD-AFC, and Ac-LEHD-AFC for caspases 3, 8, and 9, respectively). The fluorescence signals were detected by a fluorometer (Tecan GENios) at 400-nm excitation and 510-nm emission wavelengths. JNK activity was measured by the in vitro JNK kinase assay with glutathione S-transferase-c-Jun as the substrate as descried by the supplier (Cell Signaling).
Subcellular fractionation and analysis of mitochondrial activation.
Subcellular fractionation and analysis of mitochondrial activation were conducted as previously described (13, 64). In brief, livers were homogenized in buffer A (250 mM mannitol, 70 mM sucrose, 0.5 mM EGTA, 5 mM HEPES-NaOH, pH 7.2) and centrifuged first at 1,200 × g for 10 min to remove intact cells. The supernatants were further centrifuged at 10,000 × g for 15 min to harvest the cytosol. For cultured cells, the cytosolic fraction was obtained by brief treatment of cells with 0.05% digitonin in buffer B (10 mM HEPES, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, pH 7.4), followed by centrifugation at 10,000 × g for 10 min (13). The cytosolic fractions were analyzed for the presence of cytochrome c or Smac by an immunoblot assay (13, 64). The pellets from the above procedures contained the mitochondrial fraction. This fraction was resuspended in buffer B containing 2% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) or treated with freshly prepared 0.1 M Na2CO3 (pH 11.5) for 30 min on ice. The fraction was then centrifuged at 10,000 × g for 15 min. The recovered mitochondria were then analyzed by an immunoblot assay for Bax. The same membrane fraction was also treated with 1 mM DSP and analyzed for Bak oligomerization by an immunoblot assay as previously described (63).
To determine Bax conformation change, hepatocytes were stained with an anti-Bax conformation-sensitive antibody (clone 6A7; BD PharMingen) as described previously (13).
Measurement of ROS production and oxidized-lipid content.
Superoxide anion (O2−) was detected as described before (13). Briefly, cells were incubated with 2.5 μM dihydroethidium for 30 min at 37°C. Dihydroethidium is converted to a red fluorescent product, ethidium, in the presence of O2−. The cells were washed and resuspended in phosphate-buffered saline for flow cytometry.
The level of malondiadehyde (MDA) was determined with an assay kit (Lipid Peroxidation Kit; Oxford Biomedical Research, Oxford, MI) in accordance with the manufacturer's instructions. The amount of MDA was quantified by use of the MDA standards provided in the kit.
RESULTS
Bid-independent mitochondrial activation and apoptosis in TNF-α-mediated endotoxic liver injury.
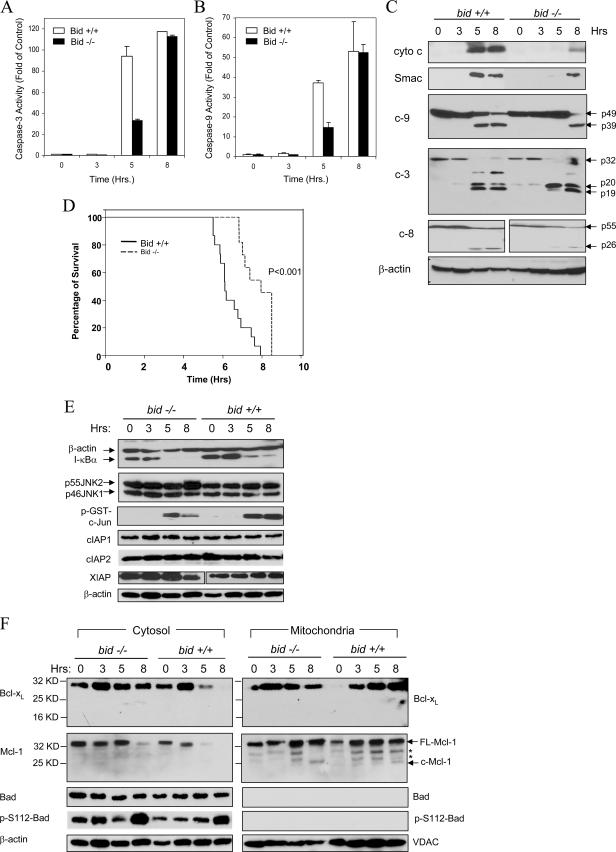
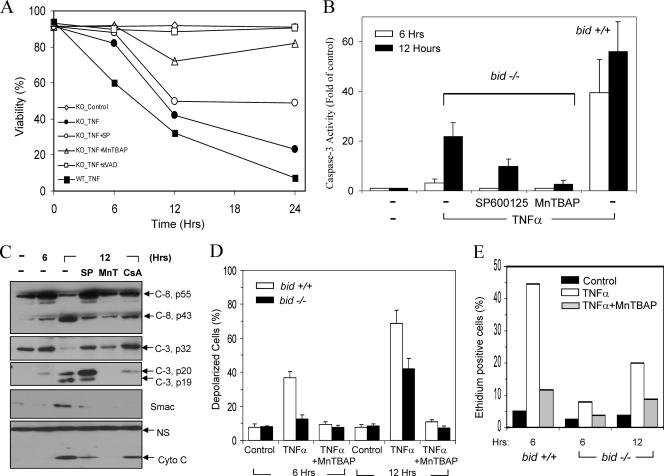
Previous studies have found that Bid, a BH3-only pro-death Bcl-2 family protein, plays an important role in death receptor-mediated hepatocyte apoptosis and liver injury (13, 20, 31, 62-64). However, it seemed that the deletion of Bid did not alter the ultimate outcome of TNF-α-mediated liver injury as much as that of Fas-mediated injury in terms of the long-term survival of mice, particularly if the mice were maintained in a C57BL/6 background (31, 62, 64). To understand the potential mechanisms, we performed a detailed study examining the kinetics of the apoptosis process with the established endotoxic model. Wild-type and bid-deficient mice were given LPS and GalN and sacrificed at different times. LPS can induce TNF-α production by macrophages, while GalN specifically suppresses transcription in the liver and therefore NF-κB activation (61). The combined treatment leads to liver-specific TNF-α-mediated apoptosis and injury. Consistent with earlier findings (64), deletion of Bid led to a significant reduction of caspase activities around 5 h after treatment (Fig. 1A, B). bid-deficient livers exhibited about 30% of the caspase 3 activity found in the livers of wild-type animals (Fig. 1A). However, by 8 h after treatment there were no significant differences in caspase activity between the wild-type and bid-deficient livers. This kinetics was well correlated with the activation of caspases. Thus, cleavage of caspase 3, caspase 9, and caspase 8 became significant in bid-deficient livers only at the later time point in the majority of mice (Fig. 1C). Thus, it is clear that caspase activation in LPS-GalN-treated livers is promoted by a bid-dependent mechanism in the early phase and by a bid-independent mechanism in the later phase. As a result, while bid-deficient C57BL/6 mice were significantly resistant to LPS-GalN-induced liver injury (data not shown) and mortality (Fig. 1D), they nevertheless could not survive for a long time.
FIG. 1.
Bid-independent mitochondrial activation and apoptosis in bid-deficient livers following LPS-GalN treatment. (A and B) Wild-type (Bid +/+, open columns) and bid-deficient (Bid −/−, solid columns) mice were treated with LPS-GalN and sacrificed at the designated time points. Liver homogenates were prepared, and the cytosolic fractions were separated for analysis of caspase 3 and caspase 9 activities. Data (mean ± standard deviation) are expressed as changes over the control at the 0-h time point. (C) The same cytosolic fractions were analyzed by an immunoblot assay with the antibodies indicated (c-9, caspase 9; c-3, caspase 3; c-8, caspase 8). cyto c, cytochrome c. (D) Wild-type (Bid +/+, n = 15) and bid-deficient (Bid −/−, n = 11) mice were treated with LPS-GalN and monitored for moribundity through the designated time points. bid-deficient mice were significantly resistant to LPS-GalN (the mean survival time was 7.78 ± 0.23 h versus 6.4 ± 0.2 h for wild-type mice; P < 0.001 by log rank analysis), but they still succumbed to the toxic effect. (E) Liver cytosol from mice treated with LPS-GalN for the indicated times were analyzed by an immunoblot assay with the antibodies indicated and also subjected to an in vitro JNK kinase assay with glutathione S-transferase-c-Jun as the substrate. (F) The mitochondrial and cytosolic fractions from mice treated with LPS-GalN for the times indicated were analyzed by an immunoblot assay with the antibodies indicated. FL-Mcl-1, full-length Mcl-1; c-Mcl-1, cleaved Mcl-1. Asterisks indicate nonspecific bands. VDAC and β-actin served as loading controls for the mitochondrial and cytosolic fractions, respectively. Hrs, hours.
