Abstract
Progesterone receptor (PR) ligand binding induces rapid and transient (5- to 10-min) activation of cytosolic c-Src-Ras-Erk1/2 mitogen-activated protein kinase (MAPK) signaling that is independent of PR functioning as transcription factors. Here, we have explored the integration of PR-dependent transcription and rapid signaling events in breast cancer cells. PR-B, but not PR-A, induced robust and sustained (6- to 72-h) Erk1/2 activation that was required for elevated cyclin D1 protein but not mRNA levels. Sustained Erk1/2 activation in response to progestins occurred via a novel mechanism distinct from rapid signaling initiated by PR/c-Src interactions and required the PR-B DNA-binding domain (DBD). PR/progestin upregulated epidermal growth factor receptor (EGFR) and Wnt-1. In response to PR-induced Wnt-1 signaling, matrix metalloprotease (MMP)-mediated membrane-proximal shedding of EGFR ligands transactivated EGFR and induced persistent downstream c-Src and Erk1/2 activities. T47D cell anchorage-independent growth was stimulated by progestins and blocked by inhibition of Erk1/2, c-Src, EGFR, or RNA interference of Wnt-1. Similarly, cell growth in soft agar required the PR DBD but was sensitive to disruption of PR/c-Src interactions, suggesting that both PR-B-induced rapid signaling events and nuclear actions contribute to this response. Our discovery that progestins are capable of robust autocrine activation of EGFR and sustained Erk1/2 signaling provides further support for the physiological linkage of growth factor and steroid hormone signaling. PR-B-induced sustained MAPK signaling may provide prosurvival or proliferative advantages to early breast cancer lesions.
Estrogen receptor (ER) studies dominate the field of hormone-responsive breast cancer research, in part due to the clinical successes of the antiestrogen tamoxifen and, more recently, aromatase inhibitors (42). Progesterone receptors (PR), encoded by a single ER-regulated gene, are primarily valued as indicators of estrogen responsiveness. Thus, PR action has been largely overlooked as an important input into the proliferation and/or survival of the epithelial component of the normal or malignant mammary gland. However, progesterone mediates alveolar proliferation during mammary gland development in the mouse (39), where PR isoforms induce the appropriate expression of potent mitogenic signaling molecules, including Wnts (7). Additionally, in humans, the peak of mammary epithelial cell proliferation and the appearance of mitotic figures coincide with high progesterone levels that occur during the luteal phase of the estrous cycle (49, 51). During pregnancy, PR-B colocalizes with cyclin D1 in dividing murine epithelial cells (1).
Factors involved in normal developmental processes are often inappropriately reasserted in cancers. Recently, progesterone exposure during hormone replacement therapy (HRT) has been recognized as an important breast cancer risk factor, with publication of numerous clinical studies (66), including the Women's Health Initiative (55) and the 2003 Million Women Study (3). Postmenopausal women who received combined HRT containing estrogen plus progesterone experienced increased breast cancer incidence relative to those who received estrogen HRT alone or placebo; the tumors detected were larger and of higher grade (11, 55). The mechanism of these effects is unknown. Progestins are not considered carcinogens. However, exposure to combined HRT may have stimulated the outgrowth of preexisting subclinical or dormant tumors and/or contributed to increased breast density, thereby delaying tumor detection. These reports underscore the practical and immediate demand for an increased understanding of the cellular response to progesterone, with clear demarcation of PR-dependent effects on signaling pathways known to be important in cell proliferation and survival.
PR-A and -B isoforms are members of a large class of steroid hormone-activated nuclear transcription factors that includes ER, androgen receptors, mineralocorticoid receptors, and glucocorticoid receptors (16). PR-C is truncated within the DNA-binding domain (DBD), but like PR-A, it can inhibit and/or modify PR-B activities (14). Ligand-bound PR dimers associate with promoter or enhancer regions of target genes and recruit coactivating enzymes, such as the steroid receptor coactivator family of acetyltransferases, to ultimately facilitate RNA Pol II-mediated transcription (38). The PR function as a ligand-activated transcription factor has been intensely studied. Like that of ER, PR expression is restricted to 7 to 10% of nonproliferating luminal epithelial cells within the normal mammary gland (60) but is found in roughly 80% of primary breast cancers. Perhaps due in part to its coexpression with functional ER, the PR-dependent mechanism(s) that may confer a proliferative and/or survival advantage on breast tumor cells remains unclear. Recently, “extranuclear” functions of PR have been described, where progestin binding to membrane-proximal PR-B induces rapid and transient (2- to 5-min) activation of the c-Src tyrosine kinase (Srcp60) (6, 41). PR extranuclear signaling to mitogen-activated protein kinase (MAPK) is extremely transient, occurring in minutes (2 to 15 min), whereas PR function as a transcription factor approaches hours. Biological responses on the order of days to months following progestin exposure have been documented (43). The question of whether rapid and transient activation of MAPKs in response to progestins can elicit sustained biological responses is a keen area of research with potential clinical significance.
PR target genes include key regulators of the cell cycle (cyclins D and E), components of growth factor signaling pathways (the epidermal growth factor receptor [EGFR] family of transmembrane receptors and their ligands), and critical mediators of developmental processes (Wnts), all of which are frequently associated with breast malignancy. We hypothesized that PR induction of these targets sets into motion a system of gene and/or protein regulation that continues in the face of rapid PR degradation by the 26S proteasome (35) to induce long-term effects on cell proliferation and/or survival. Additionally, we predicted that progestin-induced rapid signaling events contribute to PR regulation of selected target genes, thus enabling integration of cytoplasmic and nuclear PR actions. Here, we are the first to report a mechanism for progestin/PR-induced autocrine signaling, leading to sustained and robust activation of EGFR, c-Src, and Erk1/2 MAPKs. Differences in the strengths and durations of MAPK signaling are known to impart divergent cellular responses (e.g., differentiation versus proliferation in neuronal cells) (40). We further show that the sustained MAPK activation detailed here is functionally distinct from the rapid and transient PR-B/c-Src-associated signaling (6, 41) but that both pathways (rapid and sustained) may contribute to the regulation of anchorage-independent breast cancer cell growth in response to progestins.
MATERIALS AND METHODS
Constructs and RNA interference.
DNA fragments encoding base pair changes were constructed using two-step PCR mutagenesis to generate a PR DNA-binding mutant (PR-B DBD mutant C587A) (63) and an Src binding mutant (PR-B mPro; P422A, P423A, and P426A) (6) and then inserted into restriction enzyme-digested human PR-B housed in the pSG5 expression vector. Small hairpin RNAs (shRNAs) specific for candidate target sequences in the coding region of Wnt-1 were identified using the siRNA Hairpin Oligonucleotide Sequence Designer provided by the BD Knockout RNAi Clone and Confirm Kit BD Knockout RNAi (Clontech Laboratories, Inc.). Sequence similarity was searched using the NCBI BLAST server. cDNA hairpin oligonucleotides were annealed and ligated into the RNAi-Ready pSIREN vector according to the manufacturer's specifications. Three sequences were screened by retroviral infection and selection (puromycin, 2 μg/ml) for stable expression; T47D-YB cells stably expressing the small interfering RNA complementary to the Wnt-1 sequence 5′-TCAGAAGGTTCCATCGAAT-3′ were chosen for further study.
Cell culture, transfection, and retroviral infection.
The estrogen-independent PR-A- and PR-B-positive T47Dco cell line (26) and sublines, including PR-null T47D-Y cells and subsequent stable lines engineered to exclusively express either PR-B or PR-A (T47D-YB and T47D-YA, respectively), have been described previously (57). Stable expression of the PR-B mPro and C587A DBD mutants were obtained by cotransfection of PR-null T47D-Y cells with mutant PR cDNA and a neomycin resistance plasmid, using FuGENE (Roche) according to the manufacturer's instructions, and selected with neomycin analog G418 (500 μg/ml; Life Technologies, Gaithersburg, MD). T47D-YB stable Wnt-1 shRNA lines were obtained by infection with a retrovirus encoding shRNA sequence specific for Wnt-1 or with negative control (nonsense) sequence and selection in media containing 2 μg/ml puromycin. Preparation of the retrovirus and infection of target cells were performed as described previously (23). Cell lines were grown at 37°C in a humidified 5% CO2 environment in T75 flasks with minimal essential medium (Gibco, Rockville, MD) supplemented with 5% fetal bovine serum, 1% penicillin-streptomycin, 1% nonessential amino acids (Invitrogen, Carlsbad, CA), and 6 ng/ml insulin. The stable cell lines T47D-YB, T47D-YA, PR-B mPro, and T47D-C587A were additionally maintained in 200 μg/ml G418, and Wnt1 knockdowns were additionally maintained with 2 μg/ml puromycin. For experiments involving the synthetic progestin R5020, 5 × 105 to 6 × 105 cells were plated in 60-mm plates in phenol red-free Iscove's modified Eagle's medium (BioSource) supplemented with 5% dextran-coated charcoal (DCC)-stripped serum (HyClone, Logan, UT) for 48 h. EGF treatments were conducted in phenol red-free Iscove's modified Eagle's medium in the absence of serum.
Reagents.
Small-molecule inhibitor compounds, including U0126 (10 μM), AG1478 (1 μM), PD168393 (1 μM), PP2 (10 μM), SU6656 (10 μM), LY294002, and GM6001 (10 μM), were purchased from Calbiochem (La Jolla, CA) and added to cell culture media 30 min prior to the addition of R5020 (10 nM; NEN, Boston, MA), EGF (20 ng/ml; Sigma-Aldrich), or phorbol myristate acetate (PMA) (20 nM; Calbiochem). The PR antagonist RU486 (100 nM), the transcription inhibitor 5,6-dichlorobenzimidazole1-β-d-ribofuranoside (DRB) (100 μM), or the translation inhibitor cycloheximide (CHX) (10 μg/ml), obtained from Sigma-Aldrich (Saint Louis, MO), was added simultaneously with R5020. Monoclonal PR (Ab-8) and EGFR (Ab-13 for immunoprecipitation [IP]) antibodies were obtained from NeoMarkers (Fremont, CA); Srcp60 (c-Src) and the phospho- and total Erk1/2 MAPK antibodies were purchased from Cell Signaling (Beverly, MA); cyclin D1-, phospho-Y1173 EGFR-, and 4G10 phosphotyrosine-specific antibodies conjugated to agarose beads were purchased from Upstate (Lake Placid, NY); total EGFR and actin antibody were purchased from Sigma-Aldrich. Goat anti-mouse and goat anti-rabbit immunoglobulin G (IgG) horseradish peroxidase-conjugated secondary antibodies were obtained from Bio-Rad Laboratories (Hercules, CA), and goat anti-sheep IgG horseradish peroxidase-conjugated secondary antibodies were obtained from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA).
Immunoblotting and immunoprecipitation.
Whole-cell lysates were collected on ice by scraping them into RIPA buffer (10 nM sodium phosphate, pH 7.0, 150 mM NaCl, 2 mM NaCl, 2 mM EDTA, 1% [wt/vol] Nonidet P-40, 0.1% [wt/vol] sodium dodecyl sulfate [SDS], 1% sodium deoxycholate, 0.1% [vol/vol] β-mercaptoethanol, supplemented with 20 μg/ml aprotinin, 10 mM sodium pyrophosphate, 0.1 M sodium fluoride, 1 mM sodium vanadate, 1 mM phenylmethylsulfonyl fluoride), followed by sonication for 10 seconds (probe size, 2 cm deep by 12 cm long; setting 9; XL2000-Microson; Misonix, Farmingdale, IL). The lysates were clarified by centrifugation for 10 min at 14,000 RPM at 4°C. Soluble proteins were quantified by the Bradford method using Bio-Rad reagent (Hercules, CA), and equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis. The proteins were electrotransferred to a polyvinylidene difluoride membrane (Millipore, Billerica, MA), immunoblotted with specific antibodies, and developed using SuperSignal West Pico chemiluminescent substrate according to the manufacturer's protocol (Pierce, Rockford, IL). For immunoprecipitation experiments, cell lysates were collected in NP-40 lysis buffer (0.5% [vol/vol] NP-40, 50 mM Tris-HCl, 150 mM NaCl, and 1 mM EDTA, supplemented as described above) and incubated for 30 min on ice. Cell lysates containing equivalent protein concentrations were incubated for 2 h at 4°C with 1 μg appropriate antibody or control IgG, followed by overnight incubation with protein G-agarose (Roche). The immunocomplexes were washed four times with NETN buffer (0.1% [vol/vol] NP-40, 150 mM NaCl, 1 mM EDTA, and 50 mM Tris-Hcl supplemented with protease and phosphatase inhibitors as described above) and centrifuged at 12,000 × g; resuspended in Laemmli sample buffer; boiled for 5 min; and subjected to Western blotting analysis.
