Abstract
Drosophila BAP and PBAP represent two evolutionarily conserved subclasses of SWI/SNF chromatin remodelers. The two complexes share the same core subunits, including the BRM ATPase, but differ in a few signature subunits: OSA defines BAP, whereas Polybromo (PB) and BAP170 specify PBAP. Here, we present a comprehensive structure-function analysis of BAP and PBAP. An RNA interference knockdown survey revealed that the core subunits BRM and MOR are critical for the structural integrity of both complexes. Whole-genome expression profiling suggested that the SWI/SNF core complex is largely dysfunctional in cells. Regulation of the majority of target genes required the signature subunit OSA, PB, or BAP170, suggesting that SWI/SNF remodelers function mostly as holoenzymes. BAP and PBAP execute similar, independent, or antagonistic functions in transcription control and appear to direct mostly distinct biological processes. BAP, but not PBAP, is required for cell cycle progression through mitosis. Because in yeast the PBAP-homologous complex, RSC, controls cell cycle progression, our finding reveals a functional switch during evolution. BAP mediates G2/M transition through direct regulation of string/cdc25. Its signature subunit, OSA, is required for directing BAP to the string/cdc25 promoter. Our results suggest that the core subunits play architectural and enzymatic roles but that the signature subunits determine most of the functional specificity of SWI/SNF holoenzymes in general gene control.
ATP-dependent chromatin-remodeling factors (remodelers) are critical for the transmission and expression of the eukaryotic genome (8). They mediate the restructuring of chromatin, which forms an integral part of the mechanism of eukaryotic transcription control. Typically, remodelers are large multisubunit complexes defined by the presence of an ATPase “engine” subunit. Four major families, referred to as SWI/SNF, ISWI, Mi-2, and Ino80 remodelers, have been recognized based on the identities of their central ATPases (5, 24, 33, 37, 38, 47, 51). Each class executes unique biological functions by remodeling chromatin templates during DNA replication, repair, and transcription. An early example of functional diversification was the finding that the Drosophila SWI/SNF-class Brahma (BRM) remodelers, but not the ISWI remodelers, act as chromatin-specific coactivators for the transcription factor Zeste (27).
The SWI/SNF group of remodelers can be subdivided further into two distinct, evolutionarily conserved subclasses. One subfamily comprises yeast SWI/SNF (ySWI/SNF), fly BAP, and mammalian BAF, whereas the second subfamily includes yeast RSC, fly PBAP, and mammalian PBAF (35, 55). These two classes of SWI/SNF complexes are composed of highly related paralogs or identical subunits and a few subclass-specific subunits. For example, Drosophila BAP and PBAP share seven core subunits, but each is defined by unique signature subunits: the BAP-specific OSA and the PBAP-specific Polybromo (PB) and BAP170 (12, 27, 34, 42).
Work on budding yeast has established that, despite structural similarities between ySWI/SNF and RSC, each complex performs distinct cellular tasks (35, 55). ySWI/SNF is nonessential, is present in relatively small amounts, and is required for the expression of only a small portion of the yeast genome (9, 22, 49). In contrast, RSC is abundant and is essential for cell cycle progression through G2/M and for viability (1, 9, 10). RSC and ySWI/SNF also play distinct roles during DNA double-strand break repair (11). Chromatin immunolocalization studies revealed that RSC is generally recruited to RNA polymerase III promoters and to specific polymerase II promoters by transcriptional activators and repressors (14, 39). However, it is not yet clear whether the functional differences between yeast SWI/SNF subclasses can be translated directly to higher eukaryotes.
In flies and mammals, the functional distinction between the two subclasses of SWI/SNF remodelers is not well defined (35, 55). For example, in flies, both BAP and PBAP are essential for viability and appear to be more or less equally abundant. The central ATPase of both BAP and PBAP, BRM, facilitates the expression of a large portion of the fly genome (2). However, it is unclear whether this is due to a requirement for BAP or PBAP. Genomewide localization of OSA and PB on polytene chromosomes revealed that BAP and PBAP are recruited differentially, suggesting that they control distinct sets of target genes (34). The two highly related human SWI/SNF ATPases BRG1 and BRM determine functional specificity in transcription (25). The mammalian BAF- or PBAF-selective subunits have been implicated in transcription activation by specific nuclear hormone receptors (31, 40, 50) and for the expression of selective interferon-responsive genes (57). OSA, the fly BAP selective subunit, is required for repression of Wingless target genes (13). These findings suggest that the BAP/BAF and PBAP/PBAF complexes perform distinct functions in gene expression control. However, a comprehensive side-by-side analysis of gene control by both types of remodelers is lacking.
Typically, the core ATPase of the multisubunit remodelers suffices for chromatin remodeling in vitro (4, 19, 33). Therefore, we were intrigued by the roles of the remaining subunits in global transcription regulation in cells. To determine the contributions of shared and unique BAP and PBAP subunits to gene expression control, we performed a comprehensive RNA interference (RNAi) knockdown survey in Drosophila S2 cells. Epistasis analysis combined with whole-genome expression profiling suggested that SWI/SNF remodelers act mostly as holoenzymes. In cells, the core complex, without the BAP and PBAP signature subunits, appears to be dysfunctional for general transcription control. Depending on the target genes, BAP and PBAP may act coordinately or independently or antagonize each other. We dissected the pathway through which BAP, but not PBAP, controls cell cycle progression. Our results outline the functional roles of SWI/SNF complexes at the genomic level. They also demonstrate the power of integrating RNAi knockdown studies with statistical analysis of expression profiles for the structure-function analysis of multisubunit transcription-regulatory complexes.
MATERIALS AND METHODS
Cell culture, RNAi, antibodies, and immunodetection.
Drosophila S2 cells were cultured and treated with double-stranded RNA (dsRNA) for 4 days as described previously (56). Double-stranded RNA was synthesized using an Ambion Megascript T7 kit according to the manufacturer's protocol. All immunological procedures were performed by standard methods (34) and were repeated several times at different dilutions of the samples (data not shown). Antibodies against BAP and PBAP subunits and ISWI used for immunobloting have been described previously (27, 34, 58). Furthermore, we used rabbit anti-phospho-histone H3 antibodies (Upstate).
RNA extraction, RT-qPCR, and Affymetrix microarray hybridization.
RNA was extracted using the SV Total RNA Isolation System (Promega) and tested on an Agilent BioAnalyzer (Agilent). Samples with RNA integrity numbers of >8 were selected. Labeling, hybridization, washes, and staining of microarrays were performed according to Affymetrix specifications. Reverse transcription-quantitative PCR (RT-qPCR) was done with SYBR green I using a MyiQ single-color real-time PCR detection system and iQ SYBR green supermix (Bio-Rad) according to the manufacturer's protocols. First-strand cDNA was prepared with an iScript cDNA synthesis kit (Bio-Rad). Primers for RT-qPCR were selected using Beacon Designer software (Premier Biosoft). The RT-qPCR primers were as follows: CG11874, 5′-AGTGTTGCTCTGCCTAAGTGG-3′ and 5′-CGGATGATGGTGCGGATTGG-3′; string (stg), 5′-CGTTATCTAAGTTTGGGTGTTATCG-3′ and 5′-TGTGTCTGCGTCGTGTGC-3′.