A number of molecules can regulate TNF-α-mediated apoptosis (14, 54). It did not seem that there was a significant difference in the upstream activation of the NF-κB pathway between wild-type and bid-deficient mice on the basis of the pattern of I-κBα degradation (Fig. 1E). In addition, it seemed that JNK activation was largely normal in bid-deficient livers (Fig. 1E). The IAPs can suppress the activation and activity of caspases. However, we did not detect any significant and consistent changes in the levels of cIAP1, cIAP2, and XIAP following LPS-GalN treatment in either wild-type or bid-deficient livers (Fig. 1E).
The significant and consistent finding in the bid-deficient livers was the delayed mitochondrial release of cytochrome c and Smac, although the exact time point could vary, reflecting the heterogeneity in the response among the mice. Notably, the processing of caspase 3 in bid-deficient livers at the early time point usually ended at the p20 stage but almost invariably progressed to the p19 stage at the later time point, coincident with the release of cytochrome c and Smac (Fig. 1C). Earlier studies have shown that p20-p19 conversion is autocatalytic and is required for caspase 3 to acquire full activity (17, 22, 47). In addition, we and others have shown that this conversion is a result of the inactivation of XIAP by Smac released from the mitochondria in the case of death receptor activation-induced apoptosis (31, 48). Thus, this conversion is an important signature of mitochondrial activation.
We examined the potential involvement of a few other Bcl-2 family proteins in Bid-independent mitochondrial activation. The expression of two of the BH3-only pro-death molecules, Bim and PUMA, was below the detection level in either normal or LPS-GalN-treated mice (data not shown) (38). Bad and its phosphorylated form (Ser112) could be readily detected in the liver cytosol but not in the mitochondria (Fig. 1F). LPS-GalN treatment did not induce Bad dephosphorylation at Ser112 and most importantly did not cause Bad translocation to the mitochondria in either wild-type or bid-deficient livers, suggesting that Bad may not be involved in mitochondrial activation.
Among the commonly encountered anti-death Bcl-2 family proteins, Bcl-2 is not expressed in the liver (data not shown) (53). Both Bcl-xL and Mcl-1 could be readily detected, and there was an increased presence of these molecules in the mitochondria following LPS-GalN treatment (Fig. 1F), suggesting translocation (37). While no Bcl-xL cleavage could be found, cleaved mitochondrial Mcl-1 was detected at the 8-h time point following LPS-GalN treatment. The cleaved band (around 28 kDa) most likely resulted from caspase-mediated cleavage at Asp108 as previously reported (6). It is possible that this cleavage compromises the function of Mcl-1 and contributes to the susceptibility of bid-deficient mitochondria to TNF-α stimulation.
Deletion of Bid could not rescue TNF-α-dependent p65RelA deficiency-induced embryonic lethality, liver injury, and apoptosis.
p65RelA is the critical component of the p65-p50 NF-κB complex, which mediates the canonical NF-κB pathway following TNF-α stimulation (27). p65relA-deficient mice die during embryonic development at around E15.5 because of TNF-α-induced hepatocyte apoptosis (2, 15). To determine the significance of the Bid-independent mechanism in this model, we crossed bid-deficient mice with p65relA heterozygous mice and obtained offspring deficient in bid but heterozygous for relA. These mice were further intercrossed. If Bid was solely responsible for TNF-α-induced liver injury in this model, deletion of bid could inhibit liver injury and embryonic lethality and we would obtain bid/relA doubly deficient offspring. However, among more than 100 offspring screened, we never observed a single mouse doubly deficient in bid and relA in either the C57BL/6- or the 129/SVJ-dominated background (backcrossed for up to three generations) (data not shown). Further observations indicated that bid/relA-deficient embryos died at around the same time as relA-deficient embryos (data not shown). Thus, deletion of bid could not rescue relA deficiency-induced lethality.
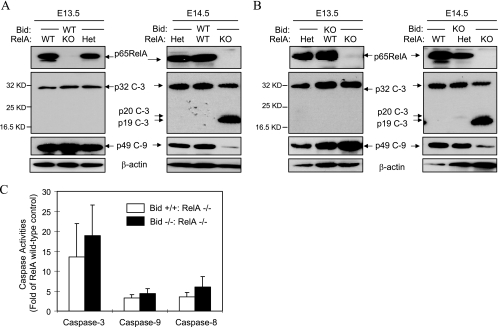
To gain further insight into the activation of apoptosis in the embryonic liver, we collected liver samples from E13.5 to E15.5 embryos and examined the activation of caspases. Because of the small size of the embryonic liver, it was not technically feasible to fractionate liver homogenates into cytosolic and mitochondrial fractions without disrupting the liver mitochondria, which would prevent accurate analysis of the mitochondrial release of apoptogenic factors. However, the data on caspase activation were sufficiently informative. Caspase activation had not been observed in E13.5 livers and began to be detected in relA-deficient livers at E14.5 (Fig. 2A). We could detect the p19 subunit of cleaved caspase 3. As an indication of mitochondrial activation, the pro-form of caspase 9 was significantly reduced in relA-deficient livers. By E15.5, all relA-deficient embryos had caspase activation in the liver (data not shown). Concomitant deletion of bid did not significantly change the time when caspases were activated or the level of caspase activity (Fig. 2B and C). These data strongly indicated that relA deficiency-triggered, TNF-α-mediated embryonic liver injury can progress by Bid-independent mechanisms.
FIG. 2.
Deletion of Bid fails to rescue relA-deficient embryos. (A) Bid wild-type embryos with different RelA genotypes were harvested at E13.5 and E14.5. Liver lysates were prepared for an immunoblot assay with the antibodies indicated (c-3, caspase 3; c-9, caspase 9). (B) Bid-deficient embryos with different RelA genotypes were harvested at E13.5 and E14.5. Liver lysates were prepared and analyzed by an immunoblot assay as in panel A. (C) Activities of caspases 3, 8, and 9 in liver lysates prepared from E14.5 singly relA-deficient embryos (open column, n = 5) or doubly relA/bid-deficient embryos (solid column, n = 7). Caspase 3, 9, and 8 activities were analyzed with Ac-DEVD-AFC, Ac-LEHD-AFC, and Ac-IETD-AFC as the substrates, respectively. Data (mean ± standard deviation) are expressed as changes over RelA wild-type livers. WT, wild type; KO, knockout, Het, heterozygous; KD, kilodaltons.
