Abstract
The kinesin spindle protein (KSP), a microtubule motor protein, is essential for the formation of bipolar spindles during mitosis. Inhibition of KSP activates the spindle checkpoint and causes apoptosis. It was shown that prolonged inhibition of KSP activates Bax and caspase-3, which requires a competent spindle checkpoint and couples with mitotic slippage. Here we investigated how Bax is activated by KSP inhibition and the roles of Bax and p53 in KSP inhibitor-induced apoptosis. We demonstrate that small interfering RNA-mediated knockdown of Bax greatly attenuates KSP inhibitor-induced apoptosis and that Bax activation is upstream of caspase activation. This indicates that Bax mediates the lethality of KSP inhibitors and that KSP inhibition provokes apoptosis via the intrinsic apoptotic pathway where Bax activation is prior to caspase activation. Although the BH3-only protein Puma is induced after mitotic slippage, suppression of de novo protein synthesis that abrogates Puma induction does not block activation of Bax or caspase-3, indicating that Bax activation is triggered by a posttranslational event. Comparison of KSP inhibitor-induced apoptosis between matched cell lines containing either functional or deficient p53 reveals that inhibition of KSP induces apoptosis independently of p53 and that p53 is dispensable for spindle checkpoint function. Thus, KSP inhibitors should be active in p53-deficient tumors.
The kinesin spindle protein (KSP), also termed kinesin-5 or Eg5, is a microtubule motor protein that is essential for the formation of bipolar spindles and the proper segregation of sister chromatids during mitosis (2, 8, 11, 34). Inhibition of KSP causes the formation of monopolar mitotic spindles, activates the spindle assembly checkpoint, and arrests cells at mitosis, which leads to subsequent cell death (2, 16, 24, 36). Since KSP functions exclusively in mitosis, KSP inhibitors should spare postmitotic cells and thus do not cause peripheral neuropathy, a major liability suffered by the microtubule inhibitors, such as taxanes and vinca alkaloids, that have been widely used in the clinic for cancer treatment. In addition, KSP inhibitors should remain efficacious in taxane-resistant tumors where the resistance may arise from acquired mutations on β-tubulin, altered expression of tubulin isoforms, or changed microtubule dynamics (12, 28, 33). As a result, KSP inhibitors have been developed as a new generation of antimitotic agents with a novel mechanism of action for cancer therapy, and several KSP inhibitors have entered clinical trials.
An in-depth understanding of the mechanism by which KSP inhibitors induce apoptosis and elucidation of the factors that determine cell sensitivity to KSP inhibitor-mediated killing will not only advance our knowledge in cell biology but also provide insights for a rational development and application of these agents in the clinic. The induction of apoptotic cell death by different death cues is mainly mediated by two pathways, the death receptor-dependent extrinsic pathway and the mitochondrion-mediated intrinsic pathway (9, 37, 38). The extrinsic apoptotic pathway is initiated by death ligand-elicited stimulation of the death receptors on the plasma membrane, such as Fas/CD95. Activated death receptors elicit activation of the initiator caspases 8 and 10, which in turn directly activate the effector caspases 3 and 7 and execute apoptosis (9, 37). In addition, the death receptor-activated caspases can trigger the intrinsic death pathway by inducing activation of the proapoptotic proteins Bax and Bak which cause permeabilization of the mitochondrial outer membrane to amplify apoptosis (19, 22). The intrinsic apoptotic pathway, however, can be provoked by various death stimuli, including DNA-damaging agents, which induce Bax/Bak activation and subsequent permeabilization of the mitochondrial membrane, leading to the activation of caspases (9, 38, 39). The induction of apoptosis is characterized by two major biochemical events, namely, the activation of Bax/Bak with subsequent mitochondrial membrane permeabilization and the activation of caspases. In the extrinsic pathway, caspases are activated prior to Bax/Bak activation, whereas in the intrinsic apoptotic pathway, Bax/Bak activation and the resultant loss of integrity of the mitochondrial membrane are upstream of and responsible for caspase activation. Recently, it was reported that, during mitotic catastrophe resulting from premature mitotic entry with either unrepaired DNA damage or incompletely replicated DNA, caspase-2 was activated prior to permeabilization of the mitochondrial membrane (4).
In the intrinsic apoptotic pathway, the BH3-only proteins of the Bcl-2 family are sentinels of various death stimuli that can trigger the activation of multidomain proteins Bax and Bak by either directly interacting with Bax/Bak or relieving the Bcl-2-like protein-mediated suppression of Bax/Bak (6, 14). Activated or derepressed Bax/Bak induces mitochondrial membrane permeabilization and the subsequent release of cytochrome c and other proapoptotic molecules from the mitochondrial intermembrane space. Activation of the BH3-only proteins can be provoked either by the induction of their expression which requires de novo protein synthesis or by posttranslational modifications, such as phosphorylation and proteolytic cleavage, that either modulate their activity and stability or alter their subcellular localizations independently of de novo protein synthesis (6, 32). The tumor suppressor p53 is a key controller of the intrinsic apoptotic program, while it also modulates the extrinsic pathway by inducing the expression of death receptors, such as Fas/CD95 and Killer/DR5 (25). p53 can activate the intrinsic death pathway by inducing the expression of proapoptotic proteins, such as the BH3-only protein Puma, or acting like a BH3-only molecule to activate Bax/Bak directly (5, 18, 26). Although it is clear that p53 mediates DNA damage-induced apoptosis, there is some evidence that microtubule inhibitors may cause cell death independently of p53 (1a), and the role of p53 in KSP inhibitor-induced lethality remains unknown.
In a previous study using a potent and specific small-molecule inhibitor of KSP, named KSP-IA, we demonstrated that prolonged inhibition of KSP activates Bax and caspase-3, committing cells to apoptosis (36). Intriguingly, activation of Bax and caspase-3 and the optimal killing by KSP-IA require a competent spindle assembly checkpoint and correlate with mitotic slippage, a process in which cells exit mitosis without segregation of sister chromatids and cytokinesis (36). This suggests that breaching an activated spindle checkpoint is responsible for triggering the apoptotic program. However, the role of Bax in KSP inhibitor-induced killing and the molecular mechanism underlying the activation of Bax and caspases by KSP inhibition were not addressed. In this report, we first showed that knockdown of Bax by small interfering RNA (siRNA) significantly reduced KSP-IA-induced apoptosis, indicating that Bax mediates the lethality of KSP inhibitors. Since Bax activation can be an apical step prior to caspase activation in the intrinsic apoptotic pathway or a late event downstream of caspase activation in the extrinsic pathway (9), and mitotic slippage may activate p53 by producing tetraploid pseudo-G1 cells with an abnormal genome (23), we investigated how Bax is activated by KSP-IA and whether p53 is required for KSP-IA-induced apoptosis. We found that KSP-IA-induced Bax activation is independent of caspase activities, demonstrating that Bax activation is an apical step upstream of caspase activation in KSP-IA-induced apoptosis. Furthermore, we showed that KSP-IA provokes the activation of Bax and caspase-3 by a posttranslational event independently of de novo protein synthesis. Finally, we demonstrated that KSP-IA activates the spindle checkpoint and induces apoptosis in a p53-independent manner.