Statistical analysis.
Statistical analysis of the microarray data was performed using the R and Bioconductor free software (http://www.bioconductor.org). Gene expression indexes were calculated using the robust multichip average (RMA) algorithm implemented in the Bioconductor affy package (23). The multiple covariance determinant algorithm is implemented in the rrcov R package and was used for filtering the genes showing low expression indexes across all experiments (46). Calculation of bootstrap P values for hierarchical clustering was performed using the pvclust R package. Heat maps were plotted using the glplots R package. PCA was performed on scaled and centered data using the R “prcomp” function. Gene ontology (GO) analysis was done with the Bioconductor GOstats library. Distances between GO terms were determined using the “simUI” (union intersection) function. Further details of the statistical analysis and R scripts will be provided upon request.
Flow cytometric analysis.
Cells were collected and fixed with 70% ethanol as described previously (7). After a minimum of 2 h on ice, the cells were washed with phosphate-buffered saline and resuspended in 400 μl phosphate-buffered saline containing 0.1% Triton X-100, 0.1 mg/ml propidium iodide, and 0.1 mg/ml RNase. The cells were incubated overnight and analyzed on a FACScan (Becton Dickinson).
ChIPs and polytene chromosome immunolocalization.
Chromatin immunoprecipitation (ChIP) assays were performed according to published procedures (53). Cross-linked chromatin was prepared from S2 cells either treated with dsRNA or untreated and sheared by sonication to an average length of 0.7 kb. After immunoprecipitation, the recovered DNA was analyzed by qPCR with SYBR green I using the MyiQ single-color real-time PCR detection system (Bio-Rad). The following primers were used in qPCRs to amplify the stg/cdc25 promoter: 5′-CAGTGGCCTCCATAGAGCTG-3′ and 5′-GTCCCGAGAAACGAGGAGA-3′. We also performed ChIP using anti-MOR antibodies on promoters of nontarget genes, such as mRpS17, mRpL16, and mRpL52, to estimate background levels (data not shown). Immunolocalization of OSA and PB on polytene chromosomes was performed as described previously (34). Chromosomes were stained with DAPI (4′,6′-diamidino-2-phenylindole), and the 99A cytological region was mapped using the photographic maps of G. Leffevre, Jr. (32).
RESULTS
Architectural functions of BAP and PBAP subunits.
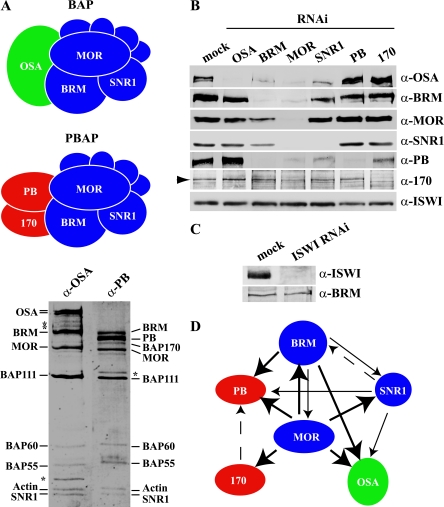
To determine the functions of individual BAP and PBAP subunits, we utilized RNAi-mediated gene knockdown by treating Drosophila S2 cells with dsRNA (56). We targeted three shared core subunits, BRM, MOR, and SNR1; the BAP-specific OSA; and the PBAP-specific subunits PB and BAP170 (Fig. 1A). As a control, we also knocked down ISWI, the central ATPase of the ISWI remodeler family. Western immunoblotting confirmed the effective reduction in protein levels of the targeted proteins (Fig. 1B and C).
FIG. 1.
The common core subunits are essential for the integrity of BAP and PBAP. (A) Drosophila contains two distinct SWI/SNF remodelers: BAP and PBAP. The two complexes share seven subunits (blue), which include the central ATPase BRM, MOR, and SNR1. The signature subunits are OSA in the BAP complex and PB and BAP170 in the PBAP complex. BAP and PBAP were immunopurified from Drosophila embryo nuclear extracts, using antibodies directed against either OSA or PB. Both complexes were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and stained with silver (bottom). All subunits are indicated; the asterisks indicate degradation products, as determined by immunoblotting. (B) S2 cells were either mock treated or incubated with dsRNA directed against selective BAP and PBAP subunits. Whole-cell extracts were prepared and analyzed by Western immunoblotting using the appropriate antibodies. An arrowhead indicates the BAP170 band. ISWI was used as a loading control. (C) Immunoblotting analysis of whole-cell extracts prepared from cells that were either mock treated or incubated with dsRNA directed against ISWI. BRM functioned as a loading control. (D) Summary of the architectural relationships between distinct BAP and PBAP subunits. The subunits from which an arrow originates are required for the incorporation of the subunits to which the arrows point. The thickness of the arrow indicates the relative importance of a given subunit for the incorporation of its partner. For example, MOR is essential for the incorporation of BRM. However, BRM makes only a modest contribution to the incorporation of MOR. Dashed arrows indicate a minor effect.
Strikingly, depletion of MOR caused a concomitant dramatic drop in the levels of all other BAP and PBAP subunits tested (Fig. 1B). The knockdown of BRM led to a strong reduction in the abundance of OSA and PB, whereas BAP170 remained unaffected. MOR and SNR1 levels were also reduced, but not as dramatically as after MOR depletion. The RNAi-mediated knockdown of SNR1 resulted in a substantial decrease in OSA and PB levels and a mild reduction in BRM levels and had little effect on MOR or BAP170. Targeting of PB did not have significant consequences for the other subunits tested, except for a slight increase in OSA levels. RNAi directed against BAP170 led to a reduction in PB levels and to a slight increase in OSA levels, but not those of the other subunits. Finally, the OSA knockdown affected neither the core subunits nor the PBAP-specific subunits.
RT-qPCR analysis revealed that changes in mRNA levels accounted only for the decreased levels of targeted proteins, not for the concomitant loss of other subunits (data not shown). Thus, a knockdown of the core subunits directly affects the stability and composition of both BAP and PBAP. Subunits that fail to assemble into a complex are apparently unstable and are quickly targeted for degradation. These results provide detailed insights into the architectural properties of BAP and PBAP subunits. A major finding was that MOR is particularly critical for the structural integrity of both complexes. On the other hand, RNAi knockdown of the signature subunits OSA (BAP), PB, and BAP170 (PBAP) resulted in the specific depletion of the respective complex. The architectural relationships between the distinct BAP and PBAP subunits are summarized in Fig. 1D.
BAP and PBAP structure-function analysis through expression profiling.
To investigate the roles of BAP and PBAP in gene expression control, we extracted RNA from S2 cells treated with dsRNA against individual subunits. Labeled RNA was hybridized on Affymetrix Drosophila Genome 2 arrays (http://www.affymetrix.com/support/technical/datasheets/drosophila2_datasheet.pdf), containing 18,500 probe sets representing all known transcripts and variants. Expression indexes were calculated using the RMA algorithm (23). Examination of RMA expression indexes revealed a bimodal distribution with low values for a large portion of the probe sets (see Fig. S1 in the supplemental material). Prior to further analysis, we removed genes that were expressed at very low levels from the data set, using the minimum covariance determinant algorithm (46).