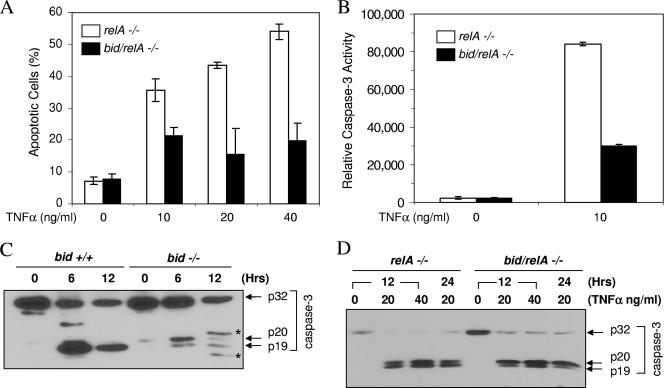
To examine the role of Bid in yet another cellular model, we compared the response of relA-deficient MEFs to TNF-α with that of bid/relA doubly deficient MEFs (Fig. 3). Deletion of Bid significantly reduced, but did not eliminate, apoptosis and caspase activation following TNF-α treatment (Fig. 3A and B). In wild-type MEFs, treatment with TNF-α and actinomycin D (ActD) led to caspase 3 cleavage at 6 h and the cleavage progressed from the p20 to the p19 stage completely by 12 h (Fig. 3C). As mentioned above, this conversion indicated mitochondrial activation. In bid-deficient MEFs, the activation of caspase 3 was much weaker but nevertheless occurred and the cleavage progressed from the p20 subunit to the p19 subunit partially, suggesting the presence of Bid-independent mitochondrial activation (Fig. 3C). When relA-deficient MEFs were analyzed following TNF-α stimulation, a similar pattern of caspase 3 processing could be observed in which deletion of bid reduced but did not eliminate the p20-p19 conversion (Fig. 3D). Thus, Bid-independent apoptosis and likely mitochondrial activation could be demonstrated in both relA-deficient embryos and MEFs.
FIG. 3.
Bid deficiency only partially protects relA-deficient MEFs from TNF-α-induced apoptosis. (A and B) MEFs deficient in RelA (open columns) or in both Bid and RelA (solid columns) were treated with TNF-α, as indicated, for 12 h (A) or 6 h (B). Percent apoptosis was determined by nuclear staining with Hoechst 33342 (A), and caspase 3 activity was determined with Ac-DEVD-AFC as the substrate. (C) Wild-type (bid +/+) and bid-deficient (bid −/−) MEFs were treated with TNF-α (10 ng/ml) in the presence of ActD (0.1 μg/ml) for the designated times. Lysates were prepared and subjected to immunoblot analysis with an anti-caspase 3 antibody. Note the weaker cleavage of caspase 3 and appearance of the p19 subunit in bid-deficient cells than in wild-type cells. Asterisks indicate nonspecific bands. (D) MEFs deficient in RelA (relA −/−) or in both Bid and RelA (bid/relA −/−) were treated with TNF-α, as indicated, for 12 h or 24 h. Lysates were prepared and subjected to immunoblot analysis with an anti-caspase 3 antibody. Note the weaker presence of the p19 subunit in bid/relA-deficient cells than in relA-deficient cells. Hrs, hours.
Bid-independent mitochondrial activation is promoted by multiple mechanisms.
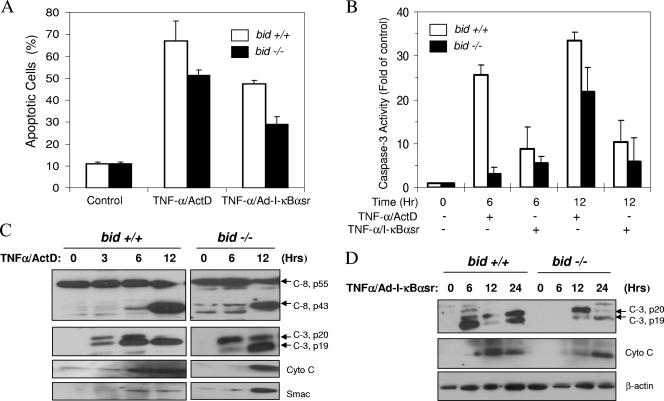
Having demonstrated the presence of a Bid-independent mitochondrial pathway in TNF-α-induced apoptosis in several models, we examined whether primary hepatocytes could be explored for the potential mechanisms involved in this Bid-independent pathway. We previously showed that bid-deficient hepatocytes demonstrated greater resistance to TNF-α-induced apoptosis and caspase activation in the presence of ActD but nevertheless still died from apoptosis at later time points (63). We confirmed that finding and further showed that this incomplete protection by bid deletion was not related to the use of ActD, since the combined use of TNF-α with an adenoviral vector expressing the superrepressor of I-κBα (Ad-I-κBαsr) still led to the activation of Bid-independent apoptosis and caspase activation (Fig. 4A and B). In wild-type hepatocytes treated with TNF-α and ActD, mitochondrial activation occurred around at 3 to 6 h on the basis of the release of apoptogenic factors. Correspondingly, the p20-p19 conversion of caspase 3 subunits occurred at around this time and was complete by 12 h. These events were delayed by about 3 h in bid-deficient hepatocytes (Fig. 4C). Caspase 8 does not require cleavage to be active, but cleavage suggests an elevated activation status (4). In TNF-α-treated cells, caspase 8 cleavage could be observed at the later time point but there were no significant differences between wild-type and bid-deficient cells. The patterns of mitochondrial activation and caspase 3 cleavage did not change in both types of hepatocytes even if cells were sensitized by Ad-I-κBαsr, although the activation kinetics was somewhat slower (Fig. 4D). Importantly, the mitochondrial activation and caspase 3 processing again occurred in the bid-deficient cells in a delayed fashion.
FIG. 4.
Bid-independent mitochondrial activation and apoptosis in TNF-α-treated, bid-deficient hepatocytes. (A and B) Wild-type (bid +/+, open columns) and bid-deficient (bid −/−, solid columns) hepatocytes were treated with TNF-α (10 ng/ml) together with ActD (0.1 μg/ml). Alternatively, hepatocytes were first infected with Ad-IκBαsr (multiplicity of infection, 50) overnight and then treated with TNF-α. Twelve hours later (A) or at the designated time points (B), percent apoptotic cells was determined by Hoechst 33342 staining (A) and caspase 3 activities were determined (B). (C and D) Wild-type (bid +/+) and bid-deficient (bid −/−) hepatocytes were sensitized with ActD (C) or Ad-I-κBαsr (D) and treated with TNF-α. At the indicated time points, the cytosolic fraction was separated and subjected to an immunoblot assay with the antibodies indicated. Note the delayed but definite release of cytochrome c (Cyto C) and Smac in the TNF-α-treated bid-deficient hepatocytes no matter whether the cells were sensitized with ActD or Ad-I-κBαsr. Correspondingly, caspase 8 (C-8) and caspase 3 (C-3) processing also occurred but in a delayed fashion. Hr or Hrs, hours.
It seemed that bid-deficient hepatocytes treated with TNF-α and ActD in vitro could reproduce the key features of Bid-independent mitochondrial activation observed in the in vivo models. We then determined what chemicals could potentially inhibit these events. z-VAD-fmk, a general caspase inhibitor, could suppress Bid-independent apoptosis in this model, supporting the idea that the death process was driven by caspases (Fig. 5A). Interestingly, we found that a broad-spectrum antioxidant, MnTBAP, had a potent inhibitory effect against both apoptosis and caspase 3 activation in bid-deficient hepatocytes (Fig. 5A and B). In addition, a specific JNK inhibitor, SP600125, had a noticeable inhibitory effect as well (Fig. 5A and B).
FIG. 5.
Inhibition of TNF-α-induced Bid-independent mitochondrial activation leads to suppression of apoptosis. (A) Wild-type (WT, ▪) and bid-deficient (KO, all other symbols) hepatocytes were treated with the vehicle control (⋄) or TNF-α-ActD (all other symbols) in the absence (•, ▪) or presence of SP600125 (25 μM, ○), MnTBAP (1 mM, Δ), or z-VAD-fmk (50 μM, □) for the indicated times. Viability was determined by propidium iodide staining. (B and C) Wild-type (bid +/+, B) and bid-deficient (bid −/−, B and C) hepatocytes were treated with TNF-α and ActD in the absence or presence of SP600125 (SP), MnTBAP (MnT), or CsA (2.5 μM), as indicated, for 6 or 12 h. Lysates were prepared to determine caspase 3 activity (B) or subjected to an immunoblot assay with the antibodies indicated (C). C-8, caspase 8; C-3, caspase 3; Cyto C, cytochrome c. A nonspecific band in the cytochrome c blot (NS) served as a loading control. (D and E) Wild-type (bid +/+) and bid-deficient (bid −/−) hepatocytes were treated with TNF-α and ActD in the absence or presence of MnTBAP for 6 or 12 h. Cells were then stained with tetramethyl rhodamine methyl ester (0.1 μM) for 30 min, and negative cells, in which mitochondria depolarized, were quantified (D). Alternatively, cells were incubated with dihydroethidium (2.5 μM) and analyzed by flow cytometry for superoxide production (E). Hrs, hours.