MATERIALS AND METHODS
Cell lines, culture, and drug treatment.
The matched pairs of TOV21G and A2780 cell lines containing either an empty vector or a construct encoding a short hairpin RNA (shRNA) against human p53 were provided by M. Cleary, M. Carleton, and P. Linsley (1). The shRNA sequence is shown below with both the sense and antisense sequences for p53 shown in bold type and the sense sequence underlined: 5′-GATCCCGACTCCAGTGGTAATCTACTTCAAGAGGTAGATTACCACTGGAGTCTTTTTAGCTT-3′. The silencing of p53 in cells expressing p53 shRNA was confirmed by both reverse transcription-PCR and Western blotting after exposure to doxorubicin (Sigma). The matched TOV21G cell lines were maintained in Dulbecco's modified Eagle medium containing 10% fetal bovine serum (FBS) and 2 μg/ml puromycin, and A2780 matched lines were grown in Dulbecco's modified Eagle medium containing 10% FBS, 10 μg/ml insulin, and 4 μg/ml blasticidin.
Nontransfected A2780 cells were grown in RPMI 1640 medium supplemented with 10% FBS and 10 μg/ml insulin, and HCT116 cells were maintained in McCoy's 5A medium containing 10% FBS. For continuous drug exposure experiments, cells were treated with vehicle (0.1% dimethyl sulfoxide [DMSO]), KSP-IA (300 nM), or paclitaxel (100 nM) (Sigma) for various times and harvested immediately after drug treatment for analysis. When z-Val-Ala-dl-Asp-fluoromethylketone (z-VAD) (Bachem) and cycloheximide (Sigma) were included in the drug treatment, they were used at concentrations of 200 μM and 10 μM, respectively. For drug washout experiments, cells were treated with KSP-IA and then incubated for a further 24 h in the absence of the drug prior to harvesting.
Western blot analysis.
Both attached and floating cells were collected and washed in phosphate-buffered saline. Cell pellets were lysed in radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.4], 1% IGEPAL, and 1% sodium deoxycholate). Total proteins in cell lysates were separated by electrophoresis on a sodium dodecyl sulfate-polyacrylamide gel, transferred to a polyvinylidene difluoride membrane (Novex), and immunostained and visualized as described previously (36). The antibodies used and their manufacturers are as follows: BubR1, p21, and Bid (BD Transduction Laboratories), phosphorylated histone H3 (p-histone H3) (Upstate Cell Signaling), cleaved poly(ADP-ribose) polymerase (PARP) (Asp214; BD Pharmingen), Bax (6A7 monoclonal antibody; Sigma), Bax (polyclonal antibody; Santa Cruz Biotechnology), caspase-2 (Cell Signaling Technology), Puma (Ab-1; Calbiochem), p53 (Ab-2; Oncogene), Bim (Sigma B-7929; EMD Biosciences and Alexis), and glyceraldehyde-3-phosphate-dehydrogenase (GAPDH) (Research Diagnostics, Inc.).
Assay for Bax conformational change (activation).
Both attached and floating cells were collected and lysed with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) lysis buffer (10 mM HEPES [pH 7.4], 150 mM NaCl, and 1% CHAPS). Activated conformers of Bax were immunoprecipitated by incubation with 6A7 monoclonal antibody as previously described (36). The immunoprecipitated Bax conformers were detected by Western blotting using an anti-Bax polyclonal antibody. The total amount of Bax (input) in cell lysate was determined by Western blotting prior to immunoprecipitation.
Flow cytometry/DNA content analysis.
Both attached and floating cells were collected and fixed with 70% ethanol. Cells were subsequently stained with 50 μg/ml propidium iodide for 1 h and analyzed by fluorescence-activated cell sorting (FACS).
Mitotic index.
After fixation with 70% ethanol, cells were incubated with the MPM2 monoclonal antibody (Upstate Technology) as described previously (36). The MPM2 antibody-stained cells were detected by FACS analysis after incubation with Alexa Fluor 488-conjugated goat against mouse immunoglobulin G (Molecular Probes). The percentage of cells stained by the MPM2 antibody was considered the mitotic index.
Gene silencing with siRNA.
A smart pool of siRNAs against human caspase-2 (catalog number M-003465) and Bax (catalog number M-003308) and a siRNA duplex against luciferase (catalog number D-001100) were purchased from Dharmacon Research, Inc. (Lafayette, CO). siRNAs were transfected into A2780 cells grown to 50% confluence using Lipofectamine (Invitrogen) according to the manufacturer's protocol. The 100 nM siRNA pool or siRNA duplex was used for each transfection. After siRNA transfection, the knockdown of the caspase-2 and Bax genes was confirmed by Western blot analysis.
RESULTS
KSP-IA initiates the apoptotic program by activating Bax in a caspase-independent manner.
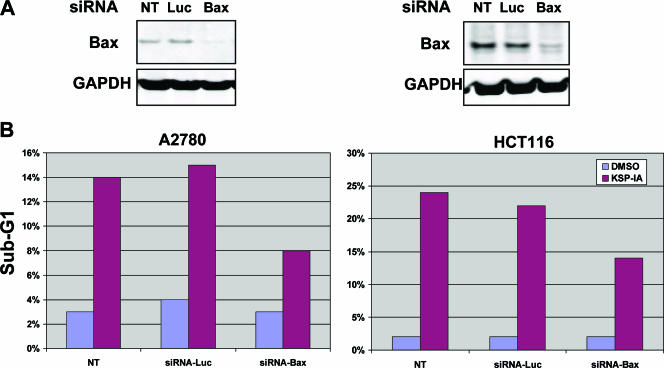
KSP-IA is a potent and specific inhibitor of KSP, which was used to investigate the biological consequence of KSP inhibition. We showed previously that prolonged inhibition of KSP by KSP-IA activated the proapoptotic protein Bax and caspase-3, leading to apoptotic cell death, and that z-VAD, a pancaspase inhibitor, completely rescued KSP-IA-induced loss of cell viability (36). To further investigate the role of Bax in KSP inhibitor-mediated cell death, we determined KSP-IA-induced apoptosis after Bax expression was silenced by Bax-specific siRNA. As shown in Fig. 1A, Bax, but not luciferase (control), siRNA was able to reduce Bax expression in both A2780 and HCT116 cells, while neither of these siRNAs affected the expression of GAPDH (Fig. 1A). Although transfection of either Bax or luciferase siRNA did not affect cell viability in the absence of KSP-IA, a pronounced reduction in the extent of apoptosis was observed in the cells where Bax was knocked down by transfection with Bax siRNA compared to either nontransfected cells or the cells transfected with luciferase siRNA after exposure to KSP-IA (Fig. 1B). This confirms that Bax mediates KSP inhibitor-induced killing. The lower levels of apoptosis observed in Bax knockdown cells may be mediated by the remaining Bax or potentially the other multidomain death protein Bak.