Intracellular staining and flow cytometry.
Cells were detached from the plate using 0.25% trypsin and collected in 1× phosphate-buffered saline plus 1% fetal bovine serum. The cells were fixed with 3% formaldehyde at 37°C for 10 min, permeabilized with 90% ice-cold MeOH for 30 min on ice, and stored at −20°C. Fixed cells (5 × 105) were stained with primary antibodies for 60 min at room temperature and then with Alexa Fluor 488-conjugated goat anti-rabbit IgG (Invitrogen) for 30 min at room temperature while protected from light. The fluorescence intensities in the FL1 channel were collected for each sample on a FACSCalibur (BD Biosciences) and analyzed using FlowJo (Tree Star) software.
Anchorage-independent growth.
Soft-agar assays were performed as described previously (56). One milliliter of 0.8% SeaPlaque agarose (BioWhitaker, Rockland, ME) in 5% DCC starvation medium was solidified in the bottom of each well of a six-well plate as the bottom agar. A total of 1 × 104 to 2 × 104 cells/well in 5% DCC starvation medium without or with 10 nM R5020 and/or dimethyl sulfoxide (DMSO) (vehicle) or 10 μM U0126, PP2, or PD168393 were mixed with 0.45% agarose, overlaid on the bottom agar, and incubated at 37°C in a humidified 5% CO2 environment. After 14 to 21 days, colonies were counted using a light microscope with a grid in the eyepiece. Using a grid size containing 100 uniform divisions (squares), colonies larger than one-third of each square in the grid were counted. Three randomly selected fields were counted for each well, and the average number of colonies is shown.
RT-PCR and real-time quantitative PCR.
Total RNA was isolated with Trizol (Invitrogen) according to the manufacturer's protocol. Two micrograms of RNA was reverse transcribed with random hexamers and Moloney murine leukemia virus reverse transcriptase (RT) (Invitrogen) according to the manufacturer's protocol. Each PCR mixture contained 1 μl of cDNA and a 0.5- to 1-μmol/liter concentration of each primer. RT-PCR was carried out using an MJ thermocycler, and samples were separated on a 2% agarose gel. Real-time quantitative PCR analysis was performed with a Light Cycler (Roche Diagnostics) according to the manufacturer's instructions. Specific detection of double-stranded DNA was obtained by preparing reactions in the presence of the fluorescent dye SYBR green I. Cumulative fluorescence was measured at the end of the extension phase of each cycle, and quantification of cycle numbers obtained at the log-linear phase of the reaction were plotted against a standard curve prepared with serially diluted samples as described previously (65). Melting-curve and agarose gel electrophoresis analyses confirmed product-specific amplification; the results were normalized to β-actin.
Transcription assay.
PR transcriptional activity in cells was measured using the Dual-Luciferase Reporter Assay (Promega, Madison WI) according to the manufacturer's protocol. HeLa cells seeded at a density of 2.25 × 105 per well (six-well plates) were transiently transfected with 0.1 μg of wild-type (wt) or mutant PR-B constructs (in pSG5), along with 1 μg progesterone-responsive element (PRE) 2× TATA in the luciferase reporter plasmid pA3-LUC and 25 ng pRL-TK, a Renilla luciferase construct for normalizing transfection efficiency. The cells were transfected with Fugene HD transfection reagent according to the manufacturer's instructions (QIAGEN Inc., Valencia, CA). After overnight incubation with DNA complexes, the medium was replaced with starvation medium and the cells were incubated for an additional 24 h. The cells were treated for 12 h with or without R5020 (10 nM), and 20 μl of lysates was analyzed for firefly/Renilla luciferase.
Zymogram assay.
Culture medium was concentrated 100 times by centrifugation using Centricon filter devices (Millipore) at 4°C. Equal amounts of protein were electrophoresed under nonreducing conditions by 10% SDS-polyacrylamide gel electrophoresis containing 0.1% gelatin (Invitrogen). The gels were incubated at 37°C for 72 h in developing buffer (Invitrogen). Proteinase activity was visualized by Coomassie blue R250 staining, where zymogram activity appears as a clear band in a dark background.
RESULTS
Progestin/PR stimulates sustained Erk1/2 MAPK activity.
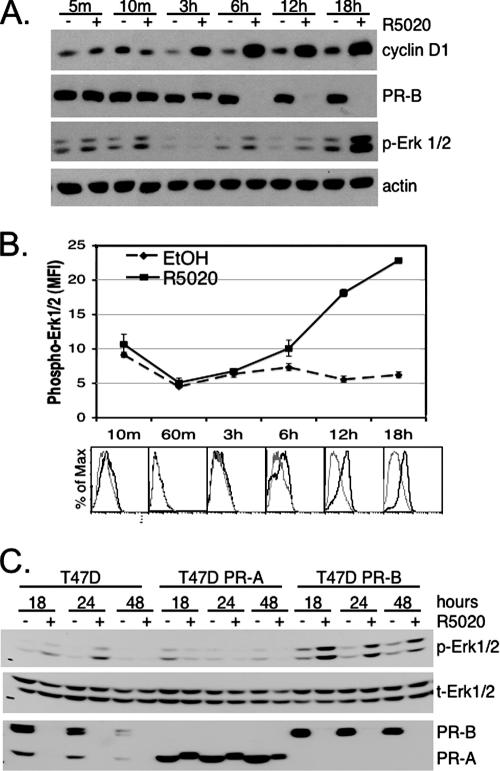
We showed previously that progestin treatment of T47D breast cancer cells stimulates increased transcription from the cyclin D1 proximal promoter region (i.e., within ∼944 bp upstream of the start site) in an Erk1/2 MAPK-dependent manner (62). Similarly, progestin-induced elevation of cyclin D1 protein levels required the activity of Erk1/2 MAPK (62). Rapid MAPK signaling induced by progestins occurs on the order of 2 to 15 min and is extremely transient and of low magnitude relative to that elicited by numerous peptide growth factors, suggesting the possibility of additional MAPK inputs to cyclin D1 upregulation. To further examine the role of progestin-induced MAPK activity in cyclin D1 expression, we treated T47D-YB breast cancer cells that exclusively express the B isoform of PR with the synthetic progestin R5020 for 0 to 18 h. PR and cyclin D1 protein expression levels were measured by Western blotting (Fig. 1A). As expected, the PR-B protein was undetectable following 6 h of progestin treatment, due to ligand-dependent receptor downregulation (Fig. 1A) (35). Despite the loss of PR protein by 6 h, PR induction of cyclin D1 continued to rise, peaking 6 to 18 h post-R5020 treatment (Fig. 1A) (21, 47). MAPK activation was measured in the same cell lysates blotted with antibodies recognizing the dually phosphorylated, active forms of Erk1/2 MAPKs. Interestingly, the results revealed oscillations in Erk1/2 MAPK activity following progestin treatment. Consistent with previous reports (6, 41), PR-ligand binding rapidly activated Erk1/2 MAPK at 5 to 10 min, and this returned to basal levels within 30 to 60 min (Fig. 1A and B). A second period of increased Erk1/2 MAPK activity was evident 6 h later and represented the beginning of a sustained signal that progressed to a peak at 18 to 24 h and was sustained for at least 72 h (not shown). Actin blotting indicated that equal amounts of protein were loaded onto the gel; no change in total Erk1/2 MAPK levels was observed (not shown) (see below).
FIG. 1.
Progestin treatment of T47D-YB cells stimulates PR-dependent sustained activation of Erk1/2 MAPKs. (A) T47D cells stably expressing wt PR-B were treated with EtOH vehicle control (−) or 10 nM R5020 (+) for the indicated times (m, minutes). Cell lysates were Western blotted for expression of cyclin D1, PR, or activated (phospho) forms of Erk1/2 MAPKs. Actin blotting was included as a protein-loading control; total MAPK did not change in the same lysates (not shown) (see the legend to panel C). (B) Time course of Erk1/2 phosphorylation as measured using flow cytometry following EtOH control (dashed lines) or R5020 (solid line) stimulation. The lines represent the mean fluorescence intensities (MFI) of triplicate measures versus time; the error bars represent the standard errors of the mean. Histogram overlays (below) at each time point represent fluorescence versus the percentage of maximum for EtOH (gray line) compared to R5020 (black line) treatment. The specificity of the assay was demonstrated using the MEK 1/2 inhibitor U0126 to block R5020-dependent increase in phospho-Erk1/2 (data not shown). The experiments were repeated three times with similar results. (C) T47D parental cells that endogenously expressed both PR-A and PR-B, or sublines engineered to stably express either PR-A or PR-B, were treated with or without R5020 for 18, 24, or 48 h; the cell lysates were Western blotted for phospho- (p) or total (t) Erk1/2 MAPK and total PR. Three independent experiments yielded identical results.
These results were confirmed by flow cytometry using phospho-Erk1/2 labeling of activated MAPKs in fixed, permeabilized cells (Fig. 1B). These data suggest that extended treatment of breast cancer cells with progestins generates a graded increase in Erk1/2 MAPK activity that is biphasic in nature. Early (5- to 10-min) MAPK activation is rapid, transient, and weak in magnitude relative to the delayed, sustained, and much more robust Erk1/2 MAPK activation observed at 18 to 72 h.
The experiments shown in Fig. 1A and B were conducted in T47D cells that exclusively express PR-B, the dominant PR isoform required for mammary gland development (44). PR-B and PR-A are functionally different isoforms with distinct transcriptional activities, depending on the cell type and promoter context (30, 64). We therefore tested for PR isoform-specific differences in the patterns of Erk1/2 MAPK activation by progestins. Parental T47D breast cancer cells that express both isoforms of PR, or variants of the cell line engineered to exclusively express either PR-A or PR-B (57), were treated with or without R5020 for 18, 24, or 48 h (Fig. 1C). Similar to T47D-YB cells, T47D parental cells expressing both PR-A and -B consistently induced sustained Erk1/2 MAPK signaling when treated with R5020 for 18 to 24 h. In contrast, the PR-A isoform expressed in T47D-YA cells failed to induce sustained Erk1/2 MAPK (Fig. 1B): indeed, extended progestin treatment of T47D-YA cells repeatedly resulted in diminished Erk1/2 MAPK signal relative to vehicle controls. Additionally, PR-A failed to undergo ligand-induced degradation in the absence of PR-B: rapid downregulation of liganded PR is MAPK and CDK2 dependent (35, 50). Thus, functional differences between PR-B and PR-A may in part be due to disparate abilities of these isoforms to activate MAPKs and input to cell cycle regulation (21, 50).
Progestin/PR-induced cyclin D1 protein upregulation requires sustained MAPK activity.
