Abstract
The import of mitochondrial preproteins requires an electric potential across the inner membrane and the hydrolysis of ATP in the matrix. We assessed the contributions of the two energy sources to the translocation driving force responsible for movement of the polypeptide chain through the translocation channel and the unfolding of preprotein domains. The import-driving activity was directly analyzed by the determination of the protease resistances of saturating amounts of membrane-spanning translocation intermediates. The ability to generate a strong translocation-driving force was solely dependent on the activity of the ATP-dependent import motor complex in the matrix. For a sustained import-driving activity on the preprotein in transit, an unstructured N-terminal segment of more than 70 to 80 amino acid residues was required. The electric potential of the inner membrane was required to maintain the import-driving activity at a high level. The electrophoretic force of the potential exhibited only a limited capacity to unfold preprotein domains. We conclude that the membrane potential increases the probability of a dynamic interaction of the preprotein with the import motor. Polypeptide translocation and unfolding are mainly driven by the inward-directed translocation activity based on the functional cooperation of the import motor components.
Although mitochondria can synthesize a small subset of proteins on their own, the vast majority have to be imported after their synthesis in the cytosol (11, 23, 36, 40). The precursor proteins are transported through specific translocase complexes residing in the outer and inner membranes (21, 37). However, the relatively small inner diameter of the translocation channels presents a major constraint for the import process (17, 49). Experimental evidence indicates that preproteins destined for the mitochondrial matrix cross the membranes in an extended conformation (42). Posttranslational import of mitochondrial preproteins therefore requires the unfolding of folded preprotein domains prior to or during the translocation reaction.
Two mitochondrial energy sources are responsible for both vectorial movement of the precursor polypeptide in the translocation channel and unfolding of preproteins. The insertion of the amino-terminal segment of the preprotein into the inner membrane is dependent on the electric potential (Δψ) across the inner membrane. It is thought that the potential exerts an electrophoretic force on the positively charged N-terminal targeting sequence (29). In addition, the Δψ has been shown to be involved in the function of the TIM23 protein complex (2). The full translocation of the polypeptide chain requires a second energy source, the hydrolysis of matrix ATP. The enzymatic machinery that couples preprotein translocation and ATP hydrolysis is provided by the import motor complex, which is located at the inner face of the inner membrane in the direct vicinity of the TIM23 translocation channel (36, 39, 48, 55). Its core component is the main mitochondrial chaperone of the Hsp70 family (mtHsp70), named Ssc1 in the yeast Saccharomyces cerevisiae. mtHsp70 drives the translocation of the preprotein by an ATP-dependent interaction with the incoming polypeptide chain (5, 13, 22, 51). The chaperone is anchored at the inner membrane by a specific interaction with the translocase component Tim44. The cochaperone Mge1 acts as a nucleotide exchange factor for mtHsp70 and enhances its ATPase activity. The mode of action of the mtHsp70 import motor is not entirely clear. It is conceivable that the import motor drives polypeptide translocation by a combination of a passive ratchet and an active pulling mechanism (31, 36). Recently, two novel membrane-associated mtHsp70 cochaperones of the DnaJ family, Pam18 and Pam16, were identified (8, 12, 24, 34, 50). Both of them regulate the activity of mtHsp70 and are required for efficient preprotein translocation (9, 25). The high number of essential components with many different molecular activities demonstrates that mitochondrial-protein import under in vivo conditions is a highly complicated process. Even the question of how the mitochondrial energy sources, membrane potential and matrix ATP, cooperate to drive preprotein import and unfolding is still not entirely clear. The mtHsp70 import motor has been shown to participate significantly in preprotein unfolding, decreasing the activation barrier of the unfolding reaction and changing unfolding pathways (20, 26, 31). However, it has been proposed that the electric potential across the inner membrane provides a driving force for translocation that contributes directly to the unfolding of preproteins on the cytosolic side of the outer membrane (19).
A characterization of the unfolding reaction can provide only indirect evidence about the relative contributions of both membrane potential and the ATP-dependent import motor to the generation of the import-driving activity. To address this question, we dissected the import reaction into specific steps, allowing the direct analysis of the translocation-driving force precursor proteins in transit. The stabilization of the folding state of precursor proteins has been shown to restrict or even inhibit the translocation reaction. Hence, the addition of a tightly bound ligand to a C-terminal dihydrofolate reductase (DHFR) domain can efficiently prevent the membrane transport of precursor-DHFR fusion proteins (10, 41). Since N-terminal parts of the preprotein are still able to insert into the translocase complexes and reach the matrix compartment, these membrane-spanning intermediates represent a critical step of the translocation reaction (4). Due to the close apposition of the methotrexate (MTX)-stabilized DHFR and the components of the outer membrane, the accumulated translocation intermediates can become inaccessible to degradation by external proteases (3, 52). Using saturating amounts of recombinant preproteins, we directly compared the roles of the inner membrane potential and matrix ATP to the import-driving activity. In contrast to the ATP-dependent import motor, the membrane potential was not able to confer an active translocation force and provided only a limited unfolding activity for the folded preprotein domain. However, maintaining a membrane potential was essential in holding preproteins in the translocation channel and supporting the ATP-dependent translocation force generated by the mtHsp70 import motor.
MATERIALS AND METHODS
Isolation of mitochondria and import of precursor proteins.
Unless otherwise stated, Saccharomyces cerevisiae strains were grown at 30°C on YPG medium (1% yeast extract, 2% Bacto peptone, and 3% glycerol). Mitochondria were isolated according to established procedures (43). The following yeast strains were used. PK81 (MATα ade2-101 lys2 ura3-52 trp1 leu2-3,112 ssc1-2::LEU2), PK82 (MATα his4-713 lys2 ura3-52 trp1 leu2-3), PK83 (MATα ade2-101 lys2 ura3-52 trp1 leu2-3,112 ssc1-3::LEU2), MB6 (MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 tim44D::LYS2 pTIM44), and tim44-8 (MATα ade2-101 his3-Δ200 leu2-Δ1 lys2-801 tim44D::LYS2 ptim44-8) (3, 13). Recombinant precursor proteins were purified from Escherichia coli cells and added to a final concentration of 25 pmol per 25 μg of mitochondrial protein (7, 26). Radiolabeled preproteins were generated by in vitro transcription and translation in rabbit reticulocyte lysate as described previously (56). Import into isolated yeast mitochondria in the presence of 2 mM NADH, 2 mM ATP, and an ATP-regenerating system (5 mM creatine phosphate and 0.1 mg/ml creatine kinase) at 25°C and subsequent treatment with proteinase K on ice were performed essentially as described previously (43). In conditional-mutant strains, the isolated mitochondria were heat shocked prior to import for 15 min at 37°C to induce nonpermissive conditions. The import buffer contained 3% (wt/vol) bovine serum albumin, 250 mM sucrose, 80 mM KCl, 5 mM MgCl2, 5 mM KPi, and 10 mM MOPS (morpholinepropanesulfonic acid)-KOH, pH 7.2. Heme (final concentration, 50 μM) was added to the import buffer as indicated. Heme stock solution (1 mM) was prepared in 90% (vol/vol) ethylene glycol, 2 mM Tris-HCl, pH 8.2, 5 mM KCl. The Δψ was dissipated by addition of 1 μM valinomycin, 8 μM antimycin, and 20 μM oligomycin. For a gradual decrease the Δψ, the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) was added at the indicated concentrations in the presence of 20 μM oligomycin. Samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) analysis, followed by digital autoradiography or immunodecoration with DHFR-specific antibodies.