FIG. 1.
Bax mediates KSP-IA-induced apoptosis. (A) siRNA-mediated knockdown of Bax. Both A2780 and HCT116 cells were transfected with either the control luciferase (Luc)- or Bax-specific siRNA. Twenty-four hours after transfection, the steady-state levels of Bax were determined by Western blot analysis. Nontransfected cells (NT) were also analyzed for comparison. (B) Suppression of KSP-IA-induced apoptosis by siRNA-mediated knockdown of Bax. At 24 h after siRNA transfection, cells transfected with either the Luc or Bax siRNA and NT cells were treated with either DMSO (vehicle) or KSP-IA (300 nM) for 48 h. The extent of apoptosis was determined by FACS analysis, and the subdiploid (sub-G1) fractions (indicative of apoptotic cells) are shown.
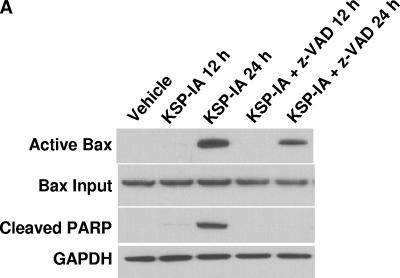
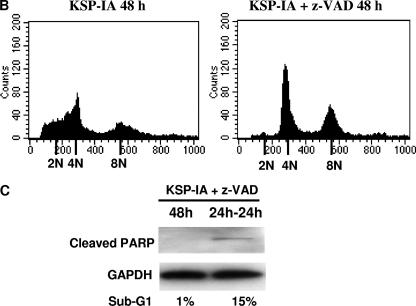
Activation of Bax involves a conformation change, which targets cytosolic Bax monomers to the mitochondrial outer membrane, where Bax oligomerizes or interacts with other channel proteins and causes permeabilization of the mitochondrial membrane (13, 29, 40). Bax can be activated via protein-protein interactions. The proteins that are able to induce Bax activation include the BH3-only proteins and p53 (14, 25, 27). Potentially, the induction of Bax expression may facilitate Bax activation as well. An examination of the steady-state levels of Bax revealed that exposure to KSP-IA did not alter the expression of this protein (Bax input in Fig. 2; also data not shown), excluding the involvement of increased Bax expression in KSP-IA-induced Bax activation. Bax can be activated by both the extrinsic and intrinsic apoptotic pathways. While in the extrinsic death pathway, the death receptor-mediated Bax activation is subsequent to and dependent on caspase activation, Bax is activated upstream of caspase activation in the intrinsic death pathway, which then results in permeabilization of the mitochondrial membrane and activation of caspases (38). To determine whether KSP-IA activates Bax prior to and independently of caspase activation, ovarian cancer A2780 cells were treated with KSP-IA in the absence or presence of z-VAD, and then Bax activation was determined by an immunoprecipitation-coupled Western blotting analysis as described before (36). As shown in Fig. 2, the pancaspase inhibitor z-VAD fails to abrogate Bax activation, although it completely blocks caspase-3-mediated cleavage of PARP and apoptotic death as measured by immunoblotting and FACS, respectively, indicating that KSP-IA-induced Bax activation is prior to and independent of caspase activation (Fig. 2A and B). A slight reduction of the levels of activated Bax in the presence of z-VAD may suggest that while caspase activities are not required for activating Bax by KSP-IA, subsequent activation of caspases may further enhance Bax activation. In addition, z-VAD does not affect KSP-IA-induced activation of the spindle checkpoint, mitotic arrest, and mitotic slippage, as determined by evaluating the phosphorylation of the spindle checkpoint component BubR1 (phosphorylated BubR1 [p-BubR1]), a marker for spindle checkpoint activation (21), the mitosis-specific phosphorylation (serine 10) of histone H3 (p-histone H3) (7), the mitotic index (the percentage of cells stained positive by the mitosis-specific antibody, anti-MPM2) (10), and cellular DNA content by FACS (Fig. 2B and 4). While the appearance of the mitotic marker, p-histone H3, and a parallel increase of mitotic index and tetraploid cells indicate mitotic arrest, maintenance of a high tetraploid fraction with a drop of mitotic index and decay of p-histone H3 indicates mitotic slippage. These observations are consistent with our former finding that mitotic slippage following mitotic arrest triggers Bax activation (36). In the presence of z-VAD, KSP-IA-treated cells underwent mitotic slippage and endoreduplication with little apoptotic death and exhibited increased polyploid population (Fig. 2B). If cells were treated with both KSP-IA and z-VAD for 24 h, washed, and further incubated in drug-free medium for another 24 h, both caspase-3 activation and apoptotic cells were detected (Fig. 2C), supporting that z-VAD blocks only caspase-mediated execution, not the caspase-independent initiation of apoptosis by KSP-IA.
FIG. 2.
KSP-IA activates Bax and initiates apoptosis independently of caspase activity. (A) A2780 cells were treated with KSP-IA (300 nM) for 12 or 24 h in the absence or presence of the pancaspase inhibitor z-VAD (200 μM). Cells were harvested and analyzed to determine the activation of Bax (Bax conformational change) by an immunoprecipitation-coupled Western blot assay, and caspase-3-mediated cleavage of PARP (cleaved PARP) was determined by Western blotting. (B) A2780 cells were treated with KSP-IA in the absence or presence of z-VAD for 48 h. Cellular DNA content was determined by FACS. Histograms of cell number versus DNA content are shown. (C) A2780 cells were incubated with both KSP-IA and z-VAD for 48 or 24 h. After 48 h of incubation, cells were harvested immediately (labeled 48h), whereas after 24 h of incubation, cells were washed and then incubated in drug-free medium for another 24 h prior to harvesting (labeled 24h-24h). Harvested cells were analyzed for caspase-3-mediated cleavage of PARP by Western blotting and for DNA content by FACS. The percentages of sub-G1 cells are shown.