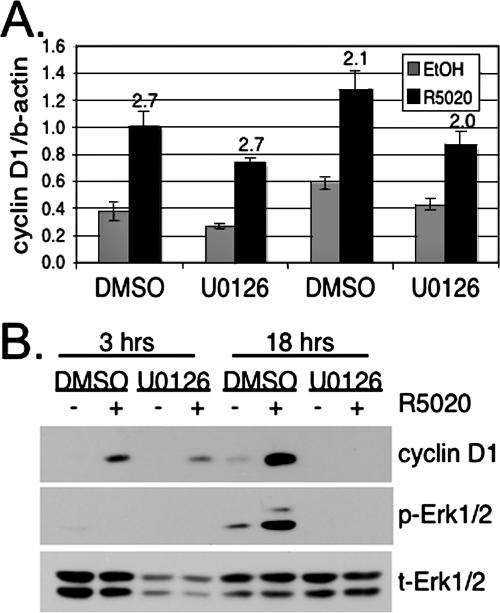
Upregulation of cyclin D1 protein is evident 3 h following progestin treatment, and protein levels remain high over the 18-h time course (Fig. 1A). The cyclin D1 protein has a short half-life, reported to be between 30 and 60 min (22). In the face of rapid PR turnover, cyclin D1 upregulation in response to progestins may be mediated by increased transcriptional activity at the level of the proximal promoter (62) or via factors acting at distant enhancer sites (8), but it likely also involves stabilization of the cyclin D1 protein in response to phosphorylation events (10) and/or translational control (54). To determine how progestins contribute to cyclin D1 protein upregulation, T47D-YB cells were treated with R5020 for 3 h, a time point when cyclin D1 levels are clearly increased but MAPK is inactive, or for 18 h, the peak in MAPK signaling and high cyclin D1 expression. Treatments were done in the presence of DMSO vehicle control or U0126, a specific inhibitor of the activating Erk1/2 MAPK kinases, MEKs 1 and 2. As measured by quantitative real-time PCR, R5020 increased cyclin D1 mRNA at both 3 h (2.7-fold) and 18 h (2.2-fold) (Fig. 2A). Basal cyclin D1 levels fluctuated somewhat and were slightly diminished in the presence of U0126. However, the overall increase in cyclin D1 mRNA with R5020 treatment at both 3 and 18 h was not significantly reduced by the MEK inhibitor (Fig. 2A). In contrast, Western blotting of cell lysates harvested from similarly treated cells revealed a minimal effect of Erk1/2 MAPK inhibition on cyclin D1 protein levels following 3 h of R5020 treatment. However, at 18 h, cyclin D1 protein expression was entirely MAPK dependent (Fig. 2B). These data suggest that in response to progestins, transcriptional upregulation of the endogenous cyclin D1 gene occurs independently of MAPKs. Progestin-induced upregulation of cyclin D1 protein requires sustained activation of MAPK at time points when PR is clearly downregulated, consistent with reports that MAPK-dependent phosphorylation events contribute to increased cyclin D1 protein stability by promoting assembly of cyclin-CDK complexes (10) and/or enhance translation (54). Cyclin D1 is not upregulated by progestins in PR-null T47D cells (not shown).
FIG. 2.
Progestin/PR-induced cyclin D1 protein, but not mRNA upregulation, requires MAPK activity. (A) Cyclin D1 mRNA levels were measured using real-time quantitative RT-PCR. The bars indicate the mean (plus standard deviation) of cyclin D1 mRNA, normalized to β-actin, measured in triplicate cultures of T47D-YB cells treated for 3 or 18 h with R5020 in the presence of DMSO or U0126; values (n-fold) over the EtOH control are indicated above the bars. (B) Whole-cell lysates from T47D-YB cells treated as in panel A were Western blotted for cyclin D1 and phospho- (p) and total (t) Erk1/2. The experiments were repeated three (A) or four (B) times with similar results.
PR-B-induced sustained activation of Erk1/2 MAPK requires transcription.
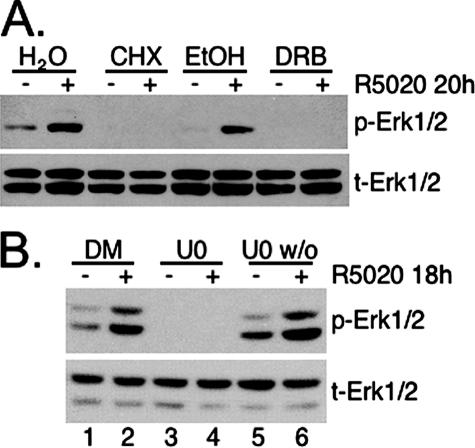
Biphasic activation of MAPK by either progestins or androgens occurs in Xenopus oocytes, where rapid activation of Erk1/2 MAPK upon hormone exposure results in a series of phosphorylation events that culminate in poly(A) addition to Mos transcripts, the homologue of mammalian MEKK1 and an upstream activator of MEK in the Erk1/2 MAPK module (36). Polyadenylation of Mos mRNA stabilizes transcripts, thereby increasing the expression of otherwise low-abundance Mos proteins to produce a second wave of MAPK activation 3 h post-hormone treatment that occurs independently of transcriptional events (29). To investigate whether progestin/PR-induced biphasic Erk1/2 MAPK activation in breast cancer cells requires transcription and/or translation, steroid-starved T47D-YB cells were treated with R5020 for 18 h in the presence or absence of DRB, an inhibitor of transcription, or CHX, an inhibitor of translation. Cell lysates were Western blotted for activated and total Erk1/2 MAPKs (Fig. 3A). The presence of either CHX or DRB prevented R5020-induced Erk1/2 MAPK activation at 18 h. The membrane was stripped and reprobed for total MAPK, indicating that no change in total MAPK expression could account for this result. Furthermore, we did not observe increased expression of MEKK1 (Xenopus Mos) protein following an extended time course of progestin treatment (not shown).
FIG. 3.
Progestin-induced sustained Erk1/2 MAPK signaling requires transcription and translation. (A) T47D-YB cells were treated for 20 h with or without R5020 in the presence of H2O vehicle control, CHX, EtOH vehicle control, or DRB, and phospho- (p) and total (t) Erk1/2 MAPKs were detected by Western blotting of whole-cell lysates. (B) T47D-YB cells were pretreated for 30 min with DMSO vehicle control or U0126 (MEK inhibitor) prior to addition of R5020. After 30 to 60 min, U0126 was washed out of the cultures represented in lanes 5 and 6 (U0 w/o; the 45-min time is shown). Cell lysates were collected after 18 h and Western blotted for phospho- or total Erk1/2 MAPKs and PR. The experiments were repeated three times with similar results.
PR is a dual-function molecule, able to rapidly stimulate cytoplasmic signaling pathways and directly or indirectly bind DNA to activate transcription of target genes. To investigate which of these PR actions are required for sustained periods of MAPK signaling, we performed washout experiments. T47D-YB cells were pretreated for 30 min with DMSO (positive control) or U0126 (negative control) prior to R5020 addition for 18 h. The plates were then left unwashed to allow (DMSO, lanes 1 and 2) or block (U0126, lanes 3 and 4) all MAPK signaling over the entire time course. For washout experiments, some U0126-containing plates were washed 30 to 45 min posttreatment, in order to block only the rapid early phase (first 30 min) of MAPK signaling in response to progestin (lanes 5 and 6). As shown in Fig. 3B, DMSO or the U0126 compound was present during the entire incubation period in positive (lanes 1 and 2) and negative (lanes 3 and 4) controls. As expected, the MEK inhibitor blocked sustained (18-h) MAPK activation by progestin in unwashed cultures (lanes 3 and 4). In contrast, when R5020 and/or U0126 was washed out of previously exposed cell cultures, R5020 (washed out after 30 to 45 min) still induced sustained MAPK in a manner that was not distinct from that of vehicle alone (Fig. 3B, lanes 5 and 6). These data suggest that the rapid, membrane-proximal PR-induced MAPK activation, occurring within 2 to 15 min of progestin exposure, is not required for the subsequent sustained MAPK activity described here (Fig. 1) and that progestin/PR-dependent events mediate latent sustained MAPK activation independently of early MAPK activities.
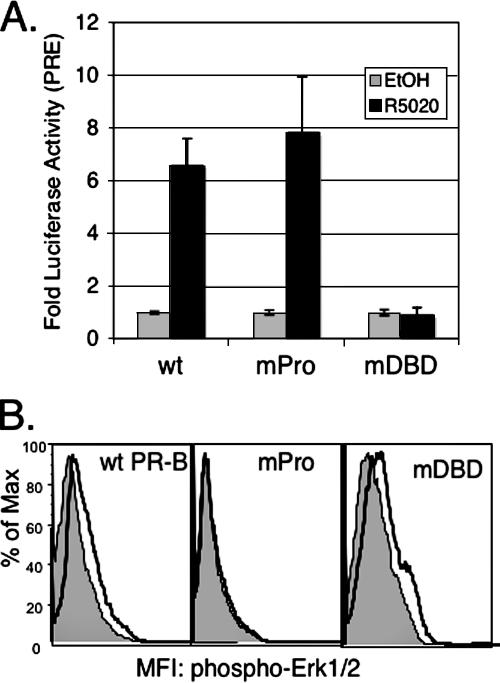
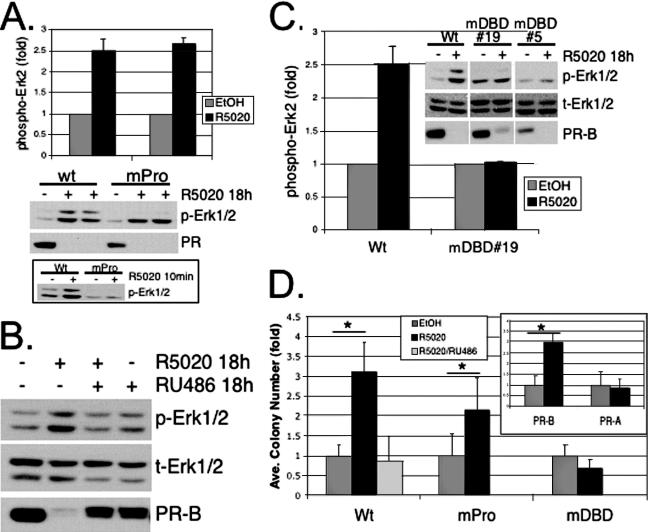
To confirm these results, we directly tested the role of the PR/c-Src interaction in sustained MAPK signaling. We made use of a mutant PR-B (PR-mPro) containing point mutations in each of three key proline residues (P422A, P423A, and P426A) in the PR N-terminal polyproline region, resulting in the disruption of the amphipathic α-helix that acts as an SH3 ligand for c-Src family kinases (6). Liganded PR-mPro is transcriptionally competent on a PRE-driven promoter (Fig. 4A) but is unable to bind to and activate c-Src and, in turn, is incapable of rapid activation of cytoplasmic signaling, including rapid (5- to 10-min) phosphorylation and activation of Erk1/2 MAPKs (Fig. 4B) (6). Conversely, a PR mutant with a disrupted DBD (Cys587 to Ala) cannot mediate transcriptional responses to progestins (Fig. 4A) (63) but is fully capable of inducing rapid (5- to 10-min) Erk1/2 MAPK activation (Fig. 4B).
FIG. 4.
Transcriptional and signaling activities of DNA-binding (mDBD) and c-Src binding (mPro) mutant PRs relative to wt PR-B. (A) Wt and mPro PR-B, but not mDBD PR-B, stimulate transcription of a transiently cotransfected PRE-luciferase reporter construct in HeLa cells. The bars indicate the averages of triplicate measurments of firefly luciferase normalized to Renilla luciferase (± standard deviations) for each condition; similar results were obtained in T47D cells (not shown). (B) Rapid Erk1/2 activation by wt PR-B and mDBD PR-B, but not mPro PR-B. Erk1/2 activity was measured using flow cytometry following 5-min R5020 treatment (heavy black line, unfilled) or treatment with EtOH vehicle control (gray-filled line). The data are represented as fluorescence (x axis) versus percent maximum (Max) (y axis).The experiments were repeated three times, in triplicate, with similar results.