Assay to assess an import-driving activity.
Cytochrome b2-DHFR fusion proteins were arrested as translocation intermediates spanning both mitochondrial membranes by pretreating the precursors with 1 μM MTX for 5 min at 0°C. After import, mitochondria were reisolated and resuspended in import buffer. In some experiments, matrix ATP levels were reduced by treating mitochondria with 20 μM oligomycin and 10 U/ml apyrase for 5 min at 0°C. The membrane potential was dissipated by addition of 1 μM valinomycin, and mitochondria were incubated for up to several min at 25°C. The residual import force exerted on the translocation intermediate was assayed by resistance against treatment with 50 μg/ml proteinase K. Samples were directly analyzed by SDS-PAGE and Western blotting using antibodies directed against DHFR. In order to monitor the release of preproteins from the translocation channel, translocation intermediates were generated as described above. After reisolation, mitochondria were resuspended in import buffer and incubated in the presence or absence of 1 μM valinomycin to deplete the inner membrane potential. Mitochondria were centrifuged for 12 min at 15,000 × g and 4°C. The pellet containing mitochondrial proteins and associated precursor proteins was analyzed by SDS-PAGE.
Generation of the TOM-TIM supercomplex and BN-PAGE.
For the accumulation of preproteins in the import sites, isolated mitochondria were incubated for 15 min at 25°C with recombinant purified cytochrome b2-DHFR fusion proteins (final concentration, 4 μg of preprotein/100 μg of mitochondria) in the presence of 5 μM MTX, unless otherwise stated. After reisolation, the mitochondria were washed with 250 mM sucrose, 1 mM EDTA, and 10 mM MOPS-KOH, pH 7.2, and lysed in ice-cold digitonin buffer (1.5% [wt/vol] digitonin, 20 mM Tris-HCl, pH 7.4, 0.1 mM EDTA, 50 mM NaCl, 10% [vol/vol] glycerol, and 1 mM phenylmethylsulfonyl fluoride). To deplete ATP, mitochondria were pretreated with 20 μM oligomycin and 10 U/ml apyrase prior to incubation with preproteins in the presence of 2 mM NADH. Soluble material was analyzed by blue-native (BN)-PAGE essentially as described previously (7, 44). Mitochondrial proteins (70 μg) were solubilized in 50 μl ice-cold 1.5% digitonin-containing buffer and resolved on a 6 to 13% gradient gel at 4°C. Protein complexes were transferred to polyvinylidene difluoride membranes and decorated with specific antibodies. For detection of the TOM-TIM supercomplex, an antibody directed against the N-terminal portion of Tim23 was used in all experiments. The High Molecular Weight Calibration Kit for native electrophoresis (GE Healthcare) was used as a molecular weight standard.
Miscellaneous.
Cytochrome b2-DHFR fusion proteins were expressed and purified as published previously (7, 26). In some figures, nonrelevant gel lanes were excised by digital treatment. SDS-PAGE and immunodecoration, using the ECL system (GE Healthcare), were performed according to standard procedures. Quantifications were performed by scanning densitometry or digital autoradiography.
RESULTS
Direct assessment of an inward-directed import-driving activity using the protease resistance of accumulated translocation intermediates.
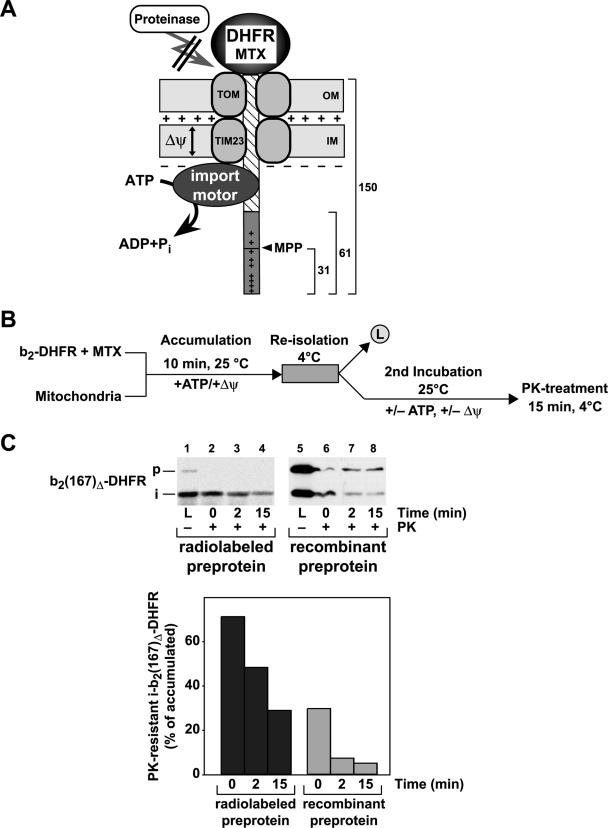
The preprotein b2(167)Δ-DHFR, containing the first 167 amino acids (aa) of cytochrome b2 fused to the entire DHFR from mouse, was used to accumulate membrane-spanning translocation intermediates in the presence of the DHFR-ligand MTX (Fig. 1A). Due to the deletion of the intermembrane space sorting signal in the presequence of cytochrome b2 (amino acids 49 to 65), the preprotein b2(167)Δ-DHFR is targeted to the mitochondrial matrix. While the stably folded DHFR domain restricts full translocation, the amino-terminal segment of the preprotein, consisting of the presequence (61 aa) and the first 89 aa of the mature part of cytochrome b2, can engage the translocation machinery and reach the matrix compartment. Previous experiments indicated that about 50 aa in a stretched conformation are sufficient to span both mitochondrial membranes (42). The MTX translocation intermediate of b2(167)Δ-DHFR therefore exposed a sufficiently large segment of the matrix to interact with the import motor complex and to be processed by the matrix-processing peptidase (MPP) (Fig. 1A). Due to the intrinsic protease resistance of the MTX-stabilized DHFR domain, the outer membrane translocase components restrict access of externally added protease to the precursor protein in case of an active translocation reaction. Therefore, after reisolation of the mitochondria to remove excess preproteins, the protease resistances of the translocation intermediates were assayed under different conditions to assess the import-driving activity acting on the preprotein polypeptide in transit (Fig. 1B).
FIG. 1.