To assess the technical variability of microarray experiments, we hybridized each RNA sample from mock- and RNAi-treated cells twice. For each replica, we found a high correlation between expression indexes (r > 0.9; P < 0.001). We also performed RT-qPCR analysis on a selection of BAP and PBAP target genes. As a reference gene, we used CG11874, encoding mannosyl-oligosaccharide mannosidase, which remained unaffected in all of our microarray experiments. We found that changes in gene expression after RNAi treatment determined by either microarray or RT-qPCR analysis were highly correlated (r = 0.96; P < 0.001; n = 72). Together, these results demonstrate that gene expression profiles obtained from microarray experiments are highly reproducible and provide a valid representation of the effects of BAP or PBAP subunit depletion on the transcriptome.
Three independent RNAi knockdown experiments, followed by RNA extraction and microarray hybridization, were performed for each subunit. For mock-treated cells, we performed six independent experiments. Next, we applied one-way analysis of variance (ANOVA) on each probe set to identify genes that changed significantly (P < 0.05) upon RNAi treatment. This analysis identified 1,714 genes, for which we determined gene expression profiles by taking the ratios between average gene expression indexes obtained from RNAi- and mock-treated cells.
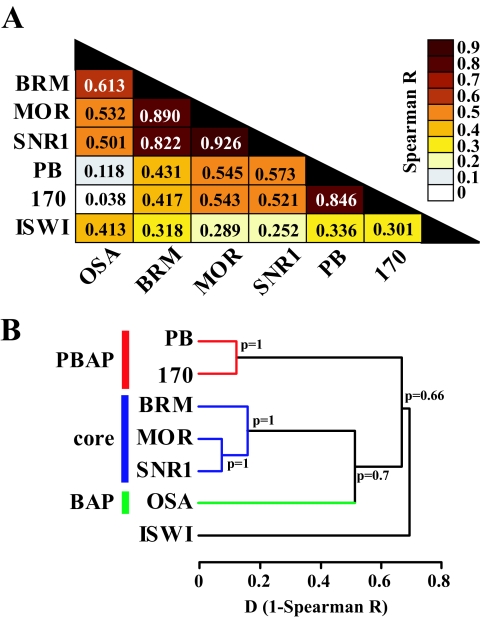
To gain insight into the relationship between the transcriptomes determined by individual BAP and PBAP subunits, we decided to use an unbiased statistical analysis of the whole data set. Spearman correlation analysis and the derived hierarchical agglomerative clustering revealed a clear separation between the transcriptomes dependent on either the core subunits, BAP, PBAP, or ISWI (Fig. 2A and B).
FIG. 2.
Expression profiling reveals functional relationships between BAP and PBAP subunits. (A) Spearman correlation matrix for microarray expression profiles obtained after RNAi knockdown of individual BAP and PBAP subunits and ISWI. The heat map reflects the indicated R values. (B) Agglomerative hierarchical cluster analysis on microarray expression profiles based on Spearman correlation coefficients (Spearman R). The BAP (green), PBAP (red), SWI/SNF core (blue), and ISWI clusters are indicated. P values indicate probabilities for each cluster calculated based on the bootstrap probability.
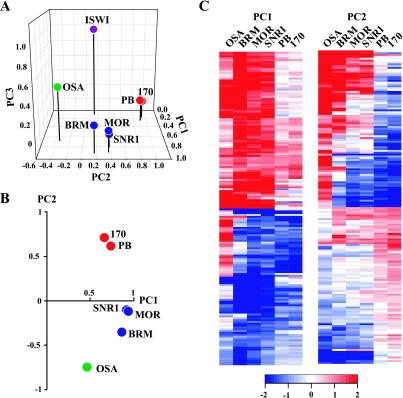
Next, we performed principal-component analysis (PCA), a powerful mathematical procedure that helps to uncover relationships in complex data sets. PCA is a linear transformation that finds and projects original variables to the fewest principal components (PCs), accounting for most of the variance in the data set. As shown in Fig. 3A, PCA revealed that the BAP and PBAP profiles were highly correlated with the first two PCs, whereas ISWI strongly correlated with PC3. Only the BAP signature subunit OSA showed some correlation with PC3, suggesting shared functions with ISWI. PC1 and PC2 explain 87.2% of the variance in the gene expression profiles obtained for BAP and PBAP. Both BAP- and PBAP-dependent expression profiles were highly correlated with PC1, suggesting that many genes are coregulated by these complexes (Fig. 3B). The core subunit profiles cluster closely together and show little correlation with PC2. The PBAP-specific PB and BAP170 transcriptomes cluster closely together and show a strong positive correlation with PC2. In clear contrast, the BAP-defining OSA transcriptome showed a strong negative correlation with PC2 (Fig. 3B). Thus, in addition to coregulated genes, others are antagonistically regulated by BAP and PBAP, i.e., genes that appear to be activated by one complex but repressed by the other.
FIG. 3.
PCA reveals coordinate and antagonistic transcription control by BAP and PBAP. (A) Representation of seven expression profiles in a three-dimensional transcriptome space. The microarray expression profiles upon RNAi-mediated knockdown of individual subunits are shown as a projection on the first three PCs after varimax rotation. The transcriptomes after depletion of the core subunits (blue), OSA (green), PB and BAP170 (red), and ISWI (purple) are indicated. (B) Projection of the microarray expression profiles for BAP and PBAP subunits on PC1 and PC2, explaining 87.2% of the variance. Note that here we did not perform varimax rotation. (C) Heat map depicting the agglomerative hierarchical clustering of genes, with the highest absolute scores for PC1 (n = 170) and PC2 (n = 170). Changes in gene expression compared to the mock-treated cells are depicted in red (up) and blue (down) on a log2 scale. White indicates no change.
BAP and PBAP perform coordinate and antagonistic functions.
The values for each PC were derived from a linear combination of the original gene expression profiles. To identify and visualize the genes that are coregulated or antagonistically regulated by BAP and PBAP, we selected the top 5% of genes at the right and left tails of the PC1 and PC2 value distributions (see Table S1 in the supplemental material). Hierarchical cluster analysis of the top-scoring PC1 genes revealed that many are coordinately up or down regulated by all BAP and PBAP subunits (Fig. 3C, left). Notably, an approximately equal number of genes are activated or repressed, reinforcing the notion that SWI/SNF remodelers can be positive, as well as negative, regulators of transcription. However, a significant portion of the genes are only regulated by either BAP or PBAP or appear to be repressed by OSA (BAP) and activated by PBAP.
The most notable feature of hierarchical clustering of the top-scoring PC2 genes is the strong antagonism between BAP and PBAP. Overall, genes activated by OSA were repressed by PB and BAP170. Conversely, OSA-repressed genes were stimulated by PBAP. The transcriptional consequence of core subunit depletion grouped with either BAP or PBAP, most likely reflecting the relative dependence of a gene on either complex (Fig. 3C, right). We suggest that in conjunction with the appropriate activators or repressors, BAP and PBAP can function as either selective coactivators or corepressors of transcription. In conclusion, our genomewide expression analysis uncovered both coordinate and antagonistic functions of BAP and PBAP in transcription control.