T47D cells stably expressing wt PR-B or PR-mPro were treated with R5020 for 18 h (Fig. 5A). A short time point (5- to 10-min) was included as a functional control for PR/c-Src-dependent signaling to MAPKs. Western blotting of whole-cell lysates further confirmed that PR-mPro failed to induce MAPK activity following 5 to 10 min of R5020 exposure. However, both wt and PR-mPro activated sustained (18-h) MAPK signaling (Fig. 5A). Reprobing of blots with antibodies specific for total Erk1/2 MAPKs indicated equal expression of the MAPK protein (not shown). PR immunoblots verified comparable levels of wt PR and PR-mPro protein expression and ligand-induced receptor downregulation. These data indicate that interaction of c-Src with PR and rapid MAPK signaling (2 to 15 min) are not required for robust latent and sustained MAPK signaling (6 to 72 h).
FIG. 5.
Contributions of rapid PR signaling and PR transcriptional events to sustained MAPK activation and anchorage-independent growth. (A) Duplicate cultures of T47D cells stably overexpressing wt PR-B or the c-Src binding mutant, PR-B mPro, were treated with EtOH vehicle control (−) or R5020 (+) for 18 h or 10 min (bottom), and whole-cell lysates were Western blotted for phospho-Erk1/2 (p-Erk1/2) MAPK and total PR. The duplicate 18-h experimental time points shown, from a representative experiment, were quantified by densitometry of visible control and experimental bands on scanned images and expressed as increased Erk2 MAPK activity (n-fold) relative to vehicle controls (the bars represent means plus standard deviations). Densitometry of multiple exposures yielded similar results. Note that Western blotting confirmed the inability of PR-B mPro to rapidly activate Erk1/2 at 10 min relative to wt PR-B (bottom). The results were repeated three times. (B) T47D-YB cells were treated with EtOH, 10 nM R5020, 10 nM R5020 plus 100 nM RU486, or 100 nM RU486 alone for 18 h; the cell lysates were subjected to Western blotting with antibodies specific for phospho- (p) or total (t) Erk1/2 MAPK and total PR. Similar results were obtained for three experiments. (C) Phospho-Erk1/2 MAPK Western blotting and quantification by densitometry of wt-PR-B-expressing T47D-YB cells and two clones (19 and 5) stably expressing the PR-DBD mutant, C587A (mDBD). The cells were treated as described above with vehicle control (−) or R5020 (+) for 18 h, and whole-cell lysates were Western blotted for phospho- or total Erk1/2 and PR-B. PR blotting indicated roughly equivalent levels of PR protein in stable cell lines. The bars represent the R5020-stimulated activation (n-fold; plus standard deviation) of Erk2 MAPK for duplicate measurments performed within the same experiment relative to controls, using stable PR-DBD mutant C587A clone 19 (mDBD). Densitometry of multiple exposures yielded similar results. The experiments were repeated three times with similar results. (D) Progestin-stimulated soft-agar colony formation of T47D cells stably expressing either wt PR-B or PR-B mutants, mPro or mDBD. Cells were plated as described in Materials and Methods, and the bars represent the increase (n-fold) (plus standard deviation) in colony numbers for each stable cell line relative to EtOH controls. The inset shows colony numbers (n-fold) for R5020-treated T47D-YB (PR-B) cells relative to cells stably expressing PR-A (T47D-YA; PR-A). The asterisks denote significance (P < 0.01) determined by an unpaired Student's t test between EtOH- and R5020-treated conditions. The results were confirmed in two independent experiments.
We next investigated whether the delayed, sustained activation of MAPK (Fig. 1) required the classical properties of PR as a ligand-activated transcription factor. The PR antagonist RU486 blocked R5020-mediated MAPK activation at 18 h and had no effect when administered alone (Fig. 5B). Ligand-dependent PR downregulation was also attenuated in the presence of RU486 but weakly stimulated by RU486 alone, consistent with previous studies (25).
Mutation of PR Cys587 to Ala, located at the base of the first zinc finger of the DBD, disrupts PR binding to DNA (63). Although unable to bind DNA and mediate transcriptional activation of PRE-containing promoters (Fig. 4A), stably expressed DBD mutant PR-B is capable of ligand binding and undergoes downregulation, similar to wt PR-B (Fig. 5C). Additionally, stably expressed DBD mutant receptors induce rapid (5- to 10-min) activation of cytosolic signaling pathways, including Erk1/2 MAPK (Fig. 4B). To test the requirement for PR transcriptional activity in sustained Erk1/2 MAPK signaling, T47D cells stably expressing wt PR-B (T47D-YB) or mutant (mDBD) PR-B (T47D-C587A) were treated with ethanol (EtOH) vehicle control or R5020 for 18 h, and the lysates were subjected to Western blotting for activated and total Erk1/2 MAPK (Fig. 5C). Again, R5020 induced sustained activation of Erk1/2 MAPKs in T47D-YB cells relative to vehicle controls. In contrast, mDBD PR-B in T47D-C587A cells failed to activate Erk1/2 MAPK at 18 h in response to progestin, while rapid (5-min) MAPK activation remained intact (Fig. 4B). Together, these results demonstrate that classical PR transcriptional activity is required to generate sustained MAPK signaling (Fig. 1).
PR interaction with c-Src and rapid activation of MAPKs is required for progestin-induced S-phase entry in T47D cells cultured on plastic (62) and also contributes to cell growth inhibition (6) during biphasic responses to progestins (21). Under serum- and steroid hormone-free conditions, progestins stimulate long-term proliferation and survival over a period of weeks to months (43). To test the contribution of PR-induced rapid MAPK signaling via the c-Src interaction and PR transcriptional activity in a “long-term” experimental setting, we assayed progestin-stimulated anchorage-independent cell growth in soft agar. In this assay, colony formation occurs over several weeks and represents a measure of the ability of cancer cells both to survive without attachment and to subsequently proliferate and grow as colonies in soft agar. T47D cells stably expressing wt PR-B, PR-mPro, or PR-mDBD were plated in soft agar in the presence or absence of R5020, and 3-week-old colonies were counted (Fig. 5D). Cells stably expressing wt PR-B formed threefold more colonies in the presence of progestin than untreated cells, an effect abolished by RU486. However, cells stably expressing PR-mDBD were unable to support increased colony numbers in the presence of progestin. Interestingly, cells expressing the Src-binding mutant, PR-mPro, also responded to progestin, but with slightly attenuated colony numbers that approached those of wt PR-B. Notably, PR-A-expressing T47D-YA cells that do not activate sustained MAPK did not form increased colony numbers in the presence of progestin (Fig. 5D, inset). PR-null cells formed colonies in soft agar but also failed to respond to progestins (not shown). These results show that PR-B transcriptional activity is required for progestin-induced cell growth in soft agar and suggest that sustained, rather than rapid, MAPK activation is important for increased anchorage-independent growth of breast cancer cells.
Sustained MAPK signaling in response to progestins requires EGFR.
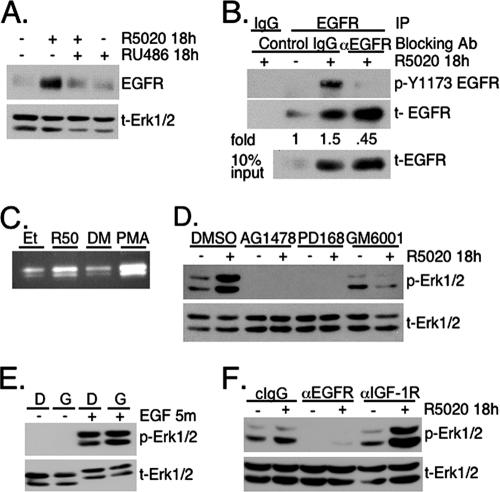
Since liganded PR-B is completely downregulated in T47D cells 6 to 18 h after R5020 treatment, additional signaling molecules must be involved in perpetuating sustained MAPK activation (Fig. 1). PR upregulation of key growth factor pathway components, including EGFR, is well established. However, how these molecules impact progestin-dependent changes in cell biology, especially with regard to tumor proliferation and/or survival, is not known. In addition to PR-dependent upregulation of EGFR levels (Fig. 6A), we wondered whether prolonged progestin treatment effected a specific increase in EGFR tyrosine kinase activity to achieve downstream sustained Erk1/2 MAPK signaling. Previously, no increase in EGFR activity was detected when total phosphotyrosine levels were tested (34). To increase the sensitivity of our assay of EGFR activation, we measured the degree of specific phosphorylation at a primary site of EGFR autophosphorylation, Tyr1173, where regulation of this site defines receptor activity (4). EGFRs were immunoprecipitated using specific or control (IgG) antibodies from lysates of T47D-YB cells that had been exposed to vehicle or R5020 for 18 h (Fig. 6B). Western blotting clearly showed an increase in EGFR Tyr1173 phosphorylation following 18 h of R5020 treatment relative to that of the vehicle control. To account for the increase in total EGFR in the presence of progestin (Fig. 6A), phospho-Tyr1173 EGFR for each treatment group was normalized to the total EGFR present in each IP, indicating a 1.5-fold increase of Tyr1173 phosphorylation following 18 h of R5020. Additionally, inclusion of an antibody that specifically blocks ligand binding to the EGFR during R5020 treatment completely abrogated EGFR activation, returning it to basal activity levels (vehicle control), without altering progestin-induced upregulation of the EGFR protein. This result demonstrates the specificity of our assay and suggests that EGFR activation in response to progestins occurs through a mechanism of EGFR-ligand binding. In summary, prolonged exposure of breast cancer cells to progestin yields an increase in both total and activated EGFRs.
FIG. 6.
Progestin-induced EGFR transactivation mediates sustained Erk1/2 MAPK signaling. (A) T47D-YB cells were treated without (−) or with (+) R5020 and/or RU486 for 18 h and then Western blotted for total (t) EGFR or total Erk1/2 MAPK as a loading control. (B) T47D-YB cells were treated for 18 h without (−) or with (+) R5020 in the presence of control IgG or with ligand-blocking antibodies (Ab) against EGFR. EGFR was then immunoprecipitated, and immune complexes were subjected to Western blotting to detect (active) phospho-Tyr1173 EGFR. The membranes were stripped and reprobed to detect the total EGFR immunoprecipitated. Monoclonal IgG served as a negative IP control, and lysate (10% of the IP input) demonstrated upregulation of total EGFR by 18-h R5020 treatment. The increase (n-fold) in EGFR activation was determined by densitometric analysis of immunoblots, where the pY-1173 signal was normalized to total EGFR in the immunoprecipitation. Densitometry of multiple exposures yielded similar results. (C) Concentrated culture medium from T47D-YB cells treated for 18 h with EtOH (Et) or R5020, or PMA as a positive control, was separated by a nondenaturing gelatin zymogram as described in Materials and Methods and stained with Coomassie blue. The clear regions indicate areas of protease activity. The results were confirmed in two independent experiments. (D) T47D-YB cells were pretreated for 30 min with DMSO vehicle control, the EGFR inhibitor AG1478 or PD168393, or the broad-range MMP inhibitor GM6001 and then treated without or with R5020 for 18 h as described above. Whole-cell lysates were Western blotted for phospho- or total Erk1/2 MAPK. (E) To control for the selectivity of the MMP inhibitor GM6001, T47D-YB cells were pretreated for 30 min with DMSO (D) or GM6001 (G), followed by a 5-min (m) exposure to 20 ng/ml EGF. The lysates were Western blotted for phospho- or total Erk1/2 MAPK. (F) T47D-YB cells were preincubated for 30 min with either control IgG or ligand-blocking antibodies against the EGFR or IGF-1R prior to 18-h R5020 treatment. Western blotting was performed as described above for phospho- or total Erk1/2. Except where noted, all experiments were repeated three or four times with similar results.