Assay used to determine the inward-directed translocation force in mitochondria. (A) Schematic drawing of the translocation-driving activity assay. The MTX-stabilized DHFR domain blocks the import of the preprotein b2(167)Δ-DHFR. The unstructured N-terminal segment of the preprotein is inserted into the import channel and can interact with the import motor if it crosses both mitochondrial membranes. The generation of an inward-directed translocation force prevents the degradation of the preprotein in transit by external proteases. The MPP cleaves off the first 31 amino acids in the presequence of cytochrome b2 as indicated. The locations of the positively charged amino acids in the presequence are indicated (+). (B) Diagram of the experimental procedure. The cytochrome b2-DHFR fusion proteins are preincubated with the specific ligand MTX. In the presence of ATP and the membrane potential, the preprotein in its MTX-stabilized state is bound to the mitochondria. Preproteins that are not inserted in the import channel are removed by a reisolation step. Subsequently, the load sample (L) is taken and the matrix ATP or inner Δψ is depleted or sustained. Samples are taken over time and subsequently treated with proteinase K. (C) Protease resistance of b2(167)Δ-DHFR translocation intermediates. Radiolabeled preproteins generated by in vitro translation and purified recombinant preproteins were stabilized by MTX and incubated with wild-type mitochondria (p, precursor; i, processing intermediate). PK was added at the indicated time points in the absence of a Δψ. The amount of the remaining fusion proteins bound to mitochondria was determined by autoradiography or by Western blotting, using antibodies against DHFR. The value obtained for the accumulated i-b2(167)Δ-DHFR (L) in the absence of PK was set to 100%.
In the vast majority of import studies, mitochondrial preproteins were generated in a radiolabeled form by cell-free translation systems. While radiolabeled preproteins are usually imported in substoichiometric amounts, the import sites can be saturated by using recombinant preproteins purified from Escherichia coli cells (7). To obtain information about the number of preproteins in transit that are subjected to a strong import force, we directly compared the radiolabeled preprotein with the identical precursor purified as a recombinant preprotein in large amounts from E. coli cells. Under the conditions used, a large part of the accumulated radiolabeled translocation intermediates, up to 70%, remained resistant to external protease, indicating an efficient generation of import-driving activity in wild-type mitochondria (Fig. 1C). When we used the purified recombinant preprotein at saturation concentrations, MTX translocation intermediates were accumulated efficiently even in the absence of any cytosolic protein components (Fig. 1C). Between 20 and 25 pmol of precursor per mg mitochondrial protein were found as intermediates, representing the approximate number of TIM23 translocation channels (7). However, after dissipation of the membrane potential and protease treatment, the amount of protease-resistant material was significantly smaller, reaching only between 25% and 35% of the initially accumulated form. Hence, only a subset of the polypeptides in transit are actually subjected to an active translocation force despite being fully inserted into the translocation channel. However, with both types of precursor proteins, the protease-resistant material decreased to background levels after prolonged incubation times. We conclude that the import force assay represents a dynamic reaction in which the amount of protease-resistant material reflected the equilibrium between the generation of an inward-directed import activity and the retrograde release of preproteins from the translocation channel. In order to obtain quantitative data about the import-driving activity in mitochondria, all further experiments were performed using saturating amounts of recombinant preproteins.
The ATP-dependent import motor is crucial for the generation of an import-driving activity.
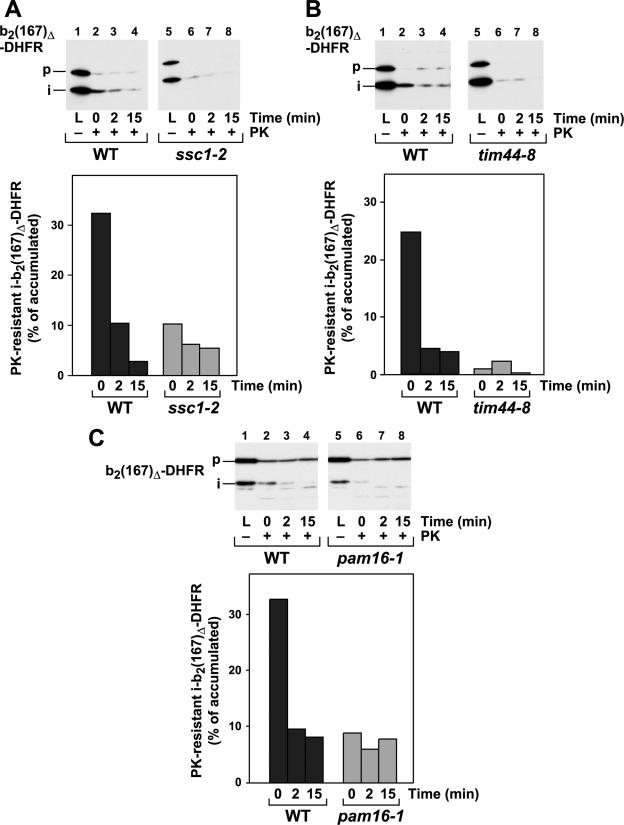
The mitochondrial import motor complex with its core component, mtHsp70, the sole ATPase required for the import reaction, couples ATP hydrolysis with the preprotein translocation reaction. To directly determine the contribution of the mtHsp70 import motor to the generation of the import-driving activity, we assayed the stabilities of the MTX translocation intermediates in mitochondria from different temperature-sensitive mutant yeast strains that are defective in different components of the motor complex. In the ssc1-2 strain, the mtHsp70 protein carries the mutation P419S in the peptide-binding domain, resulting in a stabilized interaction with substrate proteins and a folding defect (28). Mitochondria isolated from ssc1-2 exhibit a residual import activity that is limited to unfolded preproteins (13, 22, 52). Although the ssc1-2 mutant mitochondria were able to accumulate translocation intermediates in amounts comparable to those of wild-type mitochondria, their resistance to external protease was strongly reduced (Fig. 2A). Less than 10% of the accumulated preproteins were detected after the protease treatment. The preprotein translocation function of mtHsp70 is performed in close collaboration with the inner membrane protein Tim44. Hence, we tested the influence of Tim44 on the generation of an import force by the analysis of the temperature-sensitive mutant tim44-8 (3). The behavior of the mutant Tim44 was identical to that of the mtHsp70 mutant, showing a strong defect in the resistance of accumulated translocation intermediates (Fig. 2B). The essential protein Pam16 has recently been shown to be important for the import-specific regulation of the mtHsp70 ATPase activity (12, 24, 25). Similar to mutants of the core components Ssc1 and Tim44, mitochondria isolated from the temperature-sensitive mutant pam16-1 showed a strongly reduced protease resistance of the accumulated translocation intermediates, indicating a strong defect in the generation of an import-driving force (Fig. 2C). Irrespective of the individual functions of the import motor complex components, all three proteins form a single functional unit localized at the inner face of the inner membrane that is directly required for the generation of an import-driving activity on preproteins in the translocation channel.
FIG. 2.
Matrix ATP levels and conditional mutants of the import motor complex determine the protease resistance of translocation intermediates. Experiments and quantifications were performed as described in the legend to Fig. 1. The reactions were performed with conditional-mutant mitochondria from the ssc1-2 (A), tim44-8 (B), and pam16-1 (C) strains. The temperature-sensitive phenotype was induced by incubation of the isolated mitochondria for 15 min at 37°C prior to the import reaction. The protease-resistant processed form of b2(167)Δ-DHFR was quantified, and the amount obtained in the absence of protease was set to 100%.
Generation of an import-driving activity is dependent on the properties of the preprotein.