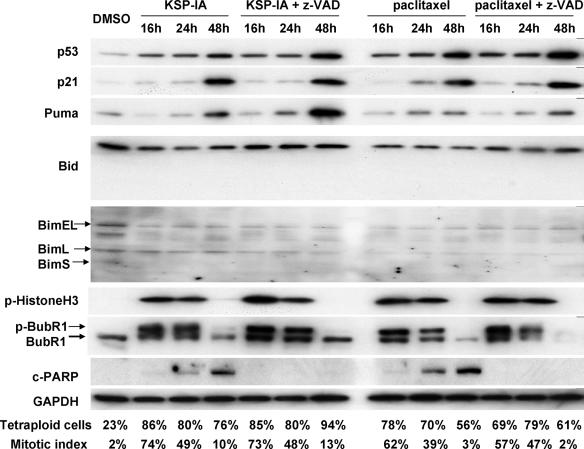
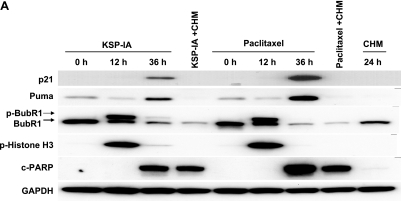
FIG. 4.
Expression of the BH3-only proteins, p53 and p21, after exposure to KSP-IA or paclitaxel and the influence of z-VAD on mitotic arrest and mitotic slippage. A2780 cells were treated with KSP-IA (300 nM) or paclitaxel (100 nM) in the presence or absence of z-VAD (200 μM) for 16, 24, or 48 h. Harvested cells were analyzed for cellular DNA content, mitotic index, the expression and phosphorylation of proteins as indicated, and caspase-3 activity (caspase-3-mediated cleavage of PARP [c-PARP]). The percentages of tetraploid cells (4N DNA content) and mitotic index are shown.
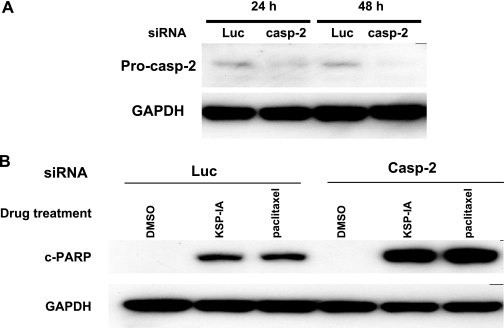
Caspase-2 is an initiator caspase. It was reported that in DNA damage-induced mitotic catastrophe, activation of caspase-2 was an initiating event upstream of the mitochondrial membrane permeabilization (4), which was responsible for inducing permeabilization of the mitochondrial membrane and activation of effector caspases. To further examine the role of caspase-2 in KSP-IA-induced cell death, we assessed KSP-IA-mediated caspase-3 activation after knocking down caspase-2 by siRNA. We observed that knockdown of caspase-2 by siRNA did not affect KSP-IA-induced activation of caspase-3 (Fig. 3), suggesting that activation of caspase-2 is not an initiating event for KSP-IA-mediated cell death. This is consistent with our results obtained by using z-VAD to inhibit caspases.
FIG. 3.
siRNA-mediated knockdown of caspase-2 does not affect KSP-IA- and paclitaxel-induced activation of caspase-3. (A) A2780 cells were transfected with siRNAs against either luciferase (Luc) as a control or human caspase-2 (casp-2). Twenty-four and 48 h after transfection, the levels of expression of procaspase-2 (Pro-casp-2) were determined by Western blotting. GAPDH levels were monitored as a loading control. (B) Twenty-four hours after transfection with either luciferase (control) or caspase-2 siRNAs, A2780 cells were treated with DMSO (vehicle), KSP-IA (300 nM), or paclitaxel (100 nM) for 24 h. Caspase-3-mediated cleavage of PARP (c-PARP) was determined by Western blotting.
Together, the data indicate that KSP-IA induces apoptosis through the intrinsic apoptotic pathway and that Bax activation is an apical step prior to the activation of caspases during this process.
KSP-IA causes activation of Bax independently of de novo protein synthesis.
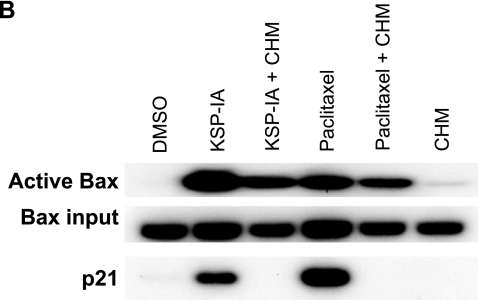
The BH3-only proteins and p53 have been shown to be able to induce Bax activation in response to various death stimuli (6, 25). To further explore how KSP-IA activates Bax, thereby triggering apoptosis, we determined the steady-state levels of the BH3-only proteins Bim, Bid, and Puma, as well as p53 and p21 (a p53-inducible gene) after exposure to KSP-IA or paclitaxel for different times in A2780 and HCT116 cells that contain wild-type p53 and a competent spindle checkpoint. Among these BH3-only proteins, Bim has been reported to be involved in the microtubule inhibitor paclitaxel-induced cell death under some circumstances (20, 35), and Puma is a p53 target gene and a major mediator of p53-induced lethality (15, 25). Since z-VAD inhibits only caspase-mediated execution of apoptosis, not the apical signaling which leads to Bax activation, we included z-VAD in KSP-IA and paclitaxel treatment to prevent potential nonspecific protein degradation caused by activated caspases as a comparison for treatment with KSP-IA or paclitaxel alone. Immunoblotting analysis reveals that, while a prolonged exposure to KSP-IA or paclitaxel activates caspase-3, neither alterations in the steady-state levels of Bim or Bid nor induction of truncated Bid was detected in A2780 cells (Fig. 4) or HCT116 cells (data not shown). To preclude the limitation in antibody specificities, we used at least two different antibodies to detect each protein, which were proved to be able to hybridize with all isoforms of Bim and Bid, including truncated Bid, respectively. A mild increase in the levels of p53 and an induction of its target genes Puma and p21 were detected after 48 h of drug treatment, which correlates with caspase-3 activation (cleaved PARP) and mitotic slippage manifested by diminished p-histone H3 and a drop of mitotic index while maintaining a high tetraploid fraction (Fig. 4). This raises the question as to whether the induction of Puma following mitotic slippage is responsible for activating Bax and thereby initiating apoptosis in response to KSP-IA and paclitaxel. To address this issue, we treated A2780 cells with KSP-IA or paclitaxel in the absence or presence of cycloheximide, which blocks de novo protein synthesis, and then assessed the levels of Puma and p21 proteins, as well as caspase-3 activity. In the absence of cycloheximide, 36 h of exposure to KSP-IA or paclitaxel caused induction of Puma and p21, and activation of caspase-3, which occurred after mitotic exit judged by fading of p-histone H3 and p-BubR1 (Fig. 5A). Addition of cycloheximide 24 h prior to harvest (after 12 h of treatment with KSP-IA or paclitaxel to avoid potential interference of cell cycle progression by cycloheximide) abrogated the induction of Puma and p21, but it did not prevent KSP-IA- and paclitaxel-induced activation of caspase-3 and cycloheximide did not cause pronounced activation of caspase-3 by itself (Fig. 5A). Since 12-h exposure to KSP-IA or paclitaxel prior to the addition of cycloheximide caused only mitotic arrest, not the activation of Bax and caspase-3 (Fig. 5A), the data indicate that induced expression of Puma is dispensable for the induction of apoptosis and that KSP-IA and paclitaxel can trigger apoptosis in the absence of de novo protein synthesis. Consistently, abrogation of de novo protein synthesis by cycloheximide failed to negate KSP-IA- and paclitaxel-induced Bax activation (Fig. 5B). A partial reduction of activated Bax in the cells cotreated with KSP-IA or paclitaxel and cycloheximide may be due to a decreased input of total Bax (Fig. 5B). Cycloheximide may decrease the levels of total Bax by inhibiting protein synthesis, but it does not prevent KSP-IA- and paclitaxel-mediated Bax activation.