BAP- and PBAP-selective subunits determine holoenzyme function.
Our results so far had established that the core subunits, but not the signature subunits, are essential for the structural integrity of BAP and PBAP. We also found that, depending on the target gene, BAP and PBAP might have similar effects, act independently, or antagonize each other. However, the function of the core complex itself in transcription control remained unclear. In other words, what activities could the core complex lacking the BAP- and PBAP signature subunits perform in the cell?
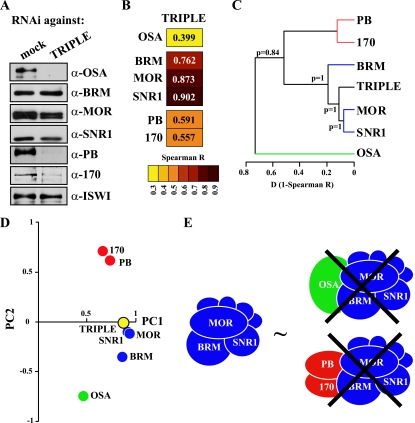
To address this question, we knocked down OSA, PB, and BAP170 simultaneously. Western immunoblotting confirmed that, although the BAP- and PBAP-defining subunits were depleted, the core complex remained (Fig. 4A). Strikingly, Spearman correlation analysis, hierarchical clustering, and PCA all revealed that the gene expression profile of cells lacking the signature subunits was very similar to that of cells depleted of the core subunits (Fig. 4B, C, and D). Genes with the highest absolute PC1 or PC2 values after depletion of core subunits were thus affected similarly by the triple OSA, PB, and BAP170 knockdown (data not shown). This observation implies that loss of the signature subunits impedes both the coordinate and antagonistic functions of BAP and PBAP. As we showed above (Fig. 1), depletion of the core subunits destabilizes both BAP and PBAP. Therefore, the effect of the triple knockdown, reducing BAP and PBAP to their common core, is largely similar to the total removal of the BAP and PBAP remodelers from the cell (Fig. 4E). The surprising implication of these findings is that, in the cell, the core SWI/SNF complex is defective for transcription control of the majority of target genes. We suggest that, for regulation of most genes, SWI/SNF remodelers act as holoenzymes, which require the signature subunits OSA or PB and BAP170 for global gene expression control.
FIG. 4.
The SWI/SNF core requires the BAP and PBAP signature subunits for global gene regulation. (A) S2 cells were either mock treated or incubated with dsRNAs directed against OSA, PB, and BAP170 (TRIPLE). Whole-cell extracts were prepared and analyzed by Western immunoblotting using the appropriate antibodies. ISWI was used as a loading control. (B) Spearman correlation matrix for microarray expression profiles obtained after the indicated RNAi-mediated depletion of either individual BAP and PBAP subunits or all three signature subunits simultaneously (TRIPLE). The heat map reflects the indicated R values. (C) Derived agglomerative hierarchical clustering of microarray expression profiles based on Spearman correlation coefficients (Spearman R). The BAP (green), PBAP (red), and SWI/SNF core (blue) clusters are indicated. The P values indicate probabilities for each cluster calculated based on the bootstrap probability. (D) Projection of the microarray expression profiles on PC1 and PC2. The transcriptomes after depletion of the individual core subunits (blue), the TRIPLE signature subunits (yellow), OSA (green), and PB and BAP170 (red) are indicated. (E) The SWI/SNF core complex stripped of BAP and PBAP signature subunits is largely dysfunctional in gene regulation.
BAP and PBAP regulate distinct biological processes.
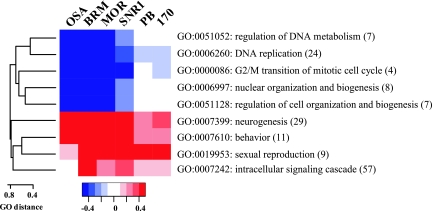
Thus far, we have analyzed BAP and PBAP functions in transcription control without considering the biological functions of their target genes. To identify the biological processes regulated by BAP and PBAP based on gene expression profiles, we used an unbiased statistical analysis of GO terms. GO terms provide a functional annotation for gene products taken from the corresponding model organism database (3). GO terms are structured into branched graphs with a common root, describing gene products according to their functional annotations. We focused our analysis on the biological process annotations and GO terms represented by at least three gene products from our data set. For each GO term, we compared the average change in gene expression after BAP or PBAP depletion with the global change in gene expression. We selected GO terms that were significantly up or down regulated as determined by the Student t test at a P value of <0.01. Next, we calculated the distance between each pair of GO terms, represented as individual nodes within the graph. To this end, we divided the number of common nodes by the sum of the nodes found between each GO term and the root. Based on the calculated distances between each pair of GO terms, we constructed a dissimilarity matrix and performed hierarchical clustering (Fig. 5). Inspection of the resulting cluster indicated that BAP might be involved in cell cycle regulation, whereas PBAP might be part of signal transduction cascades.
FIG. 5.
GO analysis of biological pathways regulated by BAP and PBAP. Agglomerative hierarchical clustering of the GO terms significantly affected by BAP or PBAP (P ≤ 0.01). The heat map represents the average change in gene expression on a log2 scale within each GO cluster after RNAi treatment targeting individual BAP or PBAP subunits.
Evolutionary switch in the role of SWI/SNF remodelers during cell cycle control.
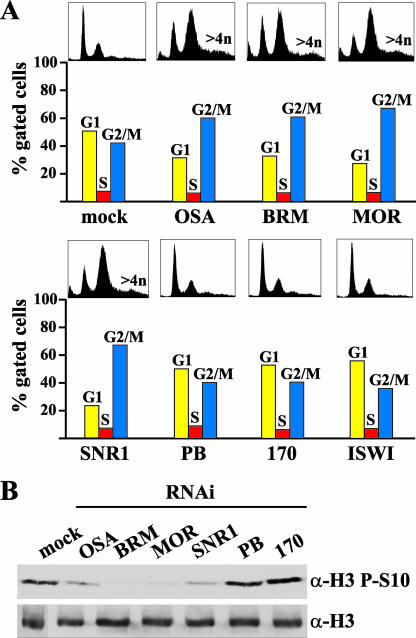
Obviously, GO predictions have to be confirmed by direct experiments. To this end, we investigated the roles of BAP and PBAP in cell cycle regulation. Our GO term analysis predicted that BAP, but not PBAP, would be involved in cell cycle control. This was unexpected, because previous work in budding yeast had established that the PBAP-related RSC, rather than the BAP-related ySWI/SNF, is essential for cell cycle progression (1, 9, 10). To test the prediction of our GO terms analysis, we examined cell cycle progression following depletion of either BAP or PBAP. Fluorescence-activated cell sorter (FACS) analysis showed that S2 cells depleted for OSA or core subunits accumulated at the G2/M phase of the cell cycle (Fig. 6A). Moreover, targeting of BAP led to an increased number of polyploid and aneuploid cells. In contrast, cells lacking PBAP-specific subunits or ISWI displayed no cell cycle defect. Consistent with a blocked transition from G2 to mitosis, BAP-depleted cells display a reduced level of histone H3 phosphorylation (Fig. 6B). In conclusion, an unbiased statistical analysis of GO terms correctly predicted the critical role of BAP, but not PBAP, in cell cycle control. In yeast, cell cycle progression through mitosis is regulated by RSC (corresponding to PBAP), but not by SWI/SNF (corresponding to BAP) (1, 9, 10). Thus, during evolution, distinct SWI/SNF-class remodelers acquired critical roles in cell cycle control.