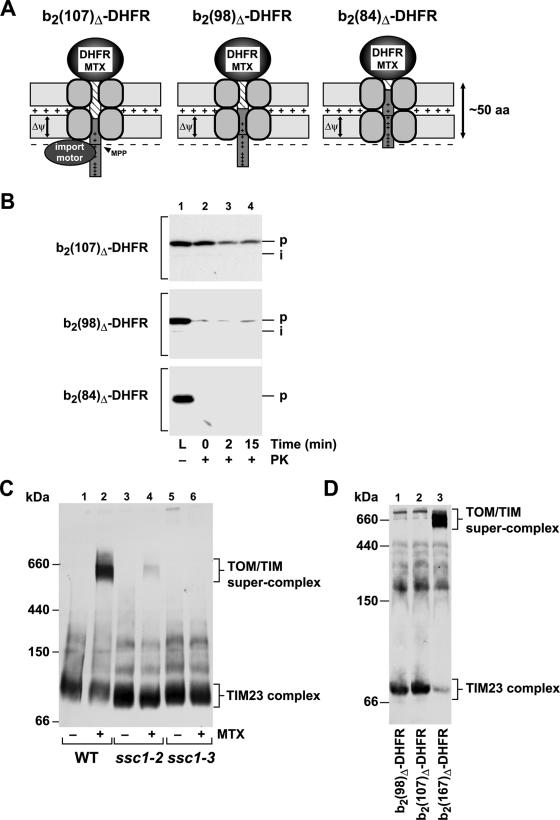
Since the generation of an inward-directed import activity is a crucial factor determining import rates, we asked if the functional interaction of the preprotein with the motor complex is the sole source of the translocation force. The interaction with mtHsp70 and the effect of the membrane potential on the preprotein in transit can be experimentally distinguished by varying the length of the N-terminal segment of the imported preprotein in front of the folded DHFR domain. For these experiments, we employed a set of specifically generated cytochrome b2-DHFR fusion proteins that contained N-terminal extensions clustered around the critical length of 50 aa required to reach the matrix compartment as translocation intermediates. The lengths of the unstructured N-terminal extensions of the following preproteins are given in parentheses: b2(107)Δ-DHFR (precursor, 92 aa; processed intermediate, 61 aa), b2(98)Δ-DHFR (precursor, 83 aa; processed intermediate, 52), and b2(84)Δ-DHFR (precursor, 72 aa; processed intermediate, 41 aa). The precursor forms of all three preproteins should be able to reach the matrix. However, the processing of the three precursor constructs is strongly reduced, since the processing site is not fully accessible (Fig. 3A). All preproteins were purified as recombinant precursors in large amounts and accumulated in wild-type mitochondria in the presence of MTX as described above. The preprotein b2(107)Δ-DHFR showed highly efficient accumulation of translocation intermediates. However, due to the shorter cytochrome b2 part, a strongly reduced processing efficiency was observed compared to the longer b2(167)Δ-DHFR (Fig. 3B). In fact, only the full-length precursor protein, and not the processed form, showed significant protease resistance that indicated the action of a translocation-driving activity. A further reduction of the N terminus by just nine residues, as in the preprotein b2(98)Δ-DHFR, completely abolished the protease resistance of the translocation intermediates. Finally, the preprotein b2(84)Δ-DHFR, which would expose about 20 residues in the matrix, was not processed by MPP, and no proteinase K (PK)-resistant signal could be detected, indicating the complete absence of an ATP-dependent import-driving activity. These results indicate that an efficient translocation force can be generated only if at least 30 to 40 residues are exposed at the matrix face of the inner membrane.
FIG. 3.
The length of the unstructured segment traversing both mitochondrial membranes is critical for the generation of an inward-directed translocation force. (A) Schematic graph of MTX-bound translocation intermediates of the preproteins b2(107)Δ-DHFR, b2(98)Δ-DHFR, and b2(84)Δ-DHFR traversing the mitochondrial membranes. Approximately 50 amino acids in an unstructured state are required to span both membranes. (B) The assay for an import-driving activity was performed as described in the legend to Fig. 1 with the cytochrome b2-DHFR fusion proteins shown in panel A. (C) Mitochondria from the wild-type (WT) and the temperature-sensitive mutant ssc1-2 and ssc1-3 strains were incubated with recombinant b2(167)Δ-DHFR in the presence or absence of MTX as described in Materials and Methods. Mitochondria were reisolated, lysed under native conditions, and analyzed by BN-PAGE. The translocase complexes were visualized by Western blotting and immunodecoration with antibodies directed against Tim23. Indicated are the molecular masses and the localizations of the protein complexes. (D) The formation of TOM-TIM supercomplexes was analyzed as described above using DHFR fusion proteins with the indicated length of the presequence and mature part derived from cytochrome b2.
The membrane-spanning translocation intermediates generated by stabilization of the DHFR domain have been shown to interact with both translocase complexes in the mitochondrial membranes, forming a large protein complex, the TOM-TIM supercomplex (4, 7, 45). This large protein complex is sufficiently stable to survive mild detergent treatment, as well as electrophoresis. Since formation of the supercomplex closely resembled the situation of the import force assay, we tested the components and energy sources required for supercomplex formation. After gentle solubilization of the mitochondria and separation of protein complexes by BN-PAGE, the TOM-TIM supercomplex could be detected by the presence of the channel component Tim23 in a high-molecular-mass complex of about 600 kDa. When we imported the fusion protein b2(167)Δ-DHFR in the presence of MTX into wild-type mitochondria, the TOM-TIM supercomplex was efficiently formed (Fig. 3C). As a control, in the absence of MTX, only the smaller TIM23 protein complex of the inner membrane, with a mass of about 90 kDa, was detected. Interestingly, the supercomplex was formed only when the import motor complex with its core component, mtHsp70, was fully functional, as demonstrated by the absence of the complex in mitochondria containing conditional-mutant forms of mtHsp70. In contrast to wild-type mitochondria, the partially import-active ssc1-2 mitochondria were able to form only a very small amount of supercomplex. The mutant ssc1-3 contains a mutation in the Hsp70 ATPase domain (G56S), rendering the protein inactive under nonpermissive conditions (13, 56). Interestingly, no signal corresponding to the TOM-TIM supercomplex could be detected in ssc1-3 mitochondria, indicating that a functional import motor is required for its formation. Previous studies established that the initial membrane potential-dependent insertion of preproteins into the translocation channels is not affected in mutant mitochondria defective in mtHsp70 (Ssc1) (13, 56). In addition, the preprotein release experiment (Fig. 4C) confirmed that wild-type and ssc1-3 mitochondria contain similar amounts of membrane-inserted preproteins. The formation of the supercomplex is therefore an mtHsp70-mediated process and requires an active translocation force. The activity of the inner membrane potential alone was not sufficient for supercomplex formation. As the generation of an import-driving activity was strongly dependent on the lengths of the N-terminal segments of the preproteins, we also tested the abilities of the different preprotein constructs to form the TOM-TIM supercomplex. While the standard protein b2(167)Δ-DHFR resulted in an almost quantitative accumulation of supercomplex, no signal for a 600-kDa complex was observed with the slightly smaller preproteins b2(107)Δ-DHFR and b2(98)Δ-DHFR (Fig. 3D). Although the precursor form of b2(107)Δ-DHFR was actively pulled across the mitochondrial membranes, the translocation force as such was not sufficient for the formation of a TOM-TIM supercomplex that is stable over prolonged experiment times. We conclude that the formation of the supercomplex under steady-state conditions requires a motor-dependent stabilization (“locking in”) that was possible only with the preprotein b2(167)Δ-DHFR.