FIG. 5.
Suppression of de novo protein synthesis by cycloheximide does not abrogate KSP-IA- and paclitaxel-induced activation of Bax and caspase-3. (A) A2780 cells were either treated with KSP-IA or paclitaxel for 12 or 36 h in the absence of cycloheximide or were pretreated with KSP-IA or paclitaxel alone for 12 h and then continuously incubated with either of these compounds for another 24 h after the addition of cycloheximide (CHM) (20 μM). Cells treated with CHM alone for 24 h were also analyzed. The induction of Puma and p21 expression, caspase-3 activity (caspase-3-mediated cleavage of PARP [c-PARP]), and levels of p-BubR1 and p-histone H3 were determined by Western blotting. (B) Cells were treated with KSP-IA or paclitaxel for 36 h with or without the addition of cycloheximide as described above for panel A. Activation of Bax was determined. The expression of p21 was detected by Western blotting to confirm the suppression of de novo protein synthesis by cycloheximide.
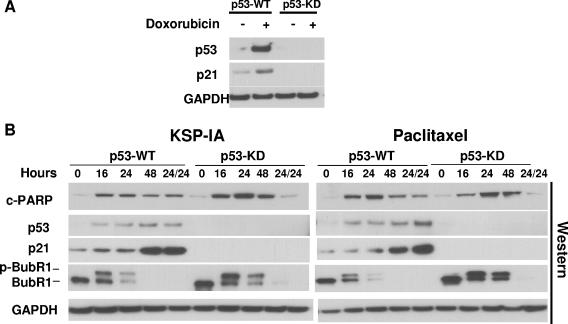
KSP-IA activates the spindle checkpoint and induces apoptosis independently of p53.
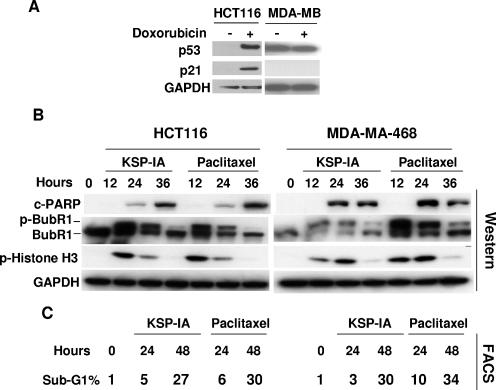
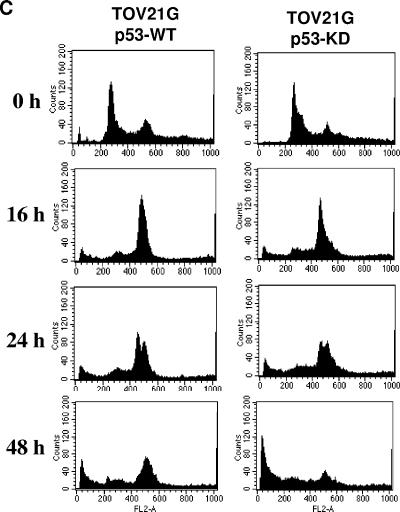
p53 can be stabilized by posttranslational modifications, and it may act like a BH3-only protein to activate Bax via protein-protein interactions, thereby inducing apoptosis through a transcription-independent mechanism (5, 18, 26). In this scenario, inhibition of de novo protein synthesis by cycloheximide may not be able to abrogate the transcription-independent lethal activity of p53. To more rigorously study the role of p53 in the induction of apoptosis by KSP-IA and paclitaxel, we compared KSP-IA- and paclitaxel-induced apoptosis between p53-proficient and -deficient cells. We found that several p53-deficient cell lines exhibited a similar, if not greater, susceptibility to KSP-IA- and paclitaxel-mediated killing to p53-intact cells that are ranked among KSP-IA- and paclitaxel-sensitive cell lines. For instance, HCT116 cells contain wild-type p53 and exhibit an intact p53 response, including transactivation of p21 expression (Fig. 6A) and cell cycle arrest (data not shown) after treatment with the DNA topoisomerase II inhibitor doxorubicin, whereas MDA-MA-468 cells that contain mutant p53 are defective in both p21 induction (Fig. 6A) and cell cycle arrest (data not shown). As shown in Fig. 6, both KSP-IA and paclitaxel activated caspase-3 in MDA-MB-468 cells as efficiently as in HCT116 cells, which resulted in similar extents of apoptosis between these cell lines (Fig. 6B and C). In addition, like HCT116 cells, MDA-MB-468 cells exhibited a competent spindle checkpoint function and mitotic arrest, which is consistent with our former observation that a competent spindle checkpoint is required for triggering apoptosis. This suggests that the sensitivity to KSP-IA and paclitaxel-mediated lethality is not affected by the p53 status. To preclude the potential influence of genetic heterogeneity on the KSP-IA responses of distinct cell lines, we determined KSP-IA-induced caspase-3 activity and cell death in matched pairs of TOV21G and A2780 cell lines in which the parental TOV21G and A2780 cell lines containing wild-type p53 were compared to the matched p53-deficient derivatives containing a p53-shRNA expression construct to knock down p53. The results obtained from matched TOV21G cell lines are shown in Fig. 7. In contrast to the parental cells containing wild-type p53 (p53-WT) and an empty vector, TOV21G derivatives expressing p53 shRNA (p53 knockdown [p53-KD]) exhibited neither p53 expression nor p21 induction after 16-h exposure to doxorubicin (Fig. 7A), confirming deficient p53 response in p53-KD cells. After incubation with either KSP-IA or paclitaxel for different intervals, the pattern of caspase-3 activation in p53-KD cells was similar to that of the parental p53-WT cells, whereas unlike p53-WT cells, p53-KD cells showed no induction of p53 and p21 expression (Fig. 7B). Moreover, p-BubR1 and mitotic arrest were induced in both p53-WT and p53-KD cells, consistent with the observation in MDA-MB-468 cells containing mutant p53. This confirms that p53 is not required for spindle checkpoint function (Fig. 6B and 7B and C). Further comparison of the extents of cell death by FACS analysis showed that even a greater fraction of subdiploid cells was detected in p53-KD than in p53-WT cells (Fig. 7C). Similar results on KSP-IA sensitivity were obtained from the A2780 matched cell lines with proficient and deficient p53 (see Fig. S1 in the supplemental material). Together, the data indicate that KSP-IA induces apoptosis in a p53-independent manner and that p53 is not required for spindle checkpoint function.