The results described above (Fig. 6B) suggest that EGFR activation following 18 h of progestin treatment requires EGFR ligand binding, as opposed to simply increased concentrations of these receptors. Matrix metalloprotease (MMP)-dependent shedding of EGFR family ligands is recognized as a method to control ligand availability in many cell types (59, 67). Following either 18 h of treatment with EtOH vehicle or R5020 or 20 min of PMA as a positive control, cell culture medium was collected and concentrated. An equal amount of protein was separated on gelatin zymograms to monitor protease activity (see Materials and Methods). Coomassie blue staining revealed increased clearing of gelatin substrate, indicative of protease activity, in the R5020 and PMA over their respective vehicle controls (Fig. 6C). Thus, 18 h of treatment of breast cancer cells with progestins stimulates proteolytic activity at time points when EGFR is activated.
To test the requirement for EGFR activity, possibly via MMP-mediated ligand shedding, in progestin-induced sustained Erk1/2 MAPK activation, T47D-YB cells were pretreated for 30 min with vehicle control (DMSO), two structurally distinct EGFR inhibitors (AG1478 and PD168393), or the broad-spectrum MMP inhibitor GM6001 (Ilomostat) to block MMP cleavage of EGFR ligands (Fig. 6D). Western blot analysis conducted with phosphospecific Erk1/2 MAPK antibodies indicated that reactivation of Erk1/2 MAPK in response to prolonged progestin treatment requires EGFR tyrosine kinase signaling. Similarly, activation of Erk1/2 MAPK at 18 h of R5020 treatment was completely blocked by pretreatment with GM6001. DMSO vehicle had no effect. Inhibition of MMP activity did not affect the ability of exogenously added EGF to signal through the EGFR and activate downstream Erk1/2 MAPK, indicating that this inhibitor acts upstream of EGFR ligand binding and receptor activation (Fig. 6E).
To address the specificity of progestin-induced sustained Erk1/2 activation via autocrine signaling to the EGFR, we employed antibodies that specifically recognize and thus block the ligand-binding domains of either EGFR (31, 58) or insulin-like growth factor-1 receptors (IGF-1R) (33). T47D-YB cells were pretreated (30 min) with blocking antibodies against the EGFR, IGF-1R, or control monoclonal IgG, followed by 18 h of treatment with R5020 (Fig. 6F). In the presence of EGFR-blocking antibodies, R5020 failed to induce sustained Erk1/2 MAPK activity relative to control IgG. In contrast, blocking antibodies to IGF-1R did not prevent sustained MAPK activation and actually increased MAPK activation in the presence of R5020, suggesting a potential transrepression of EGFR or downstream signaling to MAPK by the IGF-1R pathway. The specificity of each antibody for its receptor was confirmed in control experiments by addition of exogenous EGF or IGF-1 to serum-starved T47D cells and examination of Erk1/2 activation (not shown). In contrast to reports that rapid E2-ER transactivation of the EGFR by ligand shedding occurs through G protein-coupled receptor signaling (17), we were unable to detect an effect of pertussis toxin (a constitutive activator of Gαi-dependent G protein-coupled receptor signaling) or ICI 182 (the ERα antagonist) on progestin-PR-dependent activation of either rapid or sustained Erk1/2 MAPK signaling (not shown).
c-Src is required for sustained Erk1/2 MAPK signaling in response to progestins.
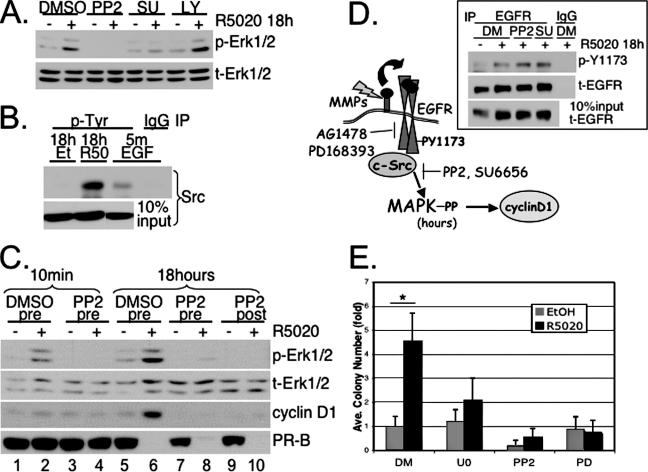
Although rapid PR/c-Src interactions did not appear to significantly contribute to sustained MAPK signaling (Fig. 5A), we observed a decrease in sustained MAPK signaling when cells were incubated with inhibitors of c-Src family members (PP2 and SU6656) but not inhibitors of PI-3K (LY294002) (Fig. 7A) or p38 MAPK (SB203580) (not shown). These results suggest that c-Src or c-Src family members act upstream of sustained Erk1/2 MAPK in response to PR ligand binding.
FIG. 7.
Persistent c-Src activation is required for progestin-induced sustained Erk1/2 activity. (A) T47D-YB cells were pretreated for 30 min with DMSO vehicle control, U0126, the c-Src family inhibitor PP2 or SU6656 (SU), or the PI3-K inhibitor, LY294002 (LY), followed by 18 h of R5020 treatment, and whole-cell lysates were Western blotted for phospho- (p) and total (t) Erk1/2 MAPKs. The specificity and activity of each c-Src inhibitor, and the LY294002 and SB203580 compounds, were confirmed by Western blotting for activated (phospho) forms of c-Src, Akt and p38, respectively (not shown). (B) T47D-YB cells were treated for 18 h with R5020 or vehicle control or 5 min (m) with EGF as a positive control for c-Src activation. Tyrosine-phosphorylated proteins were immunoprecipitated using 4G10 antibody-conjugated agarose beads (p-Tyr) or IP IgG control and Western blotted with c-Src-specific antibodies. Western blotting of c-Src in whole-cell lysates (10% of IP input) indicated no effect of vehicle control or R5020 treatments on total c-Src levels. The results were confirmed in two independent experiments. (C) T47D-YB cells were pretreated (pre) for 30 min with DMSO (lanes 1 and 2, 5 and 6) or PP2 (lanes 3 and 4, 7 and 8) prior to the addition of R5020 (+) or EtOH vehicle control (−) for 10 min or 18 h. In lanes 9 and 10, PP2 was added at the beginning of the 12th hour (post) of R5020 treatment. Cell lysates were Western blotted for phospho- or total Erk1/2 MAPK, cyclin D1, or total PR. (D) Progestin-induced persistent c-Src activity downstream of EGFR signaling to sustained activation Erk1/2 MAPK and cyclin D1 upregulation. (Inset) c-Src functions downstream of EGFR. T47D-YB cells pretreated for 30 min with DMSO, PP2, or SU6656 were treated for 18 h with R5020. EGFR was immunoprecipitated using specific antibodies or control IgG, and immune complexes were subjected to Western blotting to detect active (phospho-Tyr1173) EGFR. The membranes were stripped and reprobed to detect the total EGFR pulled down in each IP reaction. Whole-cell lysates indicate 10% of IP input. (E) T47D-YB cells were plated in soft agar as described in Materials and Methods and treated without (−) or with (+) R5020 in the presence of DMSO, U0126 (U0), PP2, or PD168393 (PD). The bars represent the increase (n-fold) in colony numbers (plus standard deviation) for cell cultures treated with progestin over EtOH DMSO controls, and the asterisk denotes statistical significance (P < 0.01) determined by an unpaired Student's t test. Except where noted, experiments were repeated three times with similar results.
To confirm that c-Src kinase is indeed activated during sustained signaling in response to progestins, tyrosine-phosphorylated proteins were immunoprecipitated from T47D-YB breast cancer cells treated with R5020 for 18 h (Fig. 7B). EGF (5 min) served as a positive control for c-Src activation. In contrast to EtOH vehicle control, a significant amount of phosphorylated c-Src was present in immunoprecipitates from R5020-treated cells, suggesting that c-Src activity is high during the sustained phase (18 h) of MAPK signaling. We were surprised by the magnitude of phospho-Src pulled down from lysates of cells that had been treated with R5020 for 18 h relative to lysates from EGF (5-min)-treated cells. These results are consistent with the amplification of MAPK signaling observed at 18 h of progestin treatment relative to that induced after 10 min (Fig. 1) and suggest a direct role for c-Src activity in both the rapid (5- to 10-min) (6) and sustained (18-h) phases of MAPK activation.
To verify the requirement for activated c-Src throughout the time course of progestin treatment leading to sustained MAPK signaling, we repeated the experiment shown in Fig. 7A but included both pre- and post-R5020 treatments with the c-Src inhibitor (PP2). T47D-YB cells were again pretreated (30 min) with either vehicle (DMSO) control or PP2 to confirm that the initial, rapid phase of cytosolic PR-B signaling to MAPK is c-Src dependent (6, 41) (Fig. 7C, compare lanes 2 and 4). Thirty minutes of pretreatment with PP2 again prevented the second phase of Erk1/2 activation observed at 18 h of exposure to R5020 (compare lanes 6 and 8). However, addition of the PP2 inhibitor 12 h after R5020 treatment also fully blocked delayed MAPK activation (lane 10), suggesting that c-Src tyrosine kinase acts perpetually to generate and maintain sustained Erk1/2 MAPK activation in response to progestins (Fig. 7C). Consistent with our results shown in Fig. 2B, cyclin D1 expression was also blocked following 12 h of posttreatment with PP2 (compare lanes 6 and 10), reinforcing the fact that sustained cytosolic (c-Src and MAPK) signaling is required for persistent high expression of cyclin D1.
These results implicate c-Src as a major signaling component during both the initial (10-min) and sustained (18-h) phases of progestin-induced Erk1/2 MAPK signaling. c-Src inhibitors did not affect the ability of progestin to activate EGFR at 18 h, which suggests that perpetual c-Src activation functions downstream of ligand-activated EGFR in order to amplify signaling inputs to MAPKs (Fig. 7D).
To confirm that long-term exposure of breast cancer cells to progestins influences anchorage-independent growth as a result of transactivation of EGFR and sustained activation of c-Src and Erk1/2 MAPKs, we again performed soft-agar assays. After 3 weeks of growth in soft agar, R5020 stimulated a fourfold increase in colony formation in the presence of DMSO (Fig. 7E). Inhibition of Erk1/2 MAPK signaling by inclusion of U0126, PP2, or PD168383 blocked the progestin-specific increase in colony numbers. Thus, progestin-stimulated tumor cell colony formation in soft agar is dependent on PR-mediated transactivation of EGFR, downstream activation of c-Src, and sustained Erk1/2 MAPK activity.
Wnt-1 is a transcriptional target of PR that links nuclear and sustained signaling activities.
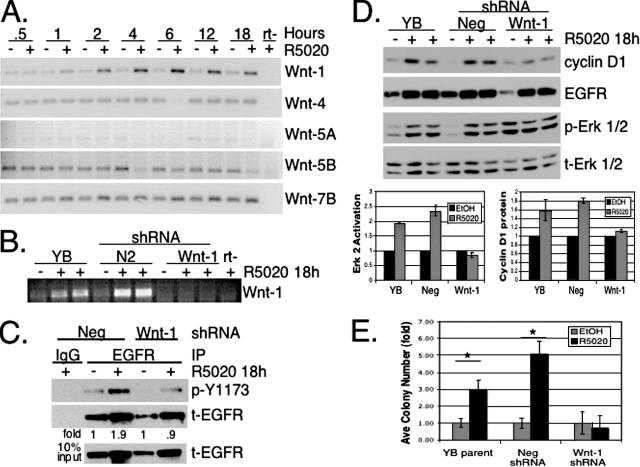
The Wnt family of secreted glycoproteins are important paracrine mediators of progesterone-directed mammary gland development (7). Recently, overexpression of Wnt-1 or Wnt-5A in immortalized mammary epithelial cells was shown to stimulate an MMP-dependent transactivation of EGFR, leading to increased MAPK activity and cyclin D1 expression (12). We hypothesized that Wnts may represent a direct link between PR transcriptional activity and sustained Erk1/2 MAPK signaling downstream of EGFR and c-Src. A time course of R5020 treatment of T47D-YB cells revealed specific upregulation of Wnt-1 mRNA, but not Wnt-4, -5A, -5B, -7B (Fig. 8A), or other Wnt family members important for mammary gland development, including Wnt-2, -3, and -7A (not shown). Wnt-1 upregulation was blocked by the PR antagonist RU486 (not shown).