FIG. 6.
BAP, but not PBAP, is required for entering mitosis. (A) Cell cycle distribution of S2 cells treated with dsRNA directed against the indicated subunits, as determined by FACS analysis. Cells with a DNA content of 4n or more are indicated. Quantification is based on gated cells. The ungated FACS profiles are also shown. (B) Western immunoblot analysis of whole-cell extracts from RNAi-treated cells with antibodies against either histone H3 phosphorylated on Ser 10 (H3 P-S10) or bulk H3.
BAP directs entry into mitosis via regulation of stg/cdc25 expression.
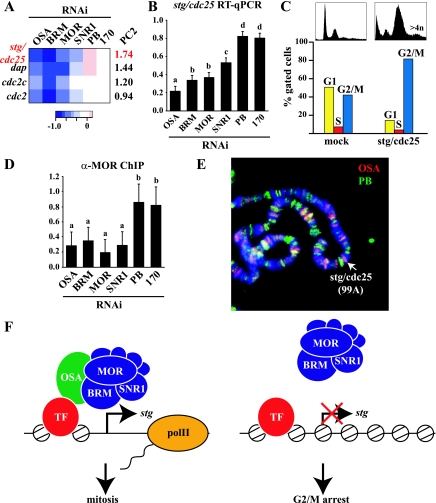
Next, we set out to determine the pathway through which BAP controls cell cycle progression. Our data set contained four genes associated with the GO term GO:0000086—G2/M transition of the mitotic cell cycle (Fig. 7A). This cluster was significantly down regulated after BAP, but not PBAP, depletion (Fig. 5). Among these potential BAP targets, stg had a particularly significant PC2 score, reflecting differential regulation by BAP and PBAP. stg encodes CDC25 phosphatase, which is critical for activation of CDC2 and entry into mitosis (41). Multiple studies have established that transcriptional regulation of stg/cdc25 plays a crucial role in the control of cell proliferation during fly development (15-17, 20, 30, 58). RT-qPCR analysis confirmed that stg/cdc25 is down regulated after BAP depletion, whereas the knockdown of PBAP-specific subunits had little or no effect (Fig. 7B). As expected based on previous studies (17), RNAi-mediated knockdown of stg/cdc25 resulted in cell accumulation in G2/M and increased aneu- and polyploidization (Fig. 7C). Thus, stg/cdc25 depletion is sufficient to recapitulate the cell cycle phenotype caused by the loss of BAP.
FIG. 7.
BAP regulates cell cycle progression via direct control of stg/cdc25 transcription. (A) Heat map depicting the expression profiles of genes involved in cell cycle progression through G2/M (GO:0000086) on a log2 scale, as determined by microarray analysis. The PC2 scores are indicated. (B) RT-qPCR determination of changes in stg/cdc25 expression following dsRNA treatment compared to mock-treated cells represented as ratios on a linear scale. The effects of targeting either BAP or PBAP subunits are significantly different as determined by one-way ANOVA [F(5,18) = 104.9; P < 0.001] on four biological replicates. A Fisher least significant difference (LSD) test revealed four homogenous groups at an α of 0.05. Groups a to c are represented by BAP, and group d by PB and BAP170. (C) Cell cycle distribution of S2 cells treated with dsRNA directed against stg/cdc25, as determined by FACS analysis. Cells with a DNA content of 4n or more are indicated. Quantification was based on gated cells. The ungated FACS profiles are also shown. (D) OSA is required for targeting BAP to the stg/cdc25 promoter. ChIP/qPCR quantification of changes in MOR binding to the stg/cdc25 promoter following dsRNA treatment compared to mock-treated cells is represented as ratios on a linear scale. Significant changes upon RNAi-mediated knockdown of individual BAP, but not PBAP, subunits were determined by one-way ANOVA [F(5,18) = 7.9; P < 0.001] on four biological replicates. The error bars represent 95% confidence intervals. The Fisher LSD test revealed two homogenous groups at an α of 0.05: group a is represented by BAP subunits and group b by PB and BAP170. (E) Distribution of OSA (red) and PB (green) larval salivary glands on polytene chromosomes, as determined by indirect immunofluorescence. DNA was visualized by DAPI staining (blue). OSA binds to the cytological region 99A, harboring the stg/cdc25 locus. (F) OSA targets BAP to the stg/cdc25 promoter to mediate transcriptional activation. The STG/CDC25 phosphatase promotes entry into mitosis through dephosphorylation and activation of CDC2. Depletion of OSA causes a failure of BAP recruitment, and the lack of stg/cdc25 expression causes arrest at the G2/M phase of the cell cycle.
To test if stg/cdc25 regulation by BAP is direct, we performed ChIP experiments, using antibodies against MOR. We extracted chromatin from S2 cells that were either mock treated or treated with RNAi directed against specific BAP or PBAP subunits (Fig. 7D). This approach allowed us to determine the contribution of each subunit to SWI/SNF recruitment while avoiding the use of different antibodies. The results of the ChIP experiments closely mirrored those of the expression analysis: SWI/SNF recruitment to the stg/cdc25 promoter was critically dependent on OSA but was unaffected by PB or BAP170 depletion (Fig. 7D). This result was supported by the determination of OSA and PB localization on larval salivary gland polytene chromosomes. OSA, but not PB, binds preferentially to cytological region 99A, the location of the stg/cdc25 gene (Fig. 7E). Collectively, these results strongly indicate that BAP is recruited to the stg/cdc25 promoter by OSA and regulates its expression.
DISCUSSION
It has become clear that the diversity among gene-specific activators and repressors is reflected by functional specification among coregulatory complexes (18, 29, 36, 45). Here, we combined functional genomics and statistical analysis for the structure-function dissection of BAP and PBAP, the fly representatives of the two evolutionarily conserved subclasses of SWI/SNF chromatin remodelers. We found that the common core subunits, particularly MOR, play critical structural roles. Epistasis analysis through whole-genome expression profiling revealed that the BAP- and PBAP-selective subunits are essential for the transcription control of many in vivo target genes. BAP and PBAP regulate distinct but overlapping transcriptional circuits, acting either independently, similarly, or antagonistically. GO term analysis indicated that BAP and PBAP control different cellular processes. Indeed, we found that BAP, but not PBAP, affects cell cycle progression through G2/M. Using both GO analysis and PCA for data mining, we determined that transcription of a key mitotic regulator, stg/cdc25, is activated by BAP. ChIP analysis revealed that BAP recruitment to the stg/cdc25 promoter was critically dependent on OSA (Fig. 7F). These results and recent studies of Mediator (52) demonstrate the value of gene expression profiling in combination with statistical data mining for structure-function analysis of multisubunit regulatory complexes. The most surprising conclusion from our work is that the core SWI/SNF complex appears largely defective for global transcription control in cells. We suggest that SWI/SNF remodelers act as holoenzymes, which require the signature subunits OSA or PB and BAP170 for control of the majority of target genes.