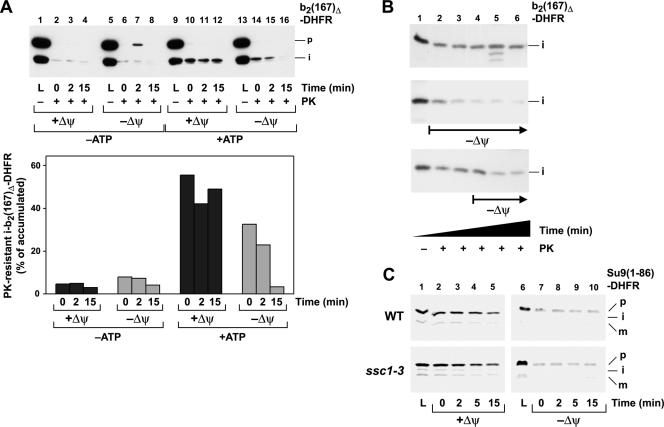
FIG. 4.
The Δψ assists the generation of an inward-directed translocation force. (A) The assay for an import-driving activity and its quantification were performed as described in the legend to Fig. 1 using the preprotein b2(167)Δ-DHFR dependent on the Δψ. The matrix ATP levels were reduced by treatment with apyrase (−ATP) as indicated. The membrane potential was depleted by the addition of valinomycin, antimycin, and oligomycin as indicated. (B) Time course of protease resistance after depletion of the membrane potential. The experiment was performed as described in the legend to Fig. 1. The Δψ was depleted by the addition of valinomycin at the indicated time points. (C) Backward release of preproteins from the translocation channel is not affected by import motor mutants. The radiolabeled preprotein Su9(86)-DHFR was accumulated as an MTX translocation intermediate in wild-type and ssc1-3 mutant mitochondria under nonpermissive conditions. Mitochondria were reisolated, and the membrane potential was depleted as indicated. The amount of precursor associated with the mitochondria was monitored over the indicated incubation times by SDS-PAGE of the mitochondrial pellet and detection by autoradiography.
The import motor and the inner membrane potential cooperate in generation of a sustained import-driving activity.
ATP hydrolysis by the import motor complex represented a major contribution to the import-driving activity. We asked if the electrophoretic force exerted by the membrane potential might play a similar role during the translocation reaction. In contrast to previous experiments that were aimed at the characterization of the mtHsp70 function, we performed the import activity assay in the presence of the inner membrane potential (Fig. 4A). Interestingly, the levels of protease-resistant intermediates of the preprotein b2(167)Δ-DHFR remained high over the complete incubation period, while in the absence of a membrane potential, the resistance was lost rapidly. In contrast, when we depleted ATP in the matrix compartment prior to the protease treatment, the amount of resistant material remained small, similar to the effect of the mutated import motor components. To test the contribution of the membrane potential to the stabilization of an import-driving activity, we added the ionophore valinomycin to dissipate the potential at different times after the accumulation of the translocation intermediates. If valinomycin was added after 5 min of incubation in the presence of high matrix ATP, the protease resistance of the intermediates was maintained up to that point and then decreased rapidly, similar to the decrease in resistance when the potential was abolished directly at the beginning of the incubation. If the membrane potential was maintained throughout the incubation period, stable amounts of resistant proteins were found even after prolonged incubation times (Fig. 4B). In order to address this supportive function of the membrane potential more directly, we performed assays in which the release of preproteins from the translocation channel was investigated. MTX translocation intermediates of the preprotein Su9(86)-DHFR were accumulated as described previously (52). The preprotein had an N-terminal segment that was just sufficiently long to completely insert into the inner membrane but did not expose a major segment to the matrix space (52). In contrast to the import activity assay, no protease was added after reisolation to monitor the total amount of preprotein inserted into the import sites. The amounts of preproteins remaining associated with mitochondria over time were determined in the presence and the absence of the membrane potential to assess the release of preprotein from the translocation channel. In this case, we had to use the preprotein in a radiolabeled form, since the excess amounts of the recombinant preproteins distorted the results of the experiments in the absence of protease treatment. In the presence of the membrane potential, we observed only a minor slow decrease in the amounts of associated preproteins (Fig. 4C). In contrast, depletion of the membrane potential caused a significant release of accumulated preproteins, essentially to background levels. Interestingly, no difference in the release of the preprotein was observed between wild-type and ssc1-3 mitochondria that do not exhibit a translocase function under nonpermissive conditions. Holding the inserted preprotein in the translocation channel per se is therefore mainly dependent on the membrane potential and is not directly affected by the activity of the mtHsp70 import motor. We conclude that the levels of matrix ATP and the correlated activity of the import motor complex determined the total amount of resistant material at the start of the reaction, while the membrane potential seemed to be responsible for the stabilization of the preproteins in the import channel. This “holding” activity increases the likelihood of an interaction between the precursor and the import motor, thereby contributing indirectly to the maintenance of the protease resistance of translocation intermediates.