SP600125 significantly reduced mitochondrial release of cytochrome c and Smac in TNF-α-ActD-treated, bid-deficient hepatocytes (Fig. 5C). It also substantially reversed the p20-p19 conversion of the caspase 3 subunits. JNK activation in the context of TNF-α stimulation could be related to ROS (9, 25, 33, 40, 43). Hence, the inhibitory effects of MnTBAP were quite potent. It almost completely suppressed the mitochondrial release of cytochrome c and Smac and caspase 3 processing (Fig. 5C). These results indicate the important involvement of ROS in the bid-independent activation of the mitochondrial apoptosis pathway and that they could have a broader effect than JNK. Since mitochondrial PT could also be triggered by ROS (3, 28), we examined the effects of CsA, a potent inhibitor of PT, which we had previously shown to be able to reduce bid-independent apoptosis (63). Consistently, CsA did inhibit mitochondrial events and caspase processing in this model, although to a lesser extent than MnTBAP (Fig. 5C). Consistently, upon TNF-α treatment bid-deficient cells exhibited delayed mitochondrial depolarization, a major sign of PT activation, which can be suppressed by CsA, as shown before (3, 26, 28, 63). Notably, this delayed depolarization could be almost completely inhibited by MnTBAP (Fig. 5D), indicating the close relationship of ROS generation and PT activation. Indeed, we did detect ROS generation in bid-deficient hepatocytes following TNF-α-GalN treatment, particularly at a later time point, although at a lower level than in wild-type cells (Fig. 5E). This increase in ROS could be effectively suppressed by MnTBAP, consistent with its protective effects.
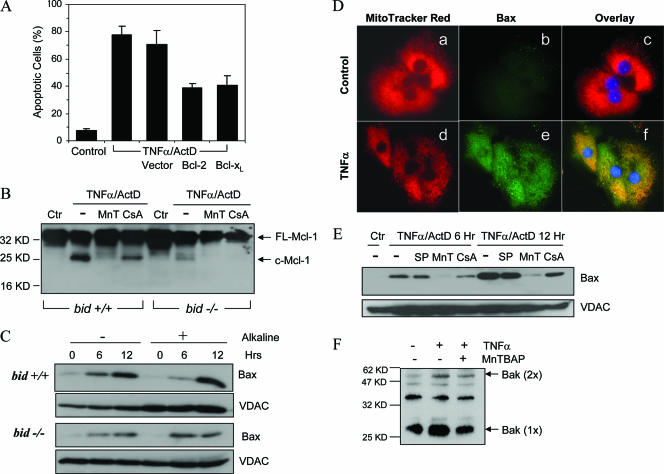
Transient expression of anti-death Bcl-2 or Bcl-xL reduced the apoptosis of bid-deficient hepatocytes treated with TNF-α (Fig. 6A), suggesting that the Bcl-2 family proteins could interact with the bid-independent mitochondrial activation mechanism. Consistent with the in vivo study, treatment of hepatocytes in vitro with TNF-α also led to cleavage of Mcl-1 on the mitochondria, independent of the status of Bid (Fig. 6B). Interestingly, this cleavage could be significantly suppressed by MnTBAP and to a lesser degree by CsA, further suggesting that Mcl-1 cleavage could be part of the events that contribute to Bid-independent mitochondrial activation.
FIG. 6.
Bid-independent alterations of Bcl-2 family proteins in TNF-α-treated hepatocytes. (A) Bid-deficient hepatocytes were first lipotransfected with a plasmid vector, Bcl-2, or Bcl-xL. Twenty-four hours later, the cells were treated with TNF-α and ActD for another 12 h. Percentages of apoptotic cells were then determined with Hoechst 33324. (B) The mitochondrion-containing membrane fractions of wild-type (bid +/+) and bid-deficient (bid −/−) hepatocytes treated with TNF-α and ActD in the presence or absence of MnTBAP (MnT) or CsA for 12 h were examined by an immunoblot assay with an anti-Mcl-1 antibody. FL-Mcl-1, full-length Mcl-1; c-Mcl-1, cleaved Mcl-1. (C) Wild-type (bid +/+) and bid-deficient (bid −/−) hepatocytes were treated with TNF-α and ActD for the indicated times. Membrane fractions containing the mitochondria were prepared and treated with 0.1 M Na2CO3 for 30 min. Control samples received no treatment. Samples were then subjected to immunoblot analysis with an anti-Bax antibody (N-20) or an anti-VDAC antibody which targeted the mitochondrial outer membrane. (D) Bid-deficient hepatocytes were treated with the vehicle (a to c) or TNF-α and ActD (d to f) for 6 h. Cells were then stained with MitoTracker Red (a, d) and the conformation-sensitive anti-Bax antibody (6A7) (b and e), followed by a Cy2-labeled secondary antibody (green). An overlay of the two signals is shown in parts c and f. (E) Bid-deficient hepatocytes were treated with the vehicle control (Ctr) or TNF-α and ActD in the absence or presence of SP600125 (SP), MnTBAP (MnT), or CsA for 6 or 12 h. Membrane fractions were then prepared and subjected to an immunoblot assay with an anti-Bax (N-20) antibody and an anti-VDAC antibody. (F) bid-deficient hepatocytes were treated as indicated for 12 h. Membrane fractions containing mitochondria were prepared and treated with a chemical cross-linker, DSP, before analysis by an immunoblot assay with an anti-Bak antibody. Bak (1×), monomeric Bak at ∼27 kDa; Bax (2×), dimerized Bak at ∼54 kDa; Hrs, hours; KD, kilodaltons.
The in vivo studies suggested that Bad, Bim, and PUMA may not participate in Bid-independent mitochondrial activation (Fig. 1F). However, since Bax could be potentially activated by JNK (52) and/or ROS (7), we examined the status of Bax in bid-deficient hepatocytes. Immunoblot analysis of mitochondrion-containing membrane fractions from TNF-α-ActD-treated hepatocytes indicated stimulation-dependent Bax translocation from the cytosol to the mitochondria (Fig. 6C). Furthermore, alkaline treatment could not dissociate Bax from the membrane, indicating that Bax had inserted itself into the membrane. Notably, in cultured cells, Bax translocation and insertion were only partially reduced in the absence of Bid (Fig. 6C). Using a different approach to examine Bax activation, we immunostained treated hepatocytes with a conformation-sensitive anti-Bax antibody (clone 6A7) and found that Bax conformation could be altered in the absence of Bid (Fig. 6D). It seemed that Bax translocation in this scenario was largely driven by ROS, as MnTBAP could significantly block such translocation, while PT or JNK inhibitors could do so to a lesser extent (Fig. 6E).
We also examined whether another multidomain pro-death molecule, Bak, could be activated in a Bid-independent way. The chemical cross-linking study revealed that, indeed, Bak could be activated in bid-deficient hepatocytes treated with TNF-α at the later time point (Fig. 6F). However, MnTBAP did not potently suppress this oligomerization, suggesting the involvement of factors other than ROS in Bak activation. Overall, it seems that Bid-independent mitochondrial activation could be mediated by a variety of different mechanisms.
Inhibition of ROS or mitochondrial PT can suppress Bid-independent apoptosis and liver injury in vivo.