FIG. 6.
Comparison of KSP-IA- and paclitaxel-induced activation of spindle checkpoint and apoptosis in cells containing wild-type or mutant p53. (A) HCT116 cells (containing wild-type p53) and MDA-MB-468 cells (containing mutant p53) were treated with vehicle or doxorubicin (200 nM) (+) for 16 h. The steady-state levels of p53 and p21 were determined by Western blotting. GAPDH was included as a loading control. The mutant p53 defective for transactivation activity was detected in MDA-MB-468 cells in the absence (−) of doxorubicin. (B and C) HCT116 and MDA-MB-468 cells were treated with KSP-IA (300 nM) or paclitaxel (100 nM) for various times and then analyzed for caspase-3 activity (caspase-3-mediated cleavage of PARP [c-PARP]), spindle checkpoint activation (p-BubR1), mitotic arrest (p-histone H3), and cellular DNA content by Western blot and FACS analyses.
FIG. 7.
Comparison of KSP-IA- and paclitaxel-induced activation of spindle checkpoint and apoptosis in isogenic TOV21G cell lines containing wild-type p53 (p53-WT, containing an empty vector) and p53-knockdown (p53-KD, containing a p53 shRNA vector). (A) p53-WT and p53-KD cells were treated with doxorubicin (200 nM) (+) for 16 h and then analyzed for the expression of p53 and p21 by Western blotting. (B) p53-WT and p53-KD cells were treated with KSP-IA (300 nM) or paclitaxel (100 nM) for 16, 24, or 48 h and then harvested immediately or treated with these compounds for 24 h followed by an incubation in compound-free medium for another 24 h prior to harvest (labeled 24/24). Cells were analyzed for caspase-3 activity (caspase-3-mediated cleavage of PARP [c-PARP]), spindle checkpoint activation (p-BubR1), and the expression of p53 and p21 by Western blotting. (C) Both p53-WT and p53-KD cells were incubated with KSP-IA for 16, 24, or 48 h and then analyzed for cellular DNA content by FACS. Histograms of cell number versus DNA content are shown.
DISCUSSION
How KSP inhibitors and other antimitotic agents, such as taxanes, induce cell death has been enigmatic. The findings that KSP-IA causes cell death by activating Bax and caspases, two characteristic biochemical events in apoptosis, and that the pancaspase inhibitor z-VAD completely blocks KSP-IA-induced cell death clearly indicate that inhibition of KSP leads to caspase-dependent apoptosis (36) (Fig. 1). Since Bax activation could be either an early event upstream of caspase activation in the intrinsic apoptotic pathway or a late event subsequent to caspase activation in the extrinsic pathway (38), ordering these two biochemical events would be important to understanding how KSP-IA initiates apoptosis. The data presented in this paper demonstrate that KSP-IA activates Bax independently of caspase activity, indicating that Bax activation is an apical step upstream of caspase activation in KSP-IA-induced apoptosis (Fig. 2 and 3). Thus understanding how Bax is activated is critical to elucidating the mechanism by which KSP inhibitors trigger the apoptotic program. It has been shown that BH3-only proteins, such as Puma, Bim, and Bid, and p53 are the major inducers of Bax activation (6, 14, 25). While the BH3-only proteins activate Bax via protein-protein interactions, p53 is able to both induce Bax expression and interact with Bax directly (25). The proapoptotic activities of the BH3-only proteins are controlled by different modes, which can be classified as transcription-dependent expression and posttranslational modification. For instance, Puma is able to activate Bax upon induction of its expression, whereas Bid is activated by proteolytic cleavage and the phosphorylation of Bim controls its stability and subcellular localization (17, 30, 32, 35). Evaluation of the steady-state levels of Puma, Bid, and Bim shows that Puma was induced following mitotic slippage while neither truncated Bid nor significant alterations in the levels of Bid and Bim were detected (Fig. 4). Notably, suppression of de novo protein synthesis that eliminates the induction of Puma and p21 does not abrogate KSP-IA- and paclitaxel-induced activation of Bax and caspase-3 (Fig. 5). This indicates that KSP-IA and paclitaxel activates Bax through a posttranslational event independently of de novo protein synthesis or Puma induction. Since p53 can be stabilized via posttranslational modification and it may activate Bax by directly interacting with Bax like a BH3-only protein (5, 18, 26), we further determined its role in the induction of apoptosis by KSP-IA and paclitaxel. The former controversy over the role of p53 in drug-induced apoptosis largely stems from the fact that cell lines that differ not only in p53 status but also in genetic background were compared. To exclude the influence of genetic heterogeneity, we compared apoptosis between matched pairs of cell lines that contain either proficient or deficient p53 in addition to the cell lines from different origins. Our data indicate that p53 is dispensable for KSP-IA- and paclitaxel-induced caspase-3 activation and apoptosis (Fig. 6 and 7).