FIG. 8.
Wnt-1 expression links PR transcriptional activity to EGFR transactivation and sustained MAPK signaling. (A) The time course of R5020-treated (+) T47D-YB cells demonstrates specific upregulation of Wnt-1 mRNA by RT-PCR. The results are representative of four independent experiments. (B) Duplicate cultures of untreated T47D-YB parental (YB) cells or cells stably expressing Neg shRNA control (N2) or Wnt-1 shRNA (Wnt-1) were treated for 18 h with R5020, and knockdown of Wnt-1 mRNA expression was measured by RT-PCR. (C) EGFR from T47D-YB cells stably expressing control shRNA (Neg) or Wnt-1 shRNA (Wnt-1) was immunoprecipitated using specific antibodies or control IgG and Western blotted using antibodies specific for phospho-Y1173 (p-Y1173) (activated) EGFR and total (t) EGFR. Lysates indicate 10% IP input. Normalization of phospho-Y1173 to total EGFR in immunoprecipitates, as measured by densitometry, indicated EGFR activity was increased 1.9-fold in R5020-treated control (Neg) cells, whereas cells expressing Wnt-1 shRNA exhibited no increase in EGFR activity (0.9-fold compared to the EtOH signal); densitometry of multiple exposures yielded similar results. The results were confirmed in two independent experiments. (D) Duplicate cultures of wt T47D-YB (YB) cells or cells stably expressing either control (Neg) or Wnt-1 shRNA (Wnt-1) were treated without (−) or with (+) R5020 for 18 h, and whole-cell lysates were Western blotted for cyclin D1, EGFR, and phospho- and total Erk1/2 MAPKs. Duplicate measurments of active Erk2 MAPK and cyclin D1 protein bands were quantified by densitometry and are represented as bars in the lower graphs (± standard deviations). (E) Triplicate cultures of T47D-YB (YB parent) cells or cells stably expressing either control shRNA (Neg) or Wnt-1 shRNA (Wnt-1) were plated in soft agar as described in Materials and Methods and treated without (EtOH) or with (black bars) R5020 for 18 h, and soft-agar colonies were counted after 3 weeks. The average colony numbers are presented as n-fold over the EtOH control for the T47D-YB parent, shRNA Neg, or Wnt-1 (± standard errors of the mean). The asterisks denote statistical significance (P < 0.01) determined by an unpaired Student's t test. The experiments were repeated twice with similar results.
Wnt-1 as a potential mediator of EGFR- and c-Src-induced sustained MAPK signaling in response to progestin was tested by stable knockdown of Wnt-1 using shRNA retroviral constructs, as described in Materials and Methods. RT-PCR demonstrated the level of Wnt-1 knockdown achieved in our clonal population compared to the parental T47D-YB or selected negative (Neg) control cells (Fig. 8B). R5020 treatment of Neg and Wnt-1 shRNA cell lines yielded an upregulation of total EGFR protein at the 18-h time point (Fig. 8C). As described above (Fig. 5), EGFR activation, measured by Tyr1173 phosphorylation, was increased 1.9-fold in the shRNA Neg control cells; Wnt-1 knockdown clearly blocked progestin-induced phosphorylation of EGFR at Tyr1173 relative to total EGFR levels (0.9-fold). Thus, in the absence of Wnt-1, EGFR activation, but not protein induction, was blocked in response to progestin.
Progestin-treated Wnt-1 knockdown cells were then tested for sustained MAPK activation. Lysates from duplicate cultures of R5020-treated T47D-YB parental, Neg shRNA, or Wnt-1 shRNA cells were Western blotted for activated Erk1/2 MAPK. Consistent with the requirement for EGFR activation upstream of sustained MAPK, both T47D-YB parental and Neg shRNA control cells activated Erk1/2 MAPK in response to progestin treatment (Fig. 8D). Despite high basal phosphorylation of Erk1/2 MAPK, Wnt-1 knockdown cells did not exhibit increased MAPK activation upon progestin treatment, as quantified in the lower left of Fig. 8D. Significantly, MAPK-dependent cyclin D1 upregulation by progestin treatment was also deficient in Wnt-1 knockdown cells (lower right of Fig. 8D). Western blotting for total MAPK demonstrated equivalent gel loading, and EGFR Western blots indicated efficient upregulation of these signaling molecules by ligand-activated PR-B.
Finally, we investigated whether PR-B-dependent Wnt-1 upregulation is essential for progestin-stimulated T47D cell growth in soft agar. T47D-YB parental cells, or Neg or Wnt-1 shRNA cells, were plated in soft agar in the presence of EtOH vehicle or R5020, and the colonies were counted after 3 weeks. The T47D-YB parental cells and Neg shRNA controls both exhibited a three- to fourfold increase in colony numbers with progestin (Fig. 8E). In contrast, Wnt-1 knockdown T47D cells failed to respond to progestin in this assay, confirming that Wnt-1 signaling, upstream of MMP, EGFR, and c-Src activities, mediates sustained MAPK activity in order to direct anchorage-independent colony formation, a long-term survival and proliferative response of breast cancer cells to progestin (Fig. 9).
FIG. 9.
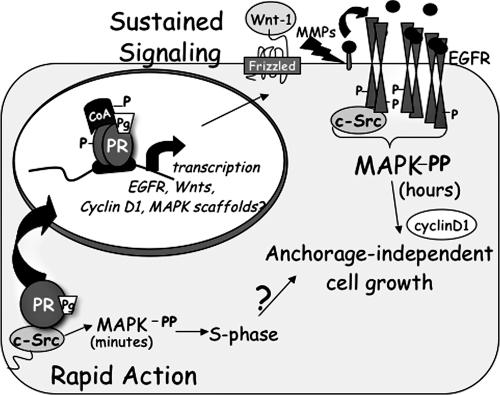
Model showing mechanism(s) of progestin/PR-mediated sustained MAPK signaling. Integration of PR rapid signaling events and PR-nuclear actions induce the transcriptional upregulation of EGFR, cyclin D1, and Wnt-1, followed by activation of MMP and transactivation of EGFR. Persistent c-Src signaling downstream of activated EGFR induces sustained Erk1/2 MAPK activity and serves as a direct input to cyclin D1 protein expression and anchorage-independent cell growth. We previously showed the MAPK dependence of progestin regulation of the proximal cyclin D1 promoter and a requirement for PR/c-Src interactions for S-phase entry of cultured T47D cells (62).
DISCUSSION
Here, we have demonstrated progestin/PR-B-dependent upregulation of growth-promoting target genes that establish a system of autocrine signaling leading to robust activation of Erk1/2 MAPKs (Fig. 9). Importantly, MAPK activation is sustained long after liganded PR are downregulated and occurs independently of rapid membrane-proximal PR signaling. Progestin-initiated sustained MAPK signaling pathways then direct anchorage-independent proliferation of breast cancer cells. Our results suggest that progestin/PR-induced classical transcriptional upregulation of the mammary gland morphogen Wnt-1 is a key event preceding stimulation of MMP-dependent EGFR transactivation (Fig. 8). Ligand-activated EGFR, together with c-Src, converges upon Erk1/2 MAPK to activate and amplify this signaling pathway as a continual input to increased cyclin D1 protein expression. These sustained signaling events (i.e., elevated Wnt-1 and increased EGFR, c-Src, and MAPK activities) are critical for anchorage-independent growth, an important readout of breast cancer cell survival and proliferation following long-term exposure to progestins, and suggest that PR actions are relevant to breast cancer progression.
PR and EGFR cross talk.
Signaling in response to both estrogen and progesterone, as well as the EGF/ErbB family of transmembrane tyrosine kinase receptors, is requisite for normal mammary gland development, and numerous studies have documented the importance of cross talk between these signaling pathways in both normal and cancerous breast cell growth (21, 34, 61). Gene targets of ER and/or PR action, including cyclin D1, c-myc, c-fos, p21, and cyclin E genes, are likewise targets of the induction of mitosis by EGFR-MAPK signaling (47). Recent reports of EGFR transactivation via autocrine feedback signaling by tumor necrosis factor and PAR receptor systems (9) prompted us to ask whether progestins coopt EGFR signaling to induce mitosis in T47D breast cancer cells. We have shown here that R5020 treatment (18 h) induced a specific increase in EGFR Tyr1173 phosphorylation, an important site of EGFR autophosphorylation (Fig. 6B). Our data (Fig. 6) strongly suggest that progestin treatment stimulates proteolytic processing of EGFR ligands to transactivate the receptor tyrosine kinase. We have not identified the specific metalloprotease activity here, but PR-B is known to increase protease expression, including ADAMTS and cathepsin L, during ovulation (53). Additionally, progestins have been shown to regulate EGF and transforming growth factor alpha mRNA, important autocrine ligands for EGFR (45, 46).
PR-induced sustained activation of MAPK is c-Src dependent.
Prolonged progestin treatment stimulated a persistent state of c-Src activation (Fig. 7B) that was required for sustained Erk1/2 MAPK activation and elevation of cyclin D1 protein levels (Fig. 7A and C). c-Src cooperates with EGFR signaling at multiple levels, including phosphorylation of EGFR Tyr845 (5). We were unable to detect EGFR Tyr845 phosphorylation in our system (not shown), and c-Src inhibitors did not affect progestin-stimulated Tyr1173 phosphorylation (Fig. 7D). c-Src-dependent activation of sustained MAPK, but not EGFR, in response to progestins places c-Src as an EGFR effector and downstream input to MAPKs. Activated c-Src may enhance EGFR signaling from endosomes by phosphorylating clathrin and dynamin (reviewed in reference 27) or promote EGFR recycling to the plasma membrane by phosphorylation and degradation of the EGFR E3 ligase Cbl (2). Interestingly, increased c-Src activity has been correlated with expression of PR, but not ER, in an early screen of 30 breast tumors (37).
Role of PR autocrine signaling in breast cancer cell biology.
Progestin treatment of breast cancer cells cultured on plastic dishes elicits a biphasic proliferative response. Initially, the cells are accelerated through one or more rounds of the cell cycle (24, 43), followed by a period of growth inhibition, during which cells accumulate in the G1 phase of the cell cycle and are resistant to further stimulation by progestins but can respond to other mitogenic stimuli (21, 48). Recently, we showed that PR-B interaction with c-Src and activation of downstream Erk1/2 MAPKs is required for S-phase entry during the proliferative response of breast cancer cells to progestins (62). Furthermore, Boonyaratanakornkit et al. (6) demonstrated a partial requirement for PR/c-Src interactions during the subsequent growth-inhibitory period observed in cells cultured on plastic (24 to 48 h). Thus, the PR functions as membrane-proximal and cytosolic signaling molecules clearly impact progestin action with regard to the regulation of cell proliferation. Interestingly, in long-term assays, continuous progestin exposure over the course of several months promoted breast cancer cell survival and resistance to chemotherapeutic agents through upregulation of the antiapoptotic molecule bcl-xl (43). Similarly, progestins clearly confer a survival and proliferative advantage on breast cancer cells expressing PR-B, as measured by anchorage-independent growth in soft agar (Fig. 5D). The effects of progestins mapped to PR-B-dependent upregulation of Wnt-1 signaling, followed by MMP-induced transactivation of EGFR and downstream signaling to c-Src, leading to sustained MAPK activity (Fig. 9). Thus, our results support reports by Moore et al. of continued proliferation and enhanced survival of T47D cells in the prolonged presence of progestins (43), a scenario that more closely resembles the hormonal environment in vivo. Interestingly, the ability of PR-mPro to activate sustained MAPK, but to support intermediate or attenuated anchorage-independent growth relative to wt PR-B, suggests that PR-induced rapid signaling events contribute to but are perhaps less important for the long-term effects of progestins on cells growing in soft agar. This result suggests that at least some degree of integration of rapid cytosolic and nuclear PR actions exists.
PR isoform-specific differences.