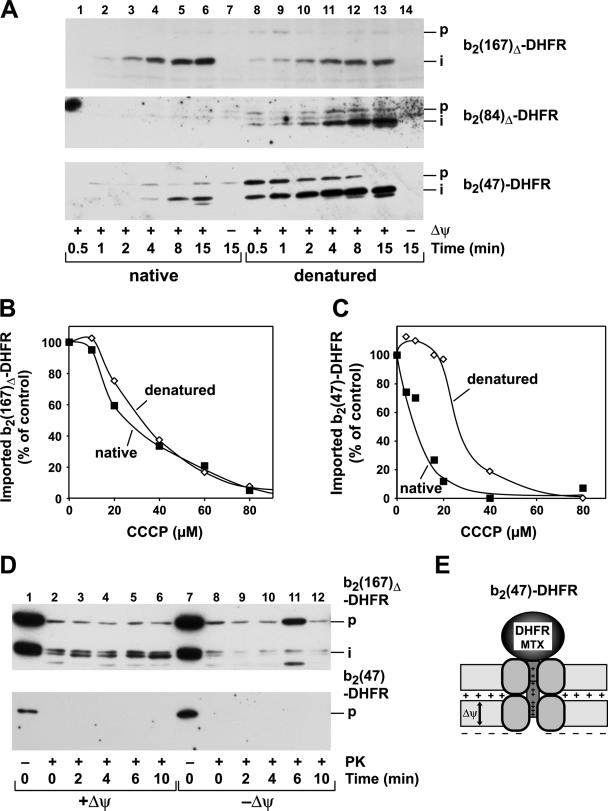
In order to assess a potential direct role of the membrane potential as an import-driving activity, we also utilized a preprotein that was not able to interact with the import motor complex when accumulated as a translocation intermediate. Any import-driving activity for this protein should result only from the electrophoretic force of the membrane potential. Using the recombinant preprotein b2(47)-DHFR, containing an N-terminal extension of 47 amino acids, we first determined its overall import efficiency compared to that of the standard fusion protein b2(167)Δ-DHFR. The precursor proteins were used in a folded, native conformation or artificially unfolded by a urea treatment prior to the import reaction (Fig. 5A). In the case of b2(167)Δ-DHFR, containing a long N-terminal extension, both folding states of the preprotein were imported with high efficiencies that were slightly increased after urea denaturation. In contrast, while the denatured preprotein b2(47)-DHFR was imported with very fast kinetics, the native precursor showed significantly slower kinetics. We reasoned that an intermediate situation is represented by the preprotein b2(84)Δ-DHFR. The N-terminal segment of b2(84)Δ-DHFR should be long enough to insert completely into the import channels and to expose some part of its targeting sequence in the matrix. Surprisingly, when we performed standard in vitro import experiments with purified saturating amounts of b2(84)Δ-DHFR in the native state, we could detect hardly any protease-resistant mature protein, even after prolonged incubation times (Fig. 5A). The import defect was directly correlated with the folding state of the preprotein, since after urea denaturation, the unfolded b2(84)Δ-DHFR was imported with high efficiency. Our analysis showed that the mtHsp70 import motor was not able to confer an import-driving activity on b2(84)Δ-DHFR translocation intermediates (Fig. 3B). However, in case of a folded C-terminal DHFR domain, b2(84)Δ-DHFR can insert deeper into the translocation channel than b2(47)-DHFR. The apparent lack of completed import is therefore consistent with a certain spatial arrangement of the N-terminal segment inside the import channel, where neither the membrane potential nor the import motor can confer a significant import-driving activity.
FIG. 5.
The rate and efficiency of mitochondrial import are dependent on the lengths of the membrane-spanning segments in front of folded domains. (A) Import of recombinant purified cytochrome b2-DHFR fusion proteins b2(167)Δ-DHFR, b2(84)Δ-DHFR, and b2(47)-DHFR in native and urea-denatured states. The import reaction was performed as described in Materials and Methods (p, precursor; i, processing intermediate). Nonimported preprotein was removed by treatment with proteinase K. The proteins were separated by SDS-PAGE and detected by Western blotting and immunodecoration with antibodies against DHFR. Import of the recombinant purified fusion proteins b2(167)Δ-DHFR (B) and b2(47)-DHFR (C) was performed dependent on the membrane potential. The proteins were imported in their native or urea-denatured state in the presence of increasing amounts of the ionophore CCCP. The imported and processed preproteins were quantified, and the value obtained for the control (0 μM CCCP) was set to 100%. (D) The assay for an import-driving activity for the fusion proteins b2(167)Δ-DHFR and b2(47)-DHFR was performed as described in the legend to Fig. 1. (E) Schematic graph of an MTX-bound translocation intermediate of b2(47)-DHFR inserted into the mitochondrial membranes.
Due to its short N-terminal segment, the motor complex cannot interact with the preprotein b2(47)-DHFR before the DHFR domain is unfolded (Fig. 5E). The initiation of the unfolding and subsequent translocation of a folded b2(47)-DHFR can be achieved only by the electric force of the membrane potential. Indeed, when we tested the dependence of the import b2(47)-DHFR on the inner Δψ, the preproteins b2(47)-DHFR and b2(167)Δ-DHFR as a control were imported as folded or unfolded precursors into isolated mitochondria. The membrane potential was gradually lowered by the incubation of the mitochondria with increasing concentrations of the protonophore CCCP (Fig. 5B and C). The import efficiency of the long construct b2(167)Δ-DHFR was decreased in the same manner for the folded and unfolded preproteins with increasing amounts of CCCP. In contrast, the import efficiency of the folded b2(47)-DHFR decreased very rapidly with increasing CCCP concentrations. Already at 20 μM, the import efficiency was almost abolished. Interestingly, the unfolded b2(47)-DHFR was still imported very efficiently and showed a CCCP sensitivity comparable to that of b2(167)Δ-DHFR. The different Δψ dependences of folded and unfolded b2(47)-DHFR indicate that not only the amino acid composition of the presequence, but also the conformational state of the preprotein, is a major factor in determining the initial translocation rate. We asked if the action of the membrane potential could replace the ATP-dependent import motor in generating an inward-directed translocation force. However, translocation intermediates of b2(47)-DHFR generated by the addition of MTX did not exhibit any resistance to external protease, indicating that the membrane potential was not able to generate an apposition of folded DHFR and translocation machinery to prevent digestion comparably close to that in the case of b2(167)Δ-DHFR (Fig. 5D). Hence, the different import kinetics of the folded and denatured precursor protein of b2(47)-DHFR were a consequence of the lack of a strong import-driving activity. This observation indicated that the location of the N-terminal segment in relation to the local environment of the translocation machinery is a major influence on the import efficiency. Depending on the environment of the presequence, either the membrane potential or the import motor, or a combination of both, became decisive for driving the polypeptide transport.
The preprotein-unfolding activity of the inner membrane potential is limited.