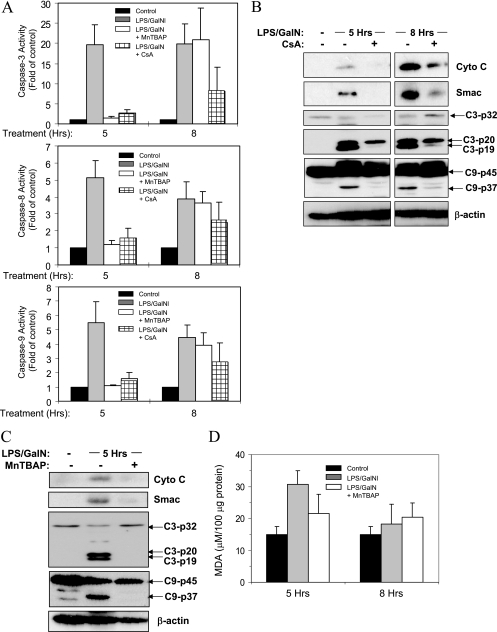
To examine whether ROS or PT could contribute to Bid-independent mitochondrial activation and hepatocyte apoptosis in vivo, we administered MnTBAP or CsA to bid-deficient mice, followed by a regular challenge with LPS-GalN. Notably, these chemicals did provide protection, reducing mitochondrial release of cytochrome c and Smac, reversing the p20-p19 conversion of caspase 3 subunits and inhibiting caspase 9 cleavage (Fig. 7).
FIG. 7.
Inhibition of ROS or PT can diminish endotoxic liver injury and mitochondrial activation in bid-deficient mice. (A) bid-deficient mice were treated with LPS-GalN alone or together with MnTBAP (10 mg/kg, intraperitoneally, 0.5 h later) or CsA (10 mg/kg, intraperitoneally, 1 h earlier). Five and eight hours later, the livers were harvested and the cytosolic fractions were analyzed for caspase activity. Data (mean ± standard deviation; n = 2 to 5 per group) are expressed as changes over the nontreated livers. (B and C) bid-deficient mice were treated as in panel A for the times indicated. The liver cytosol was subjected to immunoblot analysis with the antibodies indicated. C3, caspase 3; C9, caspase 9. (D) Liver extracts from bid-deficient mice treated with LPS-GalN alone or together with MnTBAP as in panel A for 5 or 8 h were analyzed for MDA. Hrs, hours; Cyto C, cytochrome c.
Interestingly, the protective effects of CsA in the in vivo model were more potent than those of MnTBAP, being observed at both 5 and 8 h after LPS-GalN treatment (Fig. 7A and B). MnTBAP-treated mice consistently performed better than control mice at 5 h but not at 8 h (Fig. 7A and C and data not shown). Since activation of the mitochondrial pathway in bid-deficient mice at 5 h was a less frequent event than that at 8 h, the results of MnTBAP application indicated relatively weak protection by this agent in the in vivo model. Intravenous delivery of an adenoviral vector encoding the MnSOD gene could lead to persistent overexpression of MnSOD in hepatocytes, but the protective effect was as transient as that of MnTBAP (data not shown). We then examined whether the action of ROS in the in vivo model could be relatively short-lived. We determined the level of MDA, a common end product of lipid peroxidation, since direct perturbation of mitochondrial membrane lipids could contribute to mitochondrial structure alterations and permeability changes (13). Indeed, the level of MDA was only significantly increased in livers treated with LPS-GalN at 5 h, which could be suppressed by MnTBAP, but not at 8 h. Overall, these results suggest that the direct effect of ROS in the in vivo model is relatively transient and other apoptotic events could become dominant at later time points. When the in vivo and in vitro observations are taken together, it becomes clear that multiple mechanisms contribute to the Bid-independent activation of mitochondria and apoptosis following TNF-α stimulation.
DISCUSSION
TNF-α induces mitochondrial activation in two phases, an early Bid-dependent phase and a later Bid-independent phase.
In mammalian cells, apoptosis could proceed along the extrinsic pathway (death receptor pathway) or the intrinsic pathway (mitochondrial pathway), which interact with each other broadly (8). The need for the mitochondrial pathway in the activation and progression of death receptor-mediated apoptosis varies considerably, depending on the type of cells, the type of stimuli, and the strength of the stimulation. Deletion of Bid leads to nearly complete blockage of Fas-mediated hepatocyte apoptosis and liver injury (31, 62). However, deletion of Bid only delays but does not block TNF-α-mediated hepatocyte apoptosis and liver injury (13, 63, 64). This difference could be related to the different signaling pathways activated downstream of the Fas and TNF-α receptors, and the latter recruit additional death machinery. It would thus be important to delineate these Bid-independent apoptotic mechanisms. The present study investigated this issue by utilizing several models of TNF-α toxicity in both hepatocytes and fibroblasts, where NF-κB activation was inhibited by classical inhibitors or by ablation of the p65relA gene.
Most importantly, our studies indicate that the mitochondrial pathway remains critical and contributes significantly to TNF-α-induced apoptosis in the absence of Bid. Mitochondrial activation could still be observed in the absence of Bid but occurs in a delayed fashion. When mitochondrial activation is blocked, so is apoptosis. Thus, these findings indicate that mitochondria are activated by at least two different sets of mechanisms following TNF-α administration that are mediated by Bid and other molecular events, respectively. The difference in the effective time range between the two mechanisms is relative in terms of the actual time point, as the Bid-independent mitochondrial activation could be observed earlier in some mice than in others, reflecting the heterogeneity in the population. But both types of mitochondrial activation seem to be critically important for TNF-α-induced apoptosis and tissue injury.
Multiple mechanisms contribute to Bid-independent mitochondrial activation.
Our data further demonstrate that ROS are key mediators of Bid-independent mitochondrial activation. ROS have been consistently found to be important for TNF-α-induced apoptosis, and antioxidants have been shown to alleviate cell death induced by TNF-α (13, 18, 25, 40, 43, 44, 46). TNF-α-induced ROS are mainly derived from mitochondria (13, 18, 44, 46) through Bid-mediated mechanisms (13). ROS can further damage mitochondrial structure and promote cytochrome c and Smac release (3, 13, 28). These detrimental effects can be inhibited by NF-κB-regulated MnSOD (25, 60), the anti-death Bcl-2 family proteins (18), or antioxidants (13, 44). A much smaller amount of ROS could still be detected in bid-deficient cells (Fig. 5). It is not clear how these ROS are generated; they could be derived from mitochondrial or extramitochondrial processes. It is possible that these mechanisms involve the phospholipase A2-arachidonic acid-5-lipoxygenase pathway and ceramides as previously proposed (reviewed in reference 14). In addition, the involvement of NADPH oxidase, which has been shown to be important in the early initiation of Fas-mediated apoptosis (41), could not be excluded in the case of TNF-α. Future work will be directed to understanding the source of these ROS.
It is interesting that the effects of ROS seem to be more striking in the in vitro model than in the in vivo model. Thus, while we observed that MnTBAP, a potent MnSOD mimetic, could effectively suppress mitochondrial activation and cell death in cultured bid-deficient hepatocytes, it was not as effective in the in vivo application despite the fact that it was reported to be quite effective in anti-Fas-induced liver injury (35). On the basis of a kinetics study (39), administration of MnTBAP at 10 mg/kg should maintain a steady level in serum over the course in which LPS-GalN-induced liver injury developed. We had also tried a few other regimens with different numbers of administrations, different administration times related to LPS-GalN treatment, and the administration of Ad-MnSOD without further improvement. A drop in the level of MDA at the later time point of LPS-GalN treatment in mice may suggest that the direct effect of ROS is transient, perhaps because of the fast turnover of radicals. It is thus very likely that other events, mostly TNF-α specific, could become dominant later on in the in vivo situation.
One of the key mediators of the apoptotic effects of ROS is JNK, which has been clearly indicated in TNF-α-induced apoptosis in both hepatocytes (34, 45, 57) and nonhepatocytic cells (10, 12, 43, 51). Notably, both ROS accumulation and sustained JNK phosphorylation are the major targets of NF-κB-mediated cytoprotection against TNF-α (9, 12, 25, 27, 33, 40, 51). ROS could inactivate mitogen-activated protein kinase phosphatase and thus contribute to the sustained phosphorylation of JNK (25).