It was shown previously that the optimal activation of Bax and induction of apoptosis by KSP-IA requires a proficient spindle checkpoint and couples with mitotic slippage, suggesting that mitotic slippage by overriding an activated spindle checkpoint is responsible for activating Bax and initiating apoptosis (36). Although mitotic slippage could activate the p53-dependent tetraploid checkpoint and induce gene expression (23), this report shows that activation of Bax does not rely on p53 or de novo protein synthesis, suggesting that upon mitotic slippage following prolonged mitotic arrest, there is a posttranslational event that results in Bax activation. Several reports have suggested that Bim may be involved in mediating paclitaxel-induced lethality under certain circumstances (3, 20, 35). The proapoptotic activity of Bim can be regulated posttranslationally. Under normal conditions, Bim is sequestered by association with microtubules via binding to the LC8 light chain of the dynein motor protein (31). Certain death stimuli may cause the release of Bim from dynein by inducing Bim phosphorylation, and thereby, the unleashed Bim induces Bax-dependent apoptosis (17, 30, 31). It would be interesting to determine the association between Bim and dynein/microtubules as well as the phosphorylation status of Bim after KSP-IA treatment, especially prior to and after mitotic slippage in spindle checkpoint-proficient cells. In addition, a proteomic analysis of the BH3-only proteins, other potential Bax activators, and the spindle checkpoint components prior to and after mitotic slippage could provide further insights into the mechanism underlying KSP-IA-triggered Bax activation.
Since KSP-IA kills cancer cells in a p53-independent manner, KSP inhibitors should be active and efficacious in p53-deficient tumors that are frequently seen in the clinic.
Supplementary Material
Acknowledgments
We thank Michele Cleary, Michael Carleton, and Peter Linsley for providing paired TOV21G and A2780 isogenic cell lines containing either an empty vector or a p53 shRNA construct.
Footnotes
Published ahead of print on 13 November 2006.
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Bartz, S. R., Z. Zhang, J. Burchard, M. Imakura, M. Martin, A. Palmieri, R. Needham, J. Guo, M. Gordon, N. Chung, P. Warrener, A. L. Jackson, M. Carleton, M. Oatley, L. Locco, F. Santini, T. Smith, P. Kunapuli, M. Ferrer, B. Strulovici, S. H. Friend, and P. S. Linstey. 2006. Small interfering, RNA screens reveal enhanced cisplatin cytotoxicity in tumor cells having both BRCA and TP53 disruptions. Mol. Cell. Biol. 26:9377-9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1a.Blagosklonny, M. V., and T. Fojo. 1999. Molecular effects of paclitaxel: myths and reality (a critical review). Int. J. Cancer 83:151-156. [DOI] [PubMed] [Google Scholar]
- 2.Blangy, A., H. A. Lane, P. d'Herin, M. Harper, M. Kress, and E. A. Nigg. 1995. Phosphorylation by p34cdc2 regulates spindle association of human Eg5, a kinesin-related motor essential for bipolar spindle formation in vivo. Cell 83:1159-1169. [DOI] [PubMed] [Google Scholar]
- 3.Bouillet, P., D. Metcalf, D. C. Huang, D. M. Tarlinton, T. W. Kay, F. Kontgen, J. M. Adams, and A. Strasser. 1999. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science 286:1735-1738. [DOI] [PubMed] [Google Scholar]
- 4.Castedo, M., J. L. Perfettini, T. Roumier, A. Valent, H. Raslova, K. Yakushijin, D. Horne, J. Feunteun, G. Lenoir, R. Medema, W. Vainchenker, and G. Kroemer. 2004. Mitotic catastrophe constitutes a special case of apoptosis whose suppression entails aneuploidy. Oncogene 23:4362-4370. [DOI] [PubMed] [Google Scholar]
- 5.Chipuk, J. E., T. Kuwana, L. Bouchier-Hayes, N. M. Droin, D. D. Newmeyer, M. Schuler, and D. R. Green. 2004. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science 303:1010-1014. [DOI] [PubMed] [Google Scholar]
- 6.Cory, S., and J. M. Adams. 2002. The Bcl2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 7.Crosio, C., G. M. Fimia, R. Loury, M. Kimura, Y. Okano, H. Zhou, S. Sen, C. D. Allis, and P. Sassone-Corsi. 2002. Mitotic phosphorylation of histone H3: spatio-temporal regulation by mammalian Aurora kinases. Mol. Cell. Biol. 22:874-885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagenbach, E. M., and S. A. Endow. 2004. A new kinesin tree. J. Cell Sci. 117:3-7. [DOI] [PubMed] [Google Scholar]
- 9.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 10.Davis, F. M., T. Y. Tsao, S. K. Fowler, and P. N. Rao. 1983. Monoclonal antibodies to mitotic cells. Proc. Natl. Acad. Sci. USA 80:2926-2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Enos, A. P., and N. R. Morris. 1990. Mutation of a gene that encodes a kinesin-like protein blocks nuclear division in A. nidulans. Cell 60:1019-1027. [DOI] [PubMed] [Google Scholar]
- 12.Hasegawa, S., Y. Miyoshi, C. Egawa, M. Ishitobi, T. Taguchi, Y. Tamaki, M. Monden, and S. Noguchi. 2003. Prediction of response to docetaxel by quantitative analysis of class I and III beta-tubulin isotype mRNA expression in human breast cancers. Clin. Cancer Res. 9:2992-2997. [PubMed] [Google Scholar]
- 13.Hsu, Y. T., and R. J. Youle. 1998. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J. Biol. Chem. 273:10777-10783. [DOI] [PubMed] [Google Scholar]
- 14.Huang, D. C., and A. Strasser. 2000. BH3-only proteins—essential initiators of apoptotic cell death. Cell 103:839-842. [DOI] [PubMed] [Google Scholar]