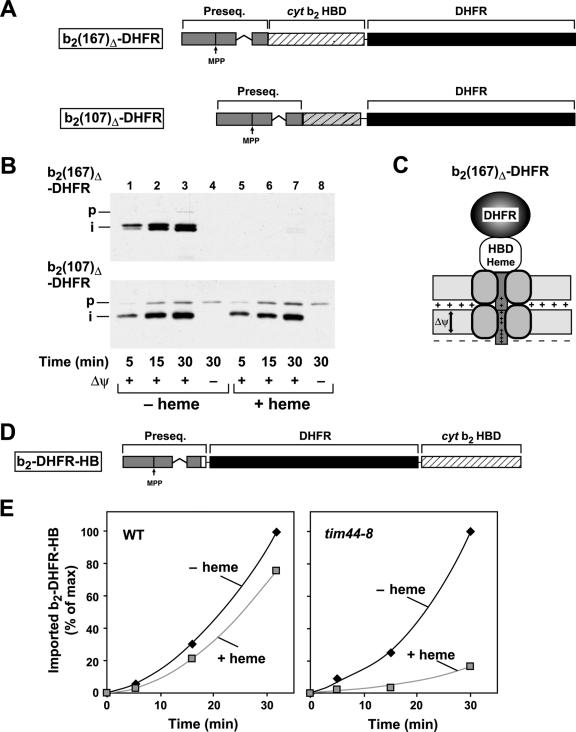
In the absence of a stabilizing ligand, the overall thermodynamic stability of the DHFR domain is relatively low (14, 53). However, due to extensive secondary-structure interactions at the N terminus, the mechanical unfolding of the DHFR domain during import requires global destabilization with a relatively high activation energy barrier (20). Based on the low unfolding activity of the membrane potential, we observed a kinetic inhibition of the import reaction in the case of b2(47)-DHFR and a strong import reduction for the longer b2(84)Δ-DHFR. To assess the relevance of the conformational properties for the import reaction, we compared the results obtained for DHFR with those for a protein domain that exhibits different structural properties and occurs in a native mitochondrial protein. The first 102 amino acid residues of the mature N-terminal part of the yeast cytochrome b2 are able to form an independently folded heme-binding domain (HBD) (Fig. 6A) that has a higher thermodynamic stability than DHFR (14). Similar to the stabilization of DHFR by MTX, the folding state of the HBD can be influenced by the addition of the ligand heme. However, previous experiments using radiolabeled preproteins showed that ligand stabilization of the HBD was not able to interfere with the import reaction into wild-type mitochondria (54). Surprisingly, when we preincubated the purified preprotein b2(167)Δ-DHFR with heme, import into wild-type mitochondria in vitro was completely abolished (Fig. 6B). The strong import inhibition was observed despite the fact that, with 89 aa of the mature part, b2(167)Δ-DHFR does not contain the complete heme-binding domain (59). However, an analysis of the three-dimensional structure (data not shown) indicated that the residual segment in b2(167)Δ-DHFR comprised the core of the heme-binding domain, which is sufficient for a stable interaction with the ligand. The control reaction using the related preprotein b2(107)Δ-DHFR, in which the heme-binding core was not present, showed no inhibition by heme, excluding an indirect effect of heme on the import machinery. The presence of a folded HBD at the N-terminal end of the mature preprotein b2(167)Δ-DHFR did result in a translocation intermediate that was too short to reach the matrix compartment (Fig. 6C). Similar to the construct b2(84)Δ-DHFR (Fig. 5A), the translocation block of b2(167)Δ-DHFR in the presence of heme indicates the limited unfolding capacity of the electrophoretic force generated by the membrane potential. These experiments show that unfolding and initiation of translocation of preproteins containing domains with high conformational stability cannot be catalyzed by the inner membrane potential alone. The inner membrane translocase component Tim44 has been shown to link the activity of the ATPase mtHsp70 to the preprotein import reaction in the matrix. Mutations in Tim44 lead to a delayed translocation reaction due to the failure of the import motor to actively unfold preprotein domains (3, 33). In order to test the activity of the import motor and its high import-driving activity on the translocation of stably folded domains, we tested the import of the HBD-containing preprotein b2-DHFR-HB in tim44-8 mutant mitochondria. In this case, the HBD was introduced at the C terminus to circumvent the problem of the weak unfolding efficiency of the Δψ, leaving a sufficient length of N-terminal extension in front of the folded domain (Fig. 6D). The fusion protein was expressed in E. coli cells, purified, and then incubated with mitochondria isolated from wild-type and tim44-8 mutant strains. The tim44-8 mutant mitochondria were treated with a short heat shock at 37°C before import to induce nonpermissive conditions. While the import of the b2-DHFR-HB into tim44-8 mitochondria was only slightly reduced in the absence of heme, the addition of the specific ligand and the subsequent stabilization of the heme domain reduced the import of the reporter construct dramatically (Fig. 6E). The close correlation of the preprotein-unfolding defect in the mutant tim44-8 with its defect in generating an import-driving activity corroborates the notion that the ATP-driven active-force generation is the main cause of preprotein unfolding.
FIG. 6.
The unfolding of the stable folded HBDs cannot be initiated by the membrane potential alone. (A) Schematic representation of the preproteins b2(167)Δ-DHFR and b2(107)Δ-DHFR. The presequence, the HBD of cytochrome b2, and the DHFR domain are labeled. (B) The import of the recombinant purified preproteins b2(167)Δ-DHFR and b2(107)Δ-DHFR was performed as described in Material and Methods in the absence or presence of heme. Nonimported proteins were removed by proteinase K. Proteins were separated by SDS-PAGE and detected by Western blotting and immunodecoration with antibodies against DHFR. (C) Schematic representation of the preprotein b2(167)Δ-DHFR with a stably folded HBD traversing both mitochondrial membranes. (D) Schematic representation of the preprotein b2-DHFR-HB. The fusion protein consists of the presequence of cytochrome (cyt) b2, the DHFR domain, and the HBD of cytochrome b2 as indicated. (E) The recombinant purified preprotein b2-DHFR-HB was imported into wild-type and tim44-8 mitochondria under nonpermissive conditions in the absence and presence of heme. The imported and processed protein was quantified. The maximal amount of protein imported was set to 100%.
DISCUSSION
The driving forces of preprotein translocation have been a central but controversial topic in the research on mitochondrial protein biogenesis (16, 31, 39). Previous studies utilized the unfolding of C-terminal preprotein domains during the translocation process as an assay to determine the generation of an inward-directed import activity (14, 19, 26, 30, 47). The unfolding process has been shown to be mechanically coupled to the translocation reaction, since the pathways of protein unfolding during import and spontaneous unfolding in solution can differ markedly (20). However, since the unfolding assays are based on a complete import reaction, too many parameters influence the reaction to allow detailed conclusions about the functions of individual components of the translocation machinery. In this study, we addressed the relative contributions of the mitochondrial energy sources, the inner Δψ and ATP hydrolysis in the matrix compartment, to the preprotein transport process. We employed a specific translocation assay that is based on the resistance of accumulated translocation intermediates against externally added proteases. The intermediates represent a specific and crucial stage of the import process in which the membrane insertion of the presequence is completed and the transport of the bulk polypeptide chain commences. Accumulation of productive translocation intermediates is achieved by the conformational stabilization of the C-terminal DHFR domain with the specific ligand MTX (10, 41). Interestingly, similar translocation intermediates can also form in intact cells, demonstrating that newly synthesized preproteins can acquire a folded conformation under in vivo conditions before being imported (57).
The assay essentially represents a two-step reaction. First, mitochondrial preproteins were accumulated as MTX-stabilized membrane-spanning translocation intermediates under standard conditions. In a second step, the generation of an inward-directed translocation activity on the polypeptide chain in transit was assayed by the resistance of the translocation intermediates to externally added proteases. At this stage of the import reaction, the mitochondrial energy sources promote polypeptide movement and unfolding. In contrast to previous experiments (3, 52), we used excess amounts of purified recombinant proteins to accumulate translocation intermediates in saturating amounts (7, 26). The use of recombinant proteins to analyze the mitochondrial import reaction offers several advantages: (i) any influence of cytosolic factors on the unfolding and translocation reactions is excluded, (ii) the possibility of saturating the translocation machinery allows quantitative data about the efficiency of the import reaction to be obtained, and (iii) the high load of substrate proteins requires recycling of the ATP-dependent import motor to sustain a strong translocation-driving activity. In contrast to the studies using radiolabeled preproteins, only a fraction of the accumulated intermediates showed protease resistance under substrate-saturating conditions. This observation could indicate that rather than the number of translocation channels, the engagement with other components of the translocation machinery, most likely the import motor complex, determined the amount of actively translocated polypeptides. Although the exact stoichiometry and the amounts of translocation components are not known, published estimates for the channel protein Tim23 are about 20 pmol/mg mitochondrial protein and 5 to 7 pmol for the import motor component Tim44 (35), while mtHsp70 as an abundant matrix chaperone is in large excess. Hence, the amount of observed protease-resistant translocation intermediates most closely reflects the amount of Tim44.