In wild-type cells, Bid could be a target of JNK to mediate mitochondrial activation since activated JNK may promote Bid cleavage either directly (10) or indirectly by inhibiting FLIP and enhancing caspase 8 activation (5, 57). However, since we found that inhibition of JNK in the absence of Bid could still reduce Bid-independent mitochondrial activation and that Bid deletion could not rescue p65relA deficiency-triggered live injury and embryonic lethality, it is plausible that JNK could have additional targets that affect mitochondrial activation (7, 52).
The multidomain pro-death Bcl-2 family proteins Bax and Bak are the common gateway to mitochondrial activation and are usually activated preferentially by Bid following death receptor activation (13, 58, 63, 64). However, in the present study, we clearly observed a Bid-independent translocation of Bax and also a reduced but observable Bax conformation change and membrane insertion in the absence of Bid in cultured primary hepatocytes. Both ROS and JNK have been shown to be able to promote Bax translocation (7, 52). These effects could overlap, and in the case of JNK, JNK could phosphorylate 14-3-3, which then releases the bound Bax protein for its translocation (52). Although Bax could be an important downstream effector of ROS and JNK, particularly in the absence of Bid, we do not think that it is the only mediator of the mitochondrial death pathway since deletion of either Bax alone (64) or Bid and Bax together (data not shown) did not confer complete resistance to TNF-α-induced liver injury, hepatocyte apoptosis, and mitochondrial activation. It is possible that Bak could substitute for Bax in these cases. Indeed, Bak oligomerization in cultured bid-deficient hepatocytes could be observed at later time points.
BH3-only pro-death molecules play an important role in activating Bax and/or Bak by direct interaction or by antagonizing anti-death Bcl-2 family proteins. While we have not exhausted our efforts to determining the role of every reported BH3-only molecule in Bid-independent TNF-α-induced mitochondrial activation, it does not seem that Bad, Bim, or PUMA is involved in the activation of Bak (or Bax) because of the lack of activation or expression in the liver. Alternatively, caspase-mediated cleavage of anti-death Bcl-2 family proteins such as Bcl-2, Bcl-xL, and Mcl-1 could convert them into proapoptosis molecules and/or disable their anti-death activities (6, 8, 21, 36, 59).
While Bcl-2 is not expressed in the murine liver (53), cleavage of Mcl-1, but not Bcl-xL, is observed in both in vivo and in vitro models following TNF-α stimulation. Several reports have indicated that specific cleavage of Mcl-1 promotes TRAIL-induced apoptosis (21, 59) and the C-terminally part of Mcl-1 possesses proapoptotic activities (36, 59). This segment has been reported to be able to interact with Bak and VDAC (59); thus, it could participate in the activation of Bak and/or PT. Initial Mcl-1 cleavage could be due to the low level of caspase activity following TNF-α receptor engagement. Notably, in the in vitro model, cleavage of Mcl-1 could be suppressed to different degrees by MnTBAP and CsA, suggesting that these events result in positive amplification through enhanced caspase activation.
The protective effects of CsA, which binds to cyclophilin D, a key component of the permeability transition pore (PTP), indicate the important involvement of the PTP in Bid-independent mitochondrial activation in both in vitro and in vivo models. The nature of the PTP and how it is activated are still controversial (3, 28). ROS may cause oxidization of key components of the PTP and keep it at the open position (3), and antioxidants have been found to suppress the PTP (Fig. 5D). The PTP could be regulated by many other factors, including the Bcl-2 family proteins (3, 26, 28). Important PTP elements such as ANT and VDAC have been found to interact with the Bcl-2 family proteins (8, 26, 59). PT can be positively regulated by Bid (63) but could still be active in the absence of Bid, as shown here. It is likely that the mitochondrial PT, which seems to be actively engaged in Bid-independent mitochondrial activation, could be another common avenue into which a diverse array of apoptotic signals merges (3, 26, 28).
In summary, the events that may mediate Bid-independent mitochondrial activation are quite diverse. Notably, these events are interconnected and can be coregulated. It is unlikely that a single event is solely responsible for mitochondrial activation. These findings demand a comprehensive strategy in mitigating TNF-α-mediated apoptosis and tissue injury.
Acknowledgments
We thank Razq Hakem (University of Toronto) for the anti-caspase 8 antibody, Gerard P. Zambetti (St. Jude Children's Research Hospital) for the anti-mouse Mcl-1 antibody, and Daniell DiFrancesca for the preparation of MEFs.
This work was in part supported by an NIH grant to X.-M. Yin (R01CA 83817). W.-X. Ding is a recipient of Liver Scholar Award from the American Liver Foundation and the Alpha-1 Foundation.
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Beg, A. A., and D. Baltimore. 1996. An essential role for NF-κB in preventing TNF-α-induced cell death. Science 274:782-784. [DOI] [PubMed] [Google Scholar]
- 2.Beg, A. A., W. C. Sha, R. T. Bronson, S. Ghosh, and D. Baltimore. 1995. Embryonic lethality and liver degeneration in mice lacking the RelA component of NF-κB. Nature 376:167-170. [DOI] [PubMed] [Google Scholar]
- 3.Bernardi, P., R. Colonna, P. Costantini, O. Eriksson, E. Fontaine, F. Ichas, S. Massari, A. Nicolli, V. Petronilli, and L. Scorrano. 1998. The mitochondrial permeability transition. Biofactors 8:273-281. [DOI] [PubMed] [Google Scholar]
- 4.Boatright, K. M., M. Renatus, F. L. Scott, S. Sperandio, H. Shin, I. M. Pedersen, J. E. Ricci, W. A. Edris, D. P. Sutherlin, D. R. Green, and G. S. Salvesen. 2003. A unified model for apical caspase activation. Mol. Cell 11:529-541. [DOI] [PubMed] [Google Scholar]
- 5.Chang, L., H. Kamata, G. Solinas, J. L. Luo, S. Maeda, K. Venuprasad, Y. C. Liu, and M. Karin. 2006. The E3 ubiquitin ligase itch couples JNK activation to TNFα-induced cell death by inducing c-FLIPL turnover. Cell 124:601-613. [DOI] [PubMed] [Google Scholar]
- 6.Clohessy, J. G., J. Zhuang, and H. J. Brady. 2004. Characterisation of Mcl-1 cleavage during apoptosis of haematopoietic cells. Br. J. Haematol. 125:655-665. [DOI] [PubMed] [Google Scholar]
- 7.D'Alessio, M., M. De Nicola, S. Coppola, G. Gualandi, L. Pugliese, C. Cerella, S. Cristofanon, P. Civitareale, M. R. Ciriolo, A. Bergamaschi, A. Magrini, and L. Ghibelli. 2005. Oxidative Bax dimerization promotes its translocation to mitochondria independently of apoptosis. FASEB J. [Online.] doi:1 0.1096/fj.04-3329fje. [DOI] [PubMed]