PR-A and -B are functionally distinct isoforms; on PRE-driven promoters, PR-B is a more potent activator of transcription than PR-A, while PR-A is a transrepressor of PR-B and ERα transcriptional activities (32, 64). However, gene array studies have revealed minimal overlap in target gene regulation between PR-A- and PR-B-expressing cell lines, regardless of the presence of progestin (28, 52). Here, we have demonstrated PR isoform-dependent responses of breast cancer cells to progestins. PR-B, but not PR-A, induced sustained Erk1/2 MAPK signaling (Fig. 1C) and increased soft-agar colony growth of isoform-specific T47D-YB, but not T47D-YA, cells (Fig. 5D, inset). Notably, in vivo, PR-B-containing tumors are significantly larger and more likely to become tamoxifen resistant than PR-A rich tumors, suggesting a link to PR-B-dependent sustained MAPK activation described here. Selective activation of MAPKs by PR-B may also explain alterations in PR isoform expression. Interestingly, human breast tumors more often overexpress PR-A than PR-B (20). Related to this finding, we observed greatly increased PR-B turnover relative to PR-A (Fig. 1C). Increased PR-B transcriptional activity signifies rapid receptor turnover (61), and these events are greatly augmented in the presence of EGF (15). It is tempting to speculate that in human breast tumors, PR-B-specific transactivation of EGFR and sustained MAPK signaling induce increased PR-B transcriptional activity coupled to its rapid turnover, thus providing the basis for the increased PR-A/PR-B ratios observed clinically (20).
Switch from paracrine to autocrine proliferation in ER/PR-positive breast tumor cells.
PR-regulated paracrine factors include Wnt family members. Wnts are a family of secreted glycoprotein morphogens, five of which are differentially expressed and regulated during pregnancy and lactation (Wnt-4, -5A, -5B, -6, and -7B) (18). Wnt-4 acts downstream of progesterone/PR to induce ductal side branching during normal mammary gland development, and Wnt-4 transgenic mice can rescue the mammary gland phenotype of PR knockout mice (7). Interestingly, constitutive expression of Wnt-1 or Wnt-5A in an immortalized but nontumorigenic mammary epithelial cell line induced transactivation of EGFR (12) by a mechanism strikingly similar to that utilized by progestin to stimulate anchorage-independent growth of breast cancer cells, detailed here. Specifically, Wnt-1 or -5A binding to Frizzled, a seven-transmembrane pass receptor, stimulated MMP-dependent cleavage of EGFR ligands, leading to Erk1/2 MAPK activation and regulation of cyclin D1 expression (12). In our system, MMP activity did not require Wnt-1 binding to LRP coreceptors, since inclusion of the soluble LRP inhibitor Dkk-1 did not block sustained MAPK signaling (not shown). Interestingly, recent data suggest that a minority population of Wnt-producing ER/PR-positive luminal epithelial cells in the normal mammary gland may act as feeder cells that provide a strong proliferative signal to neighboring or local breast progenitor stem cells (13). As these stem cells are thought to be sensitive to transformation, and may ultimately give rise to aggressive and recurrent forms of breast cancer, it is important to understand if and how progestins alter the stem cell population via Wnt production in vivo.
In contrast to the normal adult breast, where a subpopulation (7 to 10%) of ER/PR- and prolactin receptor-positive epithelial cells control the limited proliferation of adjacent ER/PR-negative cells (60), the majority of breast tumors (∼80%) are ER/PR positive and clearly proliferate (19). Progestins are not carcinogens but can act as potent breast mitogens in in vivo models (25). Furthermore, PR-B colocalization with cyclin D1 in BrdU-positive cycling murine mammary epithelial cells during pregnancy (1) indicates that ER/PR-positive cells can “switch” to a proliferative program as part of normal developmental processes. Similar events may be recapitulated during cancer transformation. In support of this idea, numerous clinical studies support a proliferative role for progestins when combined with estrogen as part of hormone replacement therapy; tumors were larger and of higher grade (3, 11, 55, 66). How ER/PR-positive breast epithelial cells gain proliferative license during neoplastic conversion is an area of active investigation. Perhaps PR coopt normally operative autocrine signaling pathways at relatively early stages during tumor promotion or progression, thus enabling the proliferation and outgrowth of formerly dormant ER/PR-positive lesions. The recurrence of endocrine-resistant tumors is a critical issue in the treatment of steroid receptor-positive tumors. Our studies suggest that breast cancer patients may benefit from combination treatment options that, in addition to inhibitors of ER activity or estrogen production, may include inhibitors of Wnt signaling, EGFR, c-Src, ERK1/2 MAPKs, and/or PR aimed at blocking autocrine mechanisms of cell proliferation.
Acknowledgments
We thank Kate Horwitz (University of Colorado Health Sciences Center, Aurora, CO) for the kind gift of the PR-null T47D cell line and T47D variants stably expressing either PR-B or PR-A (57). We acknowledge the assistance of the Flow Cytometry Core Facility of the University of Minnesota Cancer Center, a comprehensive cancer center designated by the National Cancer Institute, supported in part by P30 CA77598.
This work was supported by NIH grant R01 CA123763 (formerly DK053825) to C.A.L. and Department of Defense Predoctoral Fellowship Grant number DAMD17-03-1-0390 to E.J.F.
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Aupperlee, M. D., K. T. Smith, A. Kariagina, and S. Z. Haslam. 2005. Progesterone receptor isoforms A and B: temporal and spatial differences in expression during murine mammary gland development. Endocrinology 146:3577-3588. [DOI] [PubMed] [Google Scholar]
- 2.Bao, J., G. Gur, and Y. Yarden. 2003. Src promotes destruction of c-Cbl: implications for oncogenic synergy between Src and growth factor receptors. Proc. Natl. Acad. Sci. USA 100:2438-2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beral, V. 2003. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet 362:419-427. [DOI] [PubMed] [Google Scholar]
- 4.Bertics, P. J., W. S. Chen, L. Hubler, C. S. Lazar, M. G. Rosenfeld, and G. N. Gill. 1988. Alteration of epidermal growth factor receptor activity by mutation of its primary carboxyl-terminal site of tyrosine self-phosphorylation. J. Biol. Chem. 263:3610-3617. [PubMed] [Google Scholar]
- 5.Biscardi, J. S., M. C. Maa, D. A. Tice, M. E. Cox, T. H. Leu, and S. J. Parsons. 1999. c-Src-mediated phosphorylation of the epidermal growth factor receptor on Tyr845 and Tyr1101 is associated with modulation of receptor function. J. Biol. Chem. 274:8335-8343. [DOI] [PubMed] [Google Scholar]
- 6.Boonyaratanakornkit, V., M. P. Scott, V. Ribon, L. Sherman, S. M. Anderson, J. L. Maller, W. T. Miller, and D. P. Edwards. 2001. Progesterone receptor contains a proline-rich motif that directly interacts with SH3 domains and activates c-Src family tyrosine kinases. Mol. Cell 8:269-280. [DOI] [PubMed] [Google Scholar]
- 7.Brisken, C., A. Heineman, T. Chavarria, B. Elenbaas, J. Tan, S. K. Dey, J. A. McMahon, A. P. McMahon, and R. A. Weinberg. 2000. Essential function of Wnt-4 in mammary gland development downstream of progesterone signaling. Genes Dev. 14:650-654. [PMC free article] [PubMed] [Google Scholar]
- 8.Carroll, J. S., and M. Brown. 2006. Estrogen receptor target gene: an evolving concept. Mol. Endocrinol. 20:1707-1714. [DOI] [PubMed] [Google Scholar]
- 9.Chen, W. N., R. L. Woodbury, L. E. Kathmann, L. K. Opresko, R. C. Zangar, H. S. Wiley, and B. D. Thrall. 2004. Induced autocrine signaling through the epidermal growth factor receptor contributes to the response of mammary epithelial cells to tumor necrosis factor α. J. Biol. Chem. 279:18488-18496. [DOI] [PubMed] [Google Scholar]
- 10.Cheng, M., V. Sexl, C. J. Sherr, and M. F. Roussel. 1998. Assembly of cyclin D-dependent kinase and titration of p27Kip1 regulated by mitogen-activated protein kinase kinase (MEK1). Proc. Natl. Acad. Sci. USA 95:1091-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chlebowski, R. T., S. L. Hendrix, R. D. Langer, M. L. Stefanick, M. Gass, D. Lane, R. J. Rodabough, M. Gilligan, M. G. Cyr, C. A. Thomson, J. Khandekar, H. Petrovitch, and A. McTiernan. 2003. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women. JAMA 289:3243-3253. [DOI] [PubMed] [Google Scholar]
- 12.Civenni, G., T. Holbro, and N. E. Hynes. 2003. Wnt1 and Wnt5a induce cyclin D1 expression through ErbB1 transactivation in HC11 mammary epithelial cells. EMBO Rep. 4:166-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clarke, R. B. 2005. Isolation and characterization of human mammary stem cells. Cell Prolif. 38:375-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Condon, J. C., D. B. Hardy, K. Kovaric, and C. R. Mendelson. 2006. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-κB may contribute to the onset of labor through inhibition of PR function. Mol. Endocrinol. 20:764-775. [DOI] [PubMed] [Google Scholar]
- 15.Daniel, A. R., M. Qiu, E. J. Faivre, J. L. Hanson Ostrander, A. Skildum, and C. A. Lange. Steroids, in press. [DOI] [PMC free article] [PubMed]
- 16.Evans, R. M. 1988. The steroid and thyroid hormone receptor superfamily. Science 240:889-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Filardo, E. J., J. A. Quinn, K. I. Bland, and A. R. Frackelton, Jr. 2000. Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol. Endocrinol 14:1649-1660. [DOI] [PubMed] [Google Scholar]
- 18.Gavin, B. J., and A. P. McMahon. 1992. Differential regulation of the Wnt gene family during pregnancy and lactation suggests a role in postnatal development of the mammary gland. Mol. Cell. Biol. 12:2418-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham, J. D., and C. L. Clarke. 1997. Physiological action of progesterone in target tissues. Endocr. Rev. 18:502-519. [DOI] [PubMed] [Google Scholar]
- 20.Graham, J. D., C. Yeates, R. L. Balleine, S. S. Harvey, J. S. Milliken, A. M. Bilous, and C. L. Clarke. 1995. Characterization of progesterone receptor A and B expression in human breast cancer. Cancer Res. 55:5063-5068. [PubMed] [Google Scholar]
- 21.Groshong, S. D., G. I. Owen, B. Grimison, I. E. Schauer, M. C. Todd, T. A. Langan, R. A. Sclafani, C. A. Lange, and K. B. Horwitz. 1997. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol. Endocrinol. 11:1593-1607. [DOI] [PubMed] [Google Scholar]
- 22.Guo, Y., D. W. Stacey, and M. Hitomi. 2002. Post-transcriptional regulation of cyclin D1 expression during G2 phase. Oncogene 21:7545-7556. [DOI] [PubMed] [Google Scholar]
- 23.Hanson, J. L., N. A. Hawke, D. Kashatus, and A. S. Baldwin. 2004. The nuclear factor κB subunits RelA/p65 and c-Rel potentiate but are not required for Ras-induced cellular transformation. Cancer Res. 64:7248-7255. [DOI] [PubMed] [Google Scholar]
- 24.Hissom, J. R., and M. R. Moore. 1987. Progestin effects on growth in the human breast cancer cell line T-47D—possible therapeutic implications. Biochem. Biophys. Res. Commun. 145:706-711. [DOI] [PubMed] [Google Scholar]