- 15.Jeffers, J. R., E. Parganas, Y. Lee, C. Yang, J. Wang, J. Brennan, K. H. MacLean, J. Han, T. Chittenden, J. N. Ihle, P. J. McKinnon, J. L. Cleveland, and G. P. Zambetti. 2003. Puma is an essential mediator of p53-dependent and -independent apoptotic pathways. Cancer Cell 4:321-328. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor, T. M., T. U. Mayer, M. L. Coughlin, and T. J. Mitchison. 2000. Probing spindle assembly mechanisms with monastrol, a small molecule inhibitor of the mitotic kinesin, Eg5. J. Cell Biol. 150:975-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lei, K., and R. J. Davis. 2003. JNK phosphorylation of Bim-related members of the Bcl2 family induces Bax-dependent apoptosis. Proc. Natl. Acad. Sci. USA 100:2432-2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leu, J. I., P. Dumont, M. Hafey, M. E. Murphy, and D. L. George. 2004. Mitochondrial p53 activates Bak and causes disruption of a Bak-Mcl1 complex. Nat. Cell Biol. 6:443-450. [DOI] [PubMed] [Google Scholar]
- 19.Li, H., H. Zhu, C. J. Xu, and J. Yuan. 1998. Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94:491-501. [DOI] [PubMed] [Google Scholar]
- 20.Li, R., T. Moudgil, H. J. Ross, and H. M. Hu. 2005. Apoptosis of non-small-cell lung cancer cell lines after paclitaxel treatment involves the BH3-only proapoptotic protein Bim. Cell Death Differ. 12:292-303. [DOI] [PubMed] [Google Scholar]
- 21.Li, W., Z. Lan, H. Wu, S. Wu, J. Meadows, J. Chen, V. Zhu, and W. Dai. 1999. BUBR1 phosphorylation is regulated during mitotic checkpoint activation. Cell Growth Differ. 10:769-775. [PubMed] [Google Scholar]
- 22.Luo, X., I. Budihardjo, H. Zou, C. Slaughter, and X. Wang. 1998. Bid, a Bcl2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94:481-490. [DOI] [PubMed] [Google Scholar]
- 23.Margolis, R. L., O. D. Lohez, and P. R. Andreassen. 2003. G1 tetraploidy checkpoint and the suppression of tumorigenesis. J. Cell Biochem. 88:673-683. [DOI] [PubMed] [Google Scholar]
- 24.Mayer, T. U., T. M. Kapoor, S. J. Haggarty, R. W. King, S. L. Schreiber, and T. J. Mitchison. 1999. Small molecule inhibitor of mitotic spindle bipolarity identified in a phenotype-based screen. Science 286:971-974. [DOI] [PubMed] [Google Scholar]
- 25.Michalak, E., A. Villunger, M. Erlacher, and A. Strasser. 2005. Death squads enlisted by the tumour suppressor p53. Biochem. Biophys. Res. Commun. 331:786-798. [DOI] [PubMed] [Google Scholar]
- 26.Mihara, M., S. Erster, A. Zaika, O. Petrenko, T. Chittenden, P. Pancoska, and U. M. Moll. 2003. p53 has a direct apoptogenic role at the mitochondria. Mol. Cell 11:577-590. [DOI] [PubMed] [Google Scholar]
- 27.Moll, U. M., S. Wolff, D. Speidel, and W. Deppert. 2005. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 17:631-636. [DOI] [PubMed] [Google Scholar]
- 28.Monzo, M., R. Rosell, J. J. Sanchez, J. S. Lee, A. O'Brate, J. L. Gonzalez-Larriba, V. Alberola, J. C. Lorenzo, L. Nunez, J. Y. Ro, and C. Martin. 1999. Paclitaxel resistance in non-small-cell lung cancer associated with beta-tubulin gene mutations. J. Clin. Oncol. 17:1786-1793. [DOI] [PubMed] [Google Scholar]
- 29.Nechushtan, A., C. L. Smith, Y. T. Hsu, and R. J. Youle. 1999. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 18:2330-2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Putcha, G. V., S. Le, S. Frank, C. G. Besirli, K. Clark, B. Chu, S. Alix, R. J. Youle, A. LaMarche, A. C. Maroney, and E. M. Johnson, Jr. 2003. JNK-mediated BIM phosphorylation potentiates BAX-dependent apoptosis. Neuron 38:899-914. [DOI] [PubMed] [Google Scholar]
- 31.Puthalakath, H., D. C. Huang, L. A. O'Reilly, S. M. King, and A. Strasser. 1999. The proapoptotic activity of the Bcl-2 family member Bim is regulated by interaction with the dynein motor complex. Mol. Cell 3:287-296. [DOI] [PubMed] [Google Scholar]
- 32.Puthalakath, H., and A. Strasser. 2002. Keeping killers on a tight leash: transcriptional and post-translational control of the pro-apoptotic activity of BH3-only proteins. Cell Death Differ. 9:505-512. [DOI] [PubMed] [Google Scholar]
- 33.Rouzier, R., R. Rajan, P. Wagner, K. R. Hess, D. L. Gold, J. Stec, M. Ayers, J. S. Ross, P. Zhang, T. A. Buchholz, H. Kuerer, M. Green, B. Arun, G. N. Hortobagyi, W. F. Symmans, and L. Pusztai. 2005. Microtubule-associated protein tau: a marker of paclitaxel sensitivity in breast cancer. Proc. Natl. Acad. Sci. USA 102:8315-8320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sawin, K. E., and T. J. Mitchison. 1995. Mutations in the kinesin-like protein Eg5 disrupting localization to the mitotic spindle. Proc. Natl. Acad. Sci. USA 92:4289-4293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tan, T. T., K. Degenhardt, D. A. Nelson, B. Beaudoin, W. Nieves-Neira, P. Bouillet, A. Villunger, J. M. Adams, and E. White. 2005. Key roles of BIM-driven apoptosis in epithelial tumors and rational chemotherapy. Cancer Cell 7:227-238. [DOI] [PubMed] [Google Scholar]
- 36.Tao, W., V. J. South, Y. Zhang, J. P. Davide, L. Farrell, N. E. Kohl, L. Sepp-Lorenzino, and R. B. Lobell. 2005. Induction of apoptosis by an inhibitor of the mitotic kinesin KSP requires both activation of the spindle assembly checkpoint and mitotic slippage. Cancer Cell 8:49-59. [DOI] [PubMed] [Google Scholar]
- 37.Thorburn, A. 2004. Death receptor-induced cell killing. Cell Signal. 16:139-144. [DOI] [PubMed] [Google Scholar]
- 38.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933. [PubMed] [Google Scholar]
- 39.Wei, M. C., W. X. Zong, E. H. Cheng, T. Lindsten, V. Panoutsakopoulou, A. J. Ross, K. A. Roth, G. R. MacGregor, C. B. Thompson, and S. J. Korsmeyer. 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292:727-730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wolter, K. G., Y. T. Hsu, C. L. Smith, A. Nechushtan, X. G. Xi, and R. J. Youle. 1997. Movement of Bax from the cytosol to mitochondria during apoptosis. J. Cell Biol. 139:1281-1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.