- 8.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 9.Davis, R. J. 2000. Signal transduction by the JNK group of MAP kinases. Cell 103:239-252. [DOI] [PubMed] [Google Scholar]
- 10.Deng, Y., X. Ren, L. Yang, Y. Lin, and X. Wu. 2003. A JNK-dependent pathway is required for TNFα-induced apoptosis. Cell 115:61-70. [DOI] [PubMed] [Google Scholar]
- 11.Desagher, S., A. Osen-Sand, A. Nichols, R. Eskes, S. Montessuit, S. Lauper, K. Maundrell, B. Antonsson, and J. C. Martinou. 1999. Bid-induced conformational change of Bax is responsible for mitochondrial cytochrome c release during apoptosis. J. Cell Biol. 144:891-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Smaele, E., F. Zazzeroni, S. Papa, D. U. Nguyen, R. Jin, J. Jones, R. Cong, and G. Franzoso. 2001. Induction of gadd45β by NF-κB downregulates pro-apoptotic JNK signalling. Nature 414:308-313. [DOI] [PubMed] [Google Scholar]
- 13.Ding, W. X., H. M. Ni, D. DiFrancesca, D. B. Stolz, and X. M. Yin. 2004. Bid-dependent generation of oxygen radicals promotes death receptor activation-induced apoptosis in murine hepatocytes. Hepatology 40:403-413. [DOI] [PubMed] [Google Scholar]
- 14.Ding, W. X., and X. M. Yin. 2004. Dissection of the multiple mechanisms of TNF-α-induced apoptosis in liver injury. J. Cell. Mol. Med. 8:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doi, T. S., M. W. Marino, T. Takahashi, T. Yoshida, T. Sakakura, L. J. Old, and Y. Obata. 1999. Absence of tumor necrosis factor rescues RelA-deficient mice from embryonic lethality. Proc. Natl. Acad. Sci. USA 96:2994-2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33-42. [DOI] [PubMed] [Google Scholar]
- 17.Fernandes-Alnemri, T., R. C. Armstrong, J. Krebs, S. M. Srinivasula, L. Wang, F. Bullrich, L. C. Fritz, J. A. Trapani, K. J. Tomaselli, G. Litwack, and E. S. Alnemri. 1996. In vitro activation of CPP32 and Mch3 by Mch4, a novel human apoptotic cysteine protease containing two FADD-like domains. Proc. Natl. Acad. Sci. USA 93:7464-7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gottlieb, E., M. G. Vander Heiden, and C. B. Thompson. 2000. Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor alpha-induced apoptosis. Mol. Cell. Biol. 20:5680-5689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gross, A., X. M. Yin, K. Wang, M. C. Wei, J. Jockel, C. Milliman, H. Erdjument-Bromage, P. Tempst, and S. J. Korsmeyer. 1999. Caspase cleaved BID targets mitochondria and is required for cytochrome c release, while BCL-XL prevents this release but not tumor necrosis factor-R1/Fas death. J. Biol. Chem. 274:1156-1163. [DOI] [PubMed] [Google Scholar]
- 20.Guicciardi, M. E., S. F. Bronk, N. W. Werneburg, X. M. Yin, and G. J. Gores. 2005. Bid is upstream of lysosome-mediated caspase 2 activation in tumor necrosis factor alpha-induced hepatocyte apoptosis. Gastroenterology 129:269-284. [DOI] [PubMed] [Google Scholar]
- 21.Han, J., L. A. Goldstein, B. R. Gastman, and H. Rabinowich. 2006. Interrelated roles for Mcl-1 and BIM in regulation of TRAIL-mediated mitochondrial apoptosis. J. Biol. Chem. 281:10153-10163. [DOI] [PubMed] [Google Scholar]
- 22.Han, Z., E. A. Hendrickson, T. A. Bremner, and J. H. Wyche. 1997. A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J. Biol. Chem. 272:13432-13436. [DOI] [PubMed] [Google Scholar]
- 23.Iimuro, Y., T. Nishiura, C. Hellerbrand, K. E. Behrns, R. Schoonhoven, J. W. Grisham, and D. A. Brenner. 1998. NFκB prevents apoptosis and liver dysfunction during liver regeneration. J. Clin. Investig. 101:802-811. (Erratum, 101:1541, 1998.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Irmler, M., M. Thome, M. Hahne, P. Schneider, K. Hofmann, V. Steiner, J. L. Bodmer, M. Schroter, K. Burns, C. Mattmann, D. Rimoldi, L. E. French, and J. Tschopp. 1997. Inhibition of death receptor signals by cellular FLIP. Nature 388:190-195. [DOI] [PubMed] [Google Scholar]
- 25.Kamata, H., S. Honda, S. Maeda, L. Chang, H. Hirata, and M. Karin. 2005. Reactive oxygen species promote TNFα-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell 120:649-661. [DOI] [PubMed] [Google Scholar]
- 26.Kroemer, G., and J. C. Reed. 2000. Mitochondrial control of cell death. Nat. Med. 6:513-519. [DOI] [PubMed] [Google Scholar]
- 27.Kucharczak, J., M. J. Simmons, Y. Fan, and C. Gelinas. 2003. To be, or not to be: NF-κB is the answer—role of Rel/NF-κB in the regulation of apoptosis. Oncogene 22:8961-8982. [DOI] [PubMed] [Google Scholar]
- 28.Lemasters, J. J., A. L. Nieminen, T. Qian, L. C. Trost, and B. Herman. 1997. The mitochondrial permeability transition in toxic, hypoxic and reperfusion injury. Mol. Cell. Biochem. 174:159-165. [PubMed] [Google Scholar]
- 29.Li, L., R. M. Thomas, H. Suzuki, J. K. De Brabander, X. Wang, and P. G. Harran. 2004. A small molecule Smac mimic potentiates TRAIL- and TNFα-mediated cell death. Science 305:1471-1474. [DOI] [PubMed] [Google Scholar]
- 30.Li, Q., D. Van Antwerp, F. Mercurio, K. F. Lee, and I. M. Verma. 1999. Severe liver degeneration in mice lacking the IκB kinase 2 gene. Science 284:321-325. [DOI] [PubMed] [Google Scholar]
- 31.Li, S., Y. Zhao, X. He, T.-H. Kim, D. K. Kuharsky, H. Rabinowich, J. Chen, C. Du, and X.-M. Yin. 2002. Relief of extrinsic pathway inhibition by the Bid-dependent mitochondrial release of Smac in Fas-mediated hepatocyte apoptosis. J. Biol. Chem. 277:26912-26920. [DOI] [PubMed] [Google Scholar]
- 32.Li, Z. W., W. Chu, Y. Hu, M. Delhase, T. Deerinck, M. Ellisman, R. Johnson, and M. Karin. 1999. The IKKβ subunit of IκB kinase (IKK) is essential for nuclear factor κB activation and prevention of apoptosis. J. Exp. Med. 189:1839-1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin, A. 2003. Activation of the JNK signaling pathway: breaking the brake on apoptosis. Bioessays 25:17-24. [DOI] [PubMed] [Google Scholar]
- 34.Liu, H., C. R. Lo, and M. J. Czaja. 2002. NF-κB inhibition sensitizes hepatocytes to TNF-induced apoptosis through a sustained activation of JNK and c-Jun. Hepatology 35:772-778. [DOI] [PubMed] [Google Scholar]
- 35.Malassagne, B., P. J. Ferret, R. Hammoud, M. Tulliez, S. Bedda, H. Trebeden, P. Jaffray, Y. Calmus, B. Weill, and F. Batteux. 2001. The superoxide dismutase mimetic MnTBAP prevents Fas-induced acute liver failure in the mouse. Gastroenterology 121:1451-1459. [DOI] [PubMed] [Google Scholar]
- 36.Michels, J., J. W. O'Neill, C. L. Dallman, A. Mouzakiti, F. Habens, M. Brimmell, K. Y. Zhang, R. W. Craig, E. G. Marcusson, P. W. Johnson, and G. Packham. 2004. Mcl-1 is required for Akata6 B-lymphoma cell survival and is converted to a cell death molecule by efficient caspase-mediated cleavage. Oncogene 23:4818-4827. [DOI] [PubMed] [Google Scholar]