BAP and PBAP function as holoenzymes in cells.
Most chromatin remodelers exist as large multiprotein complexes that can be isolated as entities under stringent conditions (5, 24, 33, 37, 38, 51). Nevertheless, the central ATPase typically suffices for in vitro chromatin remodeling (4, 19, 33). In the case of human SWI/SNF, remodeling by the ATPase is stimulated by the mammalian orthologues of the MOR and SNR1 subunits (43). The human BRM and MOR homologs BRG1 and BAF155 are necessary and sufficient for EKLF-directed chromatin remodeling and transcription in vitro (26).
Here, we have investigated the functions of individual SWI/SNF subunits in cells. The core subunits, shared by BAP and PBAP, execute critical architectural and enzymatic functions. However, when stripped of OSA, PB, and BAP170, the core complex was defective in regulation of a significant portion of the target genes. Thus, the BAP- and PBAP-selective subunits are essential for global in vivo functionality of the complex. In other words, global gene expression analysis suggests that SWI/SNF remodelers predominantly act as holoenzymes. Examination of the BAP and PBAP expression profiles revealed that only a minority of SWI/SNF-regulated genes do not require an intact BAP or PBAP complex for their regulation. We suggest critical roles for OSA, PB, and BAP170 in tethering SWI/SNF to target genes. Consistent with this hypothesis, we showed that OSA is strictly required for BAP recruitment to the stg/cdc25 promoter. We propose that the core subunits provide both the structural framework and enzymatic activity, but that the BAP and PBAP signature subunits are required for regulation of the majority of SWI/SNF target genes.
Coordinate and antagonistic BAP and PBAP functions.
Our determination of their genomewide distribution on polytene chromosomes revealed that BAP and PBAP display distinct but overlapping patterns of distribution (34). This localization study was the first suggestion that BAP or PBAP had differential effects on gene expression. However, it remained unclear whether BAP and PBAP had similar or opposing activities during transcription control of common targets. PCA of gene expression profiles after BAP- or PBAP-selective depletion revealed two major classes of regulated genes. The first comprised genes coordinately activated or repressed by both complexes. The second class was composed of genes that were antagonistically regulated by BAP and PBAP. Thus, it appears that BAP and PBAP help to integrate and balance the activities of transcriptional activators and repressors to establish appropriate transcription levels.
BAP, but not PBAP, is required for cell cycle progression.
Evaluation of gene ontologies combined with expression profiling provides a valuable predictive tool for the dissection of biological pathways (3, 21). Using a statistical approach to GO analysis, we found that BAP is involved in the regulation of cell cycle progression. Indeed, depletion of BAP, but not PBAP, caused the accumulation of cells in the G2/M phase of the cell cycle and increased aneu- and polyploidization. A combination of statistical GO term analysis and PCA identified stg/cdc25 as a potential target, responsible for the BAP-dependent cell cycle phenotype. This hypothesis was confirmed experimentally by demonstrating that BAP directly binds and activates the stg/cdc25 promoter (Fig. 7B, D, and F) and by showing that a reduction of the stg/cdc25 level alone is sufficient to cause a G2/M arrest (Fig. 7C). In contrast, PBAP neither bound nor activated the stg/cdc25 promoter.
These results reveal a remarkable evolutionary switch between budding yeast and flies concerning the roles of RSC-PBAP class remodelers and ySWI/SNF-BAP in cell cycle control. In budding yeast, mutations in ySWI/SNF do not affect viability or the cell cycle, whereas RSC is essential for progression through G2/M and viability (1, 9, 10). In metazoans, BAP-BAF appear to be more abundant and perform more critical functions than their yeast counterparts (35, 55). Other studies of Drosophila have also implicated the BAP-selective OSA and several core subunits in cell cycle control (6, 48, 58). Finally, we note that insights into the role of SWI/SNF remodelers in cell cycle control will improve our understanding of certain human cancers (28, 44). For example, we found that loss of hSNF5, the human homologue of SNR1, causes a defective cell cycle and loss of ploidy control in malignant rhabdoid tumor cells (54).
In conclusion, we have used expression profiling following RNAi-mediated subunit depletion for a structure-function dissection of the BAP- and PBAP chromatin remodelers. We combined epistatic analysis and unbiased data-mining tools to explore the relations among gene expression profiles on the whole data set. We identified structural, as well as transcription-selective, functions executed by distinct subunits. Our results suggest that in general SWI/SNF remodelers act as holoenzymes, which require the BAP- or PBAP signature subunits for global transcription control. Statistical analysis of gene expression profiles and GO terms revealed that BAP and PBAP each control different biological processes. For example, BAP, but not PBAP, is required for cell cycle progression. These findings demonstrate the value of a statistical analysis of gene expression profiles for the dissection of complex biological processes controlled by multisubunit regulators.
Supplementary Material
Acknowledgments
We thank A. Maas for help with the FACS analysis, A. Dingwall for providing anti-SNR1 antibodies, E.-J. Rijkers for help with the programming, and J. Svejstrup for valuable comments on the manuscript.
This work was supported by grants from The Netherlands Organization for Scientific Research (NWO), the European Commission (to C.P.V.) and a long-term EMBO fellowship (to Y.M.M.).
Footnotes
Published ahead of print on 13 November 2006.