- 25.Horwitz, K. B. 1992. The molecular biology of RU486. Is there a role for antiprogestins in the treatment of breast cancer? Endocr. Rev. 13:146-163. [DOI] [PubMed] [Google Scholar]
- 26.Horwitz, K. B., M. B. Mockus, and B. A. Lessey. 1982. Variant T47D human breast cancer cells with high progesterone-receptor levels despite estrogen and antiestrogen resistance. Cell 28:633-642. [DOI] [PubMed] [Google Scholar]
- 27.Ishizawar, R., and S. J. Parsons. 2004. c-Src and cooperating partners in human cancer. Cancer Cell 6:209-214. [DOI] [PubMed] [Google Scholar]
- 28.Jacobsen, B. M., J. K. Richer, C. A. Sartorius, and K. B. Horwitz. 2003. Expression profiling of human breast cancers and gene regulation by progesterone receptors. J. Mammary Gland Biol. Neoplasia 8:257-268. [DOI] [PubMed] [Google Scholar]
- 29.Kanki, J. P., and D. J. Donoghue. 1991. Progression from meiosis I to meiosis II in Xenopus oocytes requires de novo translation of the mosxe protooncogene. Proc. Natl. Acad. Sci. USA 88:5794-5798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kastner, P., A. Krust, B. Turcotte, U. Stropp, L. Tora, H. Gronemeyer, and P. Chambon. 1990. Two distinct estrogen-regulated promoters generate transcripts encoding the two functionally different human progesterone receptor forms A and B. EMBO J. 9:1603-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamoto, T., J. D. Sato, A. Le, J. Polikoff, G. H. Sato, and J. Mendelsohn. 1983. Growth stimulation of A431 cells by epidermal growth factor: identification of high-affinity receptors for epidermal growth factor by an anti-receptor monoclonal antibody. Proc. Natl. Acad. Sci. USA 80:1337-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus, W. L., K. E. Weis, and B. S. Katzenellenbogen. 1995. Inhibitory cross-talk between steroid hormone receptors: differential targeting of estrogen receptor in the repression of its transcriptional activity by agonist- and antagonist-occupied progestin receptors. Mol. Cell. Biol. 15:1847-1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kull, F. C., Jr., S. Jacobs, Y. F. Su, M. E. Svoboda, J. J. Van Wyk, and P. Cuatrecasas. 1983. Monoclonal antibodies to receptors for insulin and somatomedin-C. J. Biol. Chem. 258:6561-6566. [PubMed] [Google Scholar]
- 34.Lange, C. A., J. K. Richer, T. Shen, and K. B. Horwitz. 1998. Convergence of progesterone and epidermal growth factor signaling in breast cancer. Potentiation of mitogen-activated protein kinase pathways. J. Biol. Chem. 273:31308-31316. [DOI] [PubMed] [Google Scholar]
- 35.Lange, C. A., T. Shen, and K. B. Horwitz. 2000. Phosphorylation of human progesterone receptors at serine-294 by mitogen-activated protein kinase signals their degradation by the 26S proteasome. Proc. Natl. Acad. Sci. USA 97:1032-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lange-Carter, C. A., C. M. Pleiman, A. M. Gardner, K. J. Blumer, and G. L. Johnson. 1993. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science 260:315-319. [DOI] [PubMed] [Google Scholar]
- 37.Lehrer, S., J. O'Shaughnessy, H. K. Song, E. Levine, P. Savoretti, J. Dalton, R. Lipsztein, S. Kalnicki, and W. D. Bloomer. 1989. Activity of pp60c-src protein kinase in human breast cancer. Mt. Sinai J. Med. 56:83-85. [PubMed] [Google Scholar]
- 38.Li, X., D. M. Lonard, and B. W. O'Malley. 2004. A contemporary understanding of progesterone receptor function. Mech. Ageing Dev. 125:669-678. [DOI] [PubMed] [Google Scholar]
- 39.Lydon, J. P., F. J. DeMayo, C. R. Funk, S. K. Mani, A. R. Hughes, C. A. Montgomery, G. Shyamala, O. M. Conneely, and B. W. O'Malley. 1995. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 9:2266-2278. [DOI] [PubMed] [Google Scholar]
- 40.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 41.Migliaccio, A., D. Piccolo, G. Castoria, M. Di Domenico, A. Bilancio, M. Lombardi, W. Gong, M. Beato, and F. Auricchio. 1998. Activation of the Src/p21ras/Erk pathway by progesterone receptor via cross-talk with estrogen receptor. EMBO J. 17:2008-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Miller, W. R., T. J. Anderson, S. White, A. Larionov, J. Murray, D. Evans, A. Krause, and J. M. Dixon. 2005. Aromatase inhibitors: cellular and molecular effects. J. Steroid Biochem. Mol. Biol. 95:83-89. [DOI] [PubMed] [Google Scholar]
- 43.Moore, M. R., J. L. Conover, and K. M. Franks. 2000. Progestin effects on long-term growth, death, and Bcl-xL in breast cancer cells. Biochem. Biophys. Res. Commun. 277:650-654. [DOI] [PubMed] [Google Scholar]
- 44.Mulac-Jericevic, B., J. P. Lydon, F. J. DeMayo, and O. M. Conneely. 2003. Defective mammary gland morphogenesis in mice lacking the progesterone receptor B isoform. Proc. Natl. Acad. Sci. USA 100:9744-9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Murphy, L. C., and H. Dotzlaw. 1989. Regulation of transforming growth factor alpha and transforming growth factor beta messenger ribonucleic acid abundance in T-47D, human breast cancer cells. Mol. Endocrinol. 3:611-617. [DOI] [PubMed] [Google Scholar]
- 46.Murphy, L. C., L. J. Murphy, D. Dubik, G. I. Bell, and R. P. Shiu. 1988. Epidermal growth factor gene expression in human breast cancer cells: regulation of expression by progestins. Cancer Res. 48:4555-4560. [PubMed] [Google Scholar]
- 47.Musgrove, E. A., J. A. Hamilton, C. S. Lee, K. J. Sweeney, C. K. Watts, and R. L. Sutherland. 1993. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol. Cell. Biol. 13:3577-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Musgrove, E. A., C. S. Lee, and R. L. Sutherland. 1991. Progestins both stimulate and inhibit breast cancer cell cycle progression while increasing expression of transforming growth factor alpha, epidermal growth factor receptor, c-fos, and c-myc genes. Mol. Cell. Biol. 11:5032-5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Olsson, H., H. Jernstrom, P. Alm, H. Kreipe, C. Ingvar, P. E. Jonsson, and S. Ryden. 1996. Proliferation of the breast epithelium in relation to menstrual cycle phase, hormonal use, and reproductive factors. Breast Cancer Res. Treat. 40:187-196. [DOI] [PubMed] [Google Scholar]
- 50.Pierson-Mullany, L. K., and C. A. Lange. 2004. Phosphorylation of progesterone receptor serine 400 mediates ligand-independent transcriptional activity in response to activatoin of cyclin-dependent protein kinase 2. Mol. Cell. Biol. 24:10542-10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Potten, C. S., R. J. Watson, G. T. Williams, S. Tickle, S. A. Roberts, M. Harris, and A. Howell. 1988. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br. J. Cancer 58:163-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Richer, J. K., B. M. Jacobsen, N. G. Manning, M. G. Abel, D. M. Wolf, and K. B. Horwitz. 2002. Differential gene regulation by the two progesterone receptor isoforms in human breast cancer cells. J. Biol. Chem. 277:5209-5218. [DOI] [PubMed] [Google Scholar]
- 53.Robker, R. L., D. L. Russell, L. L. Espey, J. P. Lydon, B. W. O'Malley, and J. S. Richards. 2000. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc. Natl. Acad. Sci. USA 97:4689-4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rosenwald, I. B., R. Kaspar, D. Rousseau, L. Gehrke, P. Leboulch, J. J. Chen, E. V. Schmidt, N. Sonenberg, and I. M. London. 1995. Eukaryotic translation initiation factor 4E regulates expression of cyclin D1 at transcriptional and post-transcriptional levels. J. Biol. Chem. 270:21176-21180. [DOI] [PubMed] [Google Scholar]
- 55.Rossouw, J. E., G. L. Anderson, R. L. Prentice, A. Z. LaCroix, C. Kooperberg, M. L. Stefanick, R. D. Jackson, S. A. Beresford, B. V. Howard, K. C. Johnson, J. M. Kotchen, and J. Ockene. 2002. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women's Health Initiative randomized controlled trial. JAMA 288:321-333. [DOI] [PubMed] [Google Scholar]
- 56.Sachdev, D., S. L. Li, J. S. Hartell, Y. Fujita-Yamaguchi, J. S. Miller, and D. Yee. 2003. A chimeric humanized single-chain antibody against the type I insulin-like growth factor (IGF) receptor renders breast cancer cells refractory to the mitogenic effects of IGF-I. Cancer Res. 63:627-635. [PubMed] [Google Scholar]
- 57.Sartorius, C. A., S. D. Groshong, A. Miller, R. L. Powell, L. Tung, G. Takimoto, and K. Horwitz. 1994. New T47D breast cancer cell lines for the independent study of progesterone B- and A-receptors; only antiprogestin-occupied B-receptors are switched to transcriptional agonists by cAMP. Cancer Res. 54:3868-3877. [PubMed] [Google Scholar]
- 58.Sato, J. D., T. Kawamoto, A. D. Le, J. Mendelsohn, J. Polikoff, and G. H. Sato. 1983. Biological effects in vitro of monoclonal antibodies to human epidermal growth factor receptors. Mol. Biol. Med. 1:511-529. [PubMed] [Google Scholar]
- 59.Schlondorff, J., and C. P. Blobel. 1999. Metalloprotease-disintegrins: modular proteins capable of promoting cell-cell interactions and triggering signals by protein-ectodomain shedding. J. Cell Sci. 112:3603-3617. [DOI] [PubMed] [Google Scholar]
- 60.Seagroves, T. N., J. P. Lydon, R. C. Hovey, B. K. Vonderhaar, and J. M. Rosen. 2000. C/EBPβ (CCAAT/enhancer binding protein) controls cell fate determination during mammary gland development. Mol. Endocrinol. 14:359-368. [DOI] [PubMed] [Google Scholar]
- 61.Shen, T., K. B. Horwitz, and C. A. Lange. 2001. Transcriptional hyperactivity of human progesterone receptors is coupled to their ligand-dependent down-regulation by mitogen-activated protein kinase-dependent phosphorylation of serine 294. Mol. Cell. Biol. 21:6122-6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skildum, A., E. Faivre, and C. A. Lange. 2005. Progesterone receptors induce cell cycle progression via activation of mitogen activated protein kinases. Mol. Endocrinol. 19:327-339. [DOI] [PubMed] [Google Scholar]
- 63.Takimoto, G. S., D. M. Tasset, A. C. Eppert, and K. B. Horwitz. 1992. Hormone-induced progesterone receptor phosphorylation consists of sequential DNA-independent and DNA-dependent stages: analysis with zinc finger mutants and the progesterone antagonist ZK98299. Proc. Natl. Acad. Sci. USA 89:3050-3054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tung, L., M. K. Mohamed, J. P. Hoeffler, G. S. Takimoto, and K. B. Horwitz. 1993. Antagonist-occupied human progesterone B-receptors activate transcription without binding to progesterone response elements and are dominantly inhibited by A-receptors. Mol. Endocrinol. 7:1256-1265. [DOI] [PubMed] [Google Scholar]
- 65.Wang, X., Y. Xiao, Y. Mou, Y. Zhao, W. M. Blankesteijn, and J. L. Hall. 2002. A role for the beta-catenin/T-cell factor signaling cascade in vascular remodeling. Circ. Res. 90:340-347. [DOI] [PubMed] [Google Scholar]
- 66.Warren, M. P. 2004. A comparative review of the risks and benefits of hormone replacement therapy regimens. Am. J. Obstet. Gynecol. 190:1141-1167. [DOI] [PubMed] [Google Scholar]
- 67.Xu, K., Y. Ding, J. Ling, Z. Dong, and F. X. Yu. 2004. Wound-induced HB-EGF ectodomain shedding and EGFR activation in corneal epithelial cells. Investig. Ophalmol. Vis. Sci. 45:813-820. [DOI] [PMC free article] [PubMed] [Google Scholar]