- 37.Nijhawan, D., M. Fang, E. Traer, Q. Zhong, W. Gao, F. Du, and X. Wang. 2003. Elimination of Mcl-1 is required for the initiation of apoptosis following ultraviolet irradiation. Genes Dev. 17:1475-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Reilly, L. A., L. Cullen, J. Visvader, G. J. Lindeman, C. Print, M. L. Bath, D. C. S. Huang, and A. Strasser. 2000. The proapoptotic BH3-only protein Bim is expressed in hematopoietic, epithelial, neuronal, and germ cells. Am. J. Pathol. 157:449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Oury, T. D., K. Thakker, M. Menache, L. Y. Chang, J. D. Crapo, and B. J. Day. 2001. Attenuation of bleomycin-induced pulmonary fibrosis by a catalytic antioxidant metalloporphyrin. Am. J. Respir. Cell Mol. Biol. 25:164-169. [DOI] [PubMed] [Google Scholar]
- 40.Pham, C. G., C. Bubici, F. Zazzeroni, S. Papa, J. Jones, K. Alvarez, S. Jayawardena, E. De Smaele, R. Cong, C. Beaumont, F. M. Torti, S. V. Torti, and G. Franzoso. 2004. Ferritin heavy chain upregulation by NF-κB inhibits TNFα-induced apoptosis by suppressing reactive oxygen species. Cell 119:529-542. [DOI] [PubMed] [Google Scholar]
- 41.Reinehr, R., S. Becker, A. Eberle, S. Grether-Beck, and D. Haussinger. 2005. Involvement of NADPH oxidase isoforms and Src family kinases in CD95-dependent hepatocyte apoptosis. J. Biol. Chem. 280:27179-27194. [DOI] [PubMed] [Google Scholar]
- 42.Rudolph, D., W. C. Yeh, A. Wakeham, B. Rudolph, D. Nallainathan, J. Potter, A. J. Elia, and T. W. Mak. 2000. Severe liver degeneration and lack of NF-κB activation in NEMO/IKKγ-deficient mice. Genes Dev. 14:854-862. [PMC free article] [PubMed] [Google Scholar]
- 43.Sakon, S., X. Xue, M. Takekawa, T. Sasazuki, T. Okazaki, Y. Kojima, J. H. Piao, H. Yagita, K. Okumura, T. Doi, and H. Nakano. 2003. NF-κB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 22:3898-3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulze-Osthoff, K., A. C. Bakker, B. Vanhaesebroeck, R. Beyaert, W. A. Jacob, and W. Fiers. 1992. Cytotoxic activity of tumor necrosis factor is mediated by early damage of mitochondrial functions. Evidence for the involvement of mitochondrial radical generation. J. Biol. Chem. 267:5317-5323. [PubMed] [Google Scholar]
- 45.Schwabe, R. F., H. Uchinami, T. Qian, B. L. Bennett, J. J. Lemasters, and D. A. Brenner. 2004. Differential requirement for c-Jun NH2-terminal kinase in TNFα- and Fas-mediated apoptosis in hepatocytes. FASEB J. 18:720-722. [DOI] [PubMed] [Google Scholar]
- 46.Sidoti-de Fraisse, C., V. Rincheval, Y. Risler, B. Mignotte, and J. L. Vayssiere. 1998. TNF-α activates at least two apoptotic signaling cascades. Oncogene 17:1639-1651. [DOI] [PubMed] [Google Scholar]
- 47.Stennicke, H. R., J. M. Jurgensmeier, H. Shin, Q. Deveraux, B. B. Wolf, X. Yang, Q. Zhou, H. M. Ellerby, L. M. Ellerby, D. Bredesen, D. R. Green, J. C. Reed, C. J. Froelich, and G. S. Salvesen. 1998. Pro-caspase-3 is a major physiologic target of caspase-8. J. Biol. Chem. 273:27084-27090. [DOI] [PubMed] [Google Scholar]
- 48.Sun, X. M., S. B. Bratton, M. Butterworth, M. MacFarlane, and G. M. Cohen. 2002. Bcl-2 and Bcl-xL inhibit CD95-mediated apoptosis by preventing mitochondrial release of Smac/DIABLO and subsequent inactivation of X-linked inhibitor-of-apoptosis protein. J. Biol. Chem. 277:11345-11351. [DOI] [PubMed] [Google Scholar]
- 49.Takahashi, Y., R. W. Ganster, A. Gambotto, L. Shao, T. Kaizu, T. Wu, G. P. Yagnik, A. Nakao, G. Tsoulfas, T. Ishikawa, T. Okuda, D. A. Geller, and N. Murase. 2002. Role of NF-κB on liver cold ischemia-reperfusion injury. Am. J. Physiol. Gastrointest. Liver Physiol. 283:G1175-G1184. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka, M., M. E. Fuentes, K. Yamaguchi, M. H. Durnin, S. A. Dalrymple, K. L. Hardy, and D. V. Goeddel. 1999. Embryonic lethality, liver degeneration, and impaired NF-κ B activation in IKK-β-deficient mice. Immunity 10:421-429. [DOI] [PubMed] [Google Scholar]
- 51.Tang, G., Y. Minemoto, B. Dibling, N. H. Purcell, Z. Li, M. Karin, and A. Lin. 2001. Inhibition of JNK activation through NF-κB target genes. Nature 414:313-317. [DOI] [PubMed] [Google Scholar]
- 52.Tsuruta, F., J. Sunayama, Y. Mori, S. Hattori, S. Shimizu, Y. Tsujimoto, K. Yoshioka, N. Masuyama, and Y. Gotoh. 2004. JNK promotes Bax translocation to mitochondria through phosphorylation of 14-3-3 proteins. EMBO J. 23:1889-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vail, M. E., M. L. Chaisson, J. Thompson, and N. Fausto. 2002. Bcl-2 expression delays hepatocyte cell cycle progression during liver regeneration. Oncogene 21:1548-1555. [DOI] [PubMed] [Google Scholar]
- 54.Wajant, H., K. Pfizenmaier, and P. Scheurich. 2003. Tumor necrosis factor signaling. Cell Death Differ. 10:45-65. [DOI] [PubMed] [Google Scholar]
- 55.Wang, C. Y., M. W. Mayo, R. G. Korneluk, D. V. Goeddel, and A. S. Baldwin, Jr. 1998. NF-κB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science 281:1680-1683. [DOI] [PubMed] [Google Scholar]
- 56.Wang, K., X. M. Yin, D. T. Chao, C. L. Milliman, and S. J. Korsmeyer. 1996. BID: a novel BH3 domain-only death agonist. Genes Dev. 10:2859-2869. [DOI] [PubMed] [Google Scholar]
- 57.Wang, Y., R. Singh, J. H. Lefkowitch, R. M. Rigoli, and M. J. Czaja. 2006. Tumor necrosis factor-induced toxic liver injury results from JNK2-dependent activation of caspase-8 and the mitochondrial death pathway. J. Biol. Chem. 281:15258-15267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wei, M. C., T. Lindsten, V. K. Mootha, S. Weiler, A. Gross, M. Ashiya, C. B. Thompson, and S. J. Korsmeyer. 2000. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 14:2060-2071. [PMC free article] [PubMed] [Google Scholar]
- 59.Weng, C., Y. Li, D. Xu, Y. Shi, and H. Tang. 2005. Specific cleavage of Mcl-1 by caspase-3 in tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in Jurkat leukemia T cells. J. Biol. Chem. 280:10491-10500. [DOI] [PubMed] [Google Scholar]
- 60.Wong, G. H., J. H. Elwell, L. W. Oberley, and D. V. Goeddel. 1989. Manganous superoxide dismutase is essential for cellular resistance to cytotoxicity of tumor necrosis factor. Cell 58:923-931. [DOI] [PubMed] [Google Scholar]
- 61.Yin, X. M., and W. X. Ding. 2003. Death receptor activation-induced hepatocyte apoptosis and liver injury. Curr. Mol. Med. 3:491-508. [DOI] [PubMed] [Google Scholar]
- 62.Yin, X. M., K. Wang, A. Gross, Y. Zhao, S. Zinkel, B. Klocke, K. A. Roth, and S. J. Korsmeyer. 1999. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature 400:886-891. [DOI] [PubMed] [Google Scholar]
- 63.Zhao, Y., W. X. Ding, T. Qian, S. Watkins, J. J. Lemasters, and X. M. Yin. 2003. Bid activates multiple mitochondrial apoptotic mechanisms in primary hepatocytes after death receptor engagement. Gastroenterology 125:854-867. [DOI] [PubMed] [Google Scholar]
- 64.Zhao, Y., S. Li, E. E. Childs, D. K. Kuharsky, and X.-M. Yin. 2001. Activation of Pro-death Bcl-2 family proteins and mitochondria apoptosis pathway in tumor necrosis factor-alpha-induced liver injury. J. Biol. Chem. 276:27432-27440. [DOI] [PubMed] [Google Scholar]