REFERENCES
- 1.Angus-Hill, M. L., A. Schlichter, D. Roberts, H. Erdjument-Bromage, P. Tempst, and B. R. Cairns. 2001. A Rsc3/Rsc30 zinc cluster dimer reveals novel roles for the chromatin remodeler RSC in gene expression and cell cycle control. Mol. Cell 7:741-751. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong, J. A., O. Papoulas, G. Daubresse, A. S. Sperling, J. T. Lis, M. P. Scott, and J. W. Tamkun. 2002. The Drosophila BRM complex facilitates global transcription by RNA polymerase II. EMBO J. 21:5245-5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashburner, M., C. A. Ball, J. A. Blake, D. Botstein, H. Butler, J. M. Cherry, A. P. Davis, K. Dolinski, S. S. Dwight, J. T. Eppig, M. A. Harris, D. P. Hill, L. Issel-Tarver, A. Kasarskis, S. Lewis, J. C. Matese, J. E. Richardson, M. Ringwald, G. M. Rubin, G. Sherlock, et al. 2000. Gene ontology: tool for the unification of biology. Nat. Genet. 25:25-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Becker, P. B. 2005. Nucleosome remodelers on track. Nat. Struct. Mol. Biol. 12:732-733. [DOI] [PubMed] [Google Scholar]
- 5.Becker, P. B., and W. Horz. 2002. ATP-dependent nucleosome remodeling. Annu. Rev. Biochem. 71:247-273. [DOI] [PubMed] [Google Scholar]
- 6.Brumby, A. M., C. B. Zraly, J. A. Horsfield, J. Secombe, R. Saint, A. K. Dingwall, and H. Richardson. 2002. Drosophila cyclin E interacts with components of the Brahma complex. EMBO J. 21:3377-3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Budzowska, M., I. Jaspers, J. Essers, H. de Waard, E. van Drunen, K. Hanada, B. Beverloo, R. W. Hendriks, A. de Klein, R. Kanaar, J. H. Hoeijmakers, and A. Maas. 2004. Mutation of the mouse Rad17 gene leads to embryonic lethality and reveals a role in DNA damage-dependent recombination. EMBO J. 23:3548-3558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cairns, B. R. 2005. Chromatin remodeling complexes: strength in diversity, precision through specialization. Curr. Opin. Genet. Dev. 15:185-190. [DOI] [PubMed] [Google Scholar]
- 9.Cairns, B. R., Y. Lorch, Y. Li, M. Zhang, L. Lacomis, H. Erdjument-Bromage, P. Tempst, J. Du, B. Laurent, and R. D. Kornberg. 1996. RSC, an essential, abundant chromatin-remodeling complex. Cell 87:1249-1260. [DOI] [PubMed] [Google Scholar]
- 10.Cao, Y., B. R. Cairns, R. D. Kornberg, and B. C. Laurent. 1997. Sfh1p, a component of a novel chromatin-remodeling complex, is required for cell cycle progression. Mol. Cell. Biol. 17:3323-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chai, B., J. Huang, B. R. Cairns, and B. C. Laurent. 2005. Distinct roles for the RSC and Swi/Snf ATP-dependent chromatin remodelers in DNA double-strand break repair. Genes Dev. 19:1656-1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collins, R. T., T. Furukawa, N. Tanese, and J. E. Treisman. 1999. Osa associates with the Brahma chromatin remodeling complex and promotes the activation of some target genes. EMBO J. 18:7029-7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins, R. T., and J. E. Treisman. 2000. Osa-containing Brahma chromatin remodeling complexes are required for the repression of wingless target genes. Genes Dev. 14:3140-3152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Damelin, M., I. Simon, T. I. Moy, B. Wilson, S. Komili, P. Tempst, F. P. Roth, R. A. Young, B. R. Cairns, and P. A. Silver. 2002. The genome-wide localization of Rsc9, a component of the RSC chromatin-remodeling complex, changes in response to stress. Mol. Cell 9:563-573. [DOI] [PubMed] [Google Scholar]
- 15.de Vries, H. I., L. Uyetake, W. Lemstra, J. F. Brunsting, T. T. Su, H. H. Kampinga, and O. C. Sibon. 2005. Grp/DChk1 is required for G2-M checkpoint activation in Drosophila S2 cells, whereas Dmnk/DChk2 is dispensable. J. Cell Sci. 118:1833-1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edgar, B. A., D. A. Lehman, and P. H. O'Farrell. 1994. Transcriptional regulation of string (cdc25): a link between developmental programming and the cell cycle. Development 120:3131-3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edgar, B. A., and P. H. O'Farrell. 1989. Genetic control of cell division patterns in the Drosophila embryo. Cell 57:177-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Emerson, B. M. 2002. Specificity of gene regulation. Cell 109:267-270. [DOI] [PubMed] [Google Scholar]
- 19.Flaus, A., and T. Owen-Hughes. 2004. Mechanisms for ATP-dependent chromatin remodelling: farewell to the tuna-can octamer? Curr. Opin. Genet. Dev. 14:165-173. [DOI] [PubMed] [Google Scholar]
- 20.Furnari, B., N. Rhind, and P. Russell. 1997. Cdc25 mitotic inducer targeted by chk1 DNA damage checkpoint kinase. Science 277:1495-1497. [DOI] [PubMed] [Google Scholar]
- 21.Harris, M. A., J. Clark, A. Ireland, J. Lomax, M. Ashburner, R. Foulger, K. Eilbeck, S. Lewis, B. Marshall, C. Mungall, J. Richter, G. M. Rubin, J. A. Blake, C. Bult, M. Dolan, H. Drabkin, J. T. Eppig, D. P. Hill, L. Ni, M. Ringwald, R. Balakrishnan, J. M. Cherry, K. R. Christie, M. C. Costanzo, S. S. Dwight, S. Engel, D. G. Fisk, J. E. Hirschman, E. L. Hong, R. S. Nash, A. Sethuraman, C. L. Theesfeld, D. Botstein, K. Dolinski, B. Feierbach, T. Berardini, S. Mundodi, S. Y. Rhee, R. Apweiler, D. Barrell, E. Camon, E. Dimmer, V. Lee, R. Chisholm, P. Gaudet, W. Kibbe, R. Kishore, E. M. Schwarz, P. Sternberg, M. Gwinn, L. Hannick, J. Wortman, M. Berriman, V. Wood, N. de la Cruz, P. Tonellato, P. Jaiswal, T. Seigfried, and R. White. 2004. The Gene Ontology (GO) database and informatics resource. Nucleic Acids Res. 32:D258-D261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holstege, F. C., E. G. Jennings, J. J. Wyrick, T. I. Lee, C. J. Hengartner, M. R. Green, T. R. Golub, E. S. Lander, and R. A. Young. 1998. Dissecting the regulatory circuitry of a eukaryotic genome. Cell 95:717-728. [DOI] [PubMed] [Google Scholar]
- 23.Irizarry, R. A., B. M. Bolstad, F. Collin, L. M. Cope, B. Hobbs, and T. P. Speed. 2003. Summaries of Affymetrix GeneChip probe level data. Nucleic Acids Res. 31:e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadam, S., and B. M. Emerson. 2002. Mechanisms of chromatin assembly and transcription. Curr. Opin. Cell Biol. 14:262-268. [DOI] [PubMed] [Google Scholar]
- 25.Kadam, S., and B. M. Emerson. 2003. Transcriptional specificity of human SWI/SNF BRG1 and BRM chromatin remodeling complexes. Mol. Cell 11:377-389. [DOI] [PubMed] [Google Scholar]
- 26.Kadam, S., G. S. McAlpine, M. L. Phelan, R. E. Kingston, K. A. Jones, and B. M. Emerson. 2000. Functional selectivity of recombinant mammalian SWI/SNF subunits. Genes Dev. 14:2441-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kal, A. J., T. Mahmoudi, N. B. Zak, and C. P. Verrijzer. 2000. The Drosophila brahma complex is an essential coactivator for the trithorax group protein zeste. Genes Dev. 14:1058-1071. [PMC free article] [PubMed] [Google Scholar]
- 28.Klochendler-Yeivin, A., C. Muchardt, and M. Yaniv. 2002. SWI/SNF chromatin remodeling and cancer. Curr. Opin. Genet. Dev. 12:73-79. [DOI] [PubMed] [Google Scholar]
- 29.Kornberg, R. D. 1999. Eukaryotic transcriptional control. Trends Cell Biol. 9:M46-M49. [PubMed] [Google Scholar]
- 30.Lehman, D. A., B. Patterson, L. A. Johnston, T. Balzer, J. S. Britton, R. Saint, and B. A. Edgar. 1999. cis-regulatory elements of the mitotic regulator, string/Cdc25. Development 126:1793-1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lemon, B., C. Inouye, D. S. King, and R. Tjian. 2001. Selectivity of chromatin-remodelling cofactors for ligand-activated transcription. Nature 414:924-928. [DOI] [PubMed] [Google Scholar]
- 32.Lindsley, D. L., and G. G. Zimm. 1992. The genome of Drosophila melanogaster. Academic Press, New York, NY.
- 33.Lusser, A., and J. T. Kadonaga. 2003. Chromatin remodeling by ATP-dependent molecular machines. Bioessays 25:1192-1200. [DOI] [PubMed] [Google Scholar]
- 34.Mohrmann, L., K. Langenberg, J. Krijgsveld, A. J. Kal, A. J. Heck, and C. P. Verrijzer. 2004. Differential targeting of two distinct SWI/SNF-related Drosophila chromatin-remodeling complexes. Mol. Cell. Biol. 24:3077-3088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohrmann, L., and C. P. Verrijzer. 2005. Composition and functional specificity of SWI2/SNF2 class chromatin remodeling complexes. Biochim. Biophys. Acta 1681:59-73. [DOI] [PubMed] [Google Scholar]
- 36.Naar, A. M., B. D. Lemon, and R. Tjian. 2001. Transcriptional coactivator complexes. Annu. Rev. Biochem. 70:475-501. [DOI] [PubMed] [Google Scholar]
- 37.Narlikar, G. J., H. Y. Fan, and R. E. Kingston. 2002. Cooperation between complexes that regulate chromatin structure and transcription. Cell 108:475-487. [DOI] [PubMed] [Google Scholar]
- 38.Neely, K. E., and J. L. Workman. 2002. The complexity of chromatin remodeling and its links to cancer. Biochim. Biophys. Acta 1603:19-29. [DOI] [PubMed] [Google Scholar]
- 39.Ng, H. H., F. Robert, R. A. Young, and K. Struhl. 2002. Genome-wide location and regulated recruitment of the RSC nucleosome-remodeling complex. Genes Dev. 16:806-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nie, Z., Y. Xue, D. Yang, S. Zhou, B. J. Deroo, T. K. Archer, and W. Wang. 2000. A specificity and targeting subunit of a human SWI/SNF family-related chromatin-remodeling complex. Mol. Cell. Biol. 20:8879-8888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nilsson, I., and I. Hoffmann. 2000. Cell cycle regulation by the Cdc25 phosphatase family. Prog. Cell Cycle Res. 4:107-114. [DOI] [PubMed] [Google Scholar]
- 42.Papoulas, O., S. J. Beek, S. L. Moseley, C. M. McCallum, M. Sarte, A. Shearn, and J. W. Tamkun. 1998. The Drosophila trithorax group proteins BRM, ASH1 and ASH2 are subunits of distinct protein complexes. Development 125:3955-3966. [DOI] [PubMed] [Google Scholar]
- 43.Phelan, M. L., S. Sif, G. J. Narlikar, and R. E. Kingston. 1999. Reconstitution of a core chromatin remodeling complex from SWI/SNF subunits. Mol. Cell 3:247-253. [DOI] [PubMed] [Google Scholar]
- 44.Roberts, C. W., and S. H. Orkin. 2004. The SWI/SNF complex—chromatin and cancer. Nat. Rev. Cancer 4:133-142. [DOI] [PubMed] [Google Scholar]
- 45.Roeder, R. G. 2005. Transcriptional regulation and the role of diverse coactivators in animal cells. FEBS Lett. 579:909-915. [DOI] [PubMed] [Google Scholar]
- 46.Rousseeuw, P. J., and K. van Driessen. 1999. A fast algorithm for the minimum covariance determinant estimator. Technometrics 41:212-223. [Google Scholar]
- 47.Shen, X., G. Mizuguchi, A. Hamiche, and C. Wu. 2000. A chromatin remodelling complex involved in transcription and DNA processing. Nature 406:541-544. [DOI] [PubMed] [Google Scholar]
- 48.Staehling-Hampton, K., P. J. Ciampa, A. Brook, and N. Dyson. 1999. A genetic screen for modifiers of E2F in Drosophila melanogaster. Genetics 153:275-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sudarsanam, P., V. R. Iyer, P. O. Brown, and F. Winston. 2000. Whole-genome expression analysis of snf/swi mutants of Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 97:3364-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trotter, K. W., and T. K. Archer. 2004. Reconstitution of glucocorticoid receptor-dependent transcription in vivo. Mol. Cell. Biol. 24:3347-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsukiyama, T. 2002. The in vivo functions of ATP-dependent chromatin-remodelling factors. Nat Rev. Mol. Cell. Biol. 3:422-429. [DOI] [PubMed] [Google Scholar]
- 52.van de Peppel, J., N. Kettelarij, H. van Bakel, T. T. Kockelkorn, D. van Leenen, and F. C. Holstege. 2005. Mediator expression profiling epistasis reveals a signal transduction pathway with antagonistic submodules and highly specific downstream targets. Mol. Cell 19:511-522. [DOI] [PubMed] [Google Scholar]
- 53.van der Knaap, J. A., B. R. Kumar, Y. M. Moshkin, K. Langenberg, J. Krijgsveld, A. J. Heck, F. Karch, and C. P. Verrijzer. 2005. GMP synthetase stimulates histone H2B deubiquitylation by the epigenetic silencer USP7. Mol. Cell 17:695-707. [DOI] [PubMed] [Google Scholar]
- 54.Vries, R. G., V. Bezrookove, L. M. Zuijderduijn, S. K. Kia, A. Houweling, I. Oruetxebarria, A. K. Raap, and C. P. Verrijzer. 2005. Cancer associated mutations in chromatin remodeler hSNF5 promote chromosomal instability by compromising the mitotic checkpoint. Genes Dev. 19:665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang, W. 2003. The SWI/SNF family of ATP-dependent chromatin remodelers: similar mechanisms for diverse functions. Curr. Top. Microbiol. Immunol. 274:143-169. [DOI] [PubMed] [Google Scholar]
- 56.Worby, C. A., N. Simonson-Leff, and J. E. Dixon. 2001. RNA interference of gene expression (RNAi) in cultured Drosophila cells. Sci. STKE 2001:PL1. [DOI] [PubMed]
- 57.Yan, Z., K. Cui, D. M. Murray, C. Ling, Y. Xue, A. Gerstein, R. Parsons, K. Zhao, and W. Wang. 2005. PBAF chromatin-remodeling complex requires a novel specificity subunit, BAF200, to regulate expression of selective interferon-responsive genes. Genes Dev. 19:1662-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zraly, C. B., D. R. Marenda, and A. K. Dingwall. 2004. SNR1 (INI1/SNF5) mediates important cell growth functions of the Drosophila Brahma (SWI/SNF) chromatin remodeling complex. Genetics 168:199-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.