As the sole ATP-dependent enzyme involved in the import reaction, the activities of the chaperone mtHsp70 (Ssc1) and its cofactors have been directly correlated with the transport and unfolding of preproteins (30, 31, 38, 56). The membrane protein Tim44 forms the core component of the import motor, or “presequence translocase-associated motor complex” (1, 48), connecting the TIM23 translocation channel with the ATP-hydrolyzing chaperone. In close agreement with previous results, we were able to show that the crucial components of the import motor, mtHsp70, Tim44, and the Pam18/16 system, are essential to generate a strong driving force for protein translocation in a close mechanistic collaboration. Interestingly, our results demonstrate a clear distinction between the energy sources ATP and Δψ on the generation of the import force. At high matrix ATP levels, the translocation intermediates exhibited high protease resistance, indicating that the generation of an inward-directed translocation force is an active process directly based on the ATPase activity of mtHsp70. However, if the membrane potential is depleted, the strong translocation force could not be maintained over prolonged incubation times, and the amounts of protease-resistant precursors decreased quickly. Although in the case of the MTX intermediates, the Δψ-dependent insertion steps into the inner membrane TIM23 complex had been completed, the membrane potential seemed to be required to increase the likelihood of interaction between the precursor protein and the import motor, resulting in a sustained translocation-driving activity. This conclusion is supported by the observation that accumulated translocation intermediates with short N-terminal segments are released from the translocation channel in a reaction strongly dependent on the Δψ but are not affected by the ssc1-3 mutation of mtHsp70 with its strong translocation defect.
Our experiments revealed that the location of the positively charged presequence in relation to the Δψ across the inner membrane has important consequences for the overall import reaction. The presequence of the small preprotein b2(47)-DHFR could not completely insert into the import channel and did not interact with the import motor complex in the matrix. In this case, it was postulated that the membrane potential was able to exert an inward-directed force on the presequence (46) that was based on the ionic interaction of the positively charged presequence and the negatively charged matrix side of the inner membrane. However, no protease resistance of the translocation intermediate was observed, i.e., the electric potential across the inner membrane alone was not able to generate a strong translocation force. As a result, the import of b2(47)-DHFR is strongly dependent on the Δψ. However, the electrophoretic force was involved in the unfolding of the DHFR domain, most probably by trapping spontaneous unfolding events (19), resulting in an initial lag phase in the overall translocation kinetics. Since the machinery of the outer membrane does not contribute prominently to preprotein unfolding (18), the Δψ becomes the only energy source available for the initiation of the import reaction of preproteins with small N-terminal extensions.
While the unfolding activity of the membrane potential is restricted to small preproteins with short N-terminal extensions below 50 aa, the unfolding of longer preproteins is provided by the translocation force exerted by the mtHsp70 import motor. An interesting intermediate situation is represented by the construct b2(84)Δ-DHFR. The extreme N terminus of b2(84)Δ-DHFR should be able to reach the matrix, but no translocation force due to the import motor activity could be observed. With an N-terminal segment of 72 aa, the unprocessed form of b2(84)Δ-DHFR would expose sufficient residues in the matrix to allow binding of at least one mtHsp70 molecule. However, one round of mtHsp70 interaction does not seem to be sufficient to maintain sustained translocation activity. This is in agreement with the observation that the mtHsp70 membrane anchor Tim44 and, in correlation, also the chaperone itself form a dimer in the active translocation complex (35). While the shorter b2(47)-DHFR showed an initial import lag, the translocation of the purified b2(84)Δ-DHFR in a folded state was completely blocked. In this case, the longer N-terminal segment allowed deeper insertion into the import channel. However, it is likely that this specific spatial arrangement prevented the generation of the driving force based on the membrane potential. In contrast to b2(84)Δ-DHFR, the precursor form of the preprotein b2(107)Δ-DHFR, with an N-terminal extension of 92 aa, might be able to present several mtHsp70-binding sites, resulting in an active translocation-driving force and concomitant protease resistance. The processed intermediate as such was too short to expose these binding sites, leading to the loss of protease resistance. As a result, b2(107)Δ-DHFR is efficiently imported even in a folded conformation (26).
The long preprotein b2(167)Δ-DHFR was imported efficiently and with kinetics very similar to those of b2(107)Δ-DHFR, correlating with the observed strong translocation-driving activity. We were able to show that this activity was also required for the generation of the stable TOM-TIM supercomplex that represents a “locked-in” preprotein substrate and connects the translocation complexes of the outer and inner membranes. However, the stabilization of the preprotein in the supercomplex also showed specific topological requirements. The formation of the stable TOM-TIM supercomplex required a long amino-terminal extension that is present in the fusion protein b2(167)Δ-DHFR. Here, several binding sites for mtHsp70 and/or other components of the import machinery are required to stabilize the translocation intermediate. We conclude that the membrane potential is not able to generate a substantive translocation force per se but plays an important supportive role to maintain the translocation activity of the import motor. Interestingly, it has been observed that an enhanced preprotein binding activity of a mtHsp70 mutant was able to compensate for the partial loss of Δψ during the import reaction (15), indicating a cooperative action between the membrane potential and the mtHsp70 import motor. In this case, the supportive role of the Δψ in maintaining the ability of the import motor to generate an import-driving activity was partially replaced by a high binding affinity of mtHsp70 to the preprotein in transit.
The extent to which individual preproteins are dependent on import-driving forces for initiation of translocation is certainly influenced by their different structural properties. Especially, the structural composition near the presequence has been shown to be a major influence on the unfolding reaction (58). The N terminus of DHFR is inserted deeply into the interior of the structure, exhibiting strong intramolecular interactions within a β-sheet structure. Although the overall thermodynamic stability of DHFR is relatively low, membrane translocation requires a global unfolding step of the whole domain prior to the insertion step (20). In the short b2(47)-DHFR, spontaneous unfolding events of the DHFR domain could be trapped by the membrane potential in a reaction sufficient to initiate the import reaction. In contrast, the specific spatial arrangement of the b2(84)Δ-DHFR presequence in the translocation channel precludes an import-driving activity of the membrane potential, effectively blocking translocation. Compared to DHFR, the HBD of cytochrome b2 exhibits higher thermodynamic stability (14). Due to the absence of extensive secondary-structure interactions, its conformation at the N terminus suggests a relatively low mechanical stability. However, in the presence of the ligand heme, our results show that unfolding of the HBD is effectively prevented in the absence of a strong import-driving activity like that generated by the import motor complex. Even with a long N-terminal extension, import of the HBD strongly depends on a functional Tim44 and hence on the activity of the import motor. The conformational state of the HBD exhibits properties similar to those of the enzyme barnase, which has been used as a model protein for unfolding studies. It has been shown that the α-helical N terminus of barnase can be unfolded relatively easily by the mechanical action of the import machinery (20). The fusion protein b2(65)-barnase, was unfolded and imported by mitochondria dependent on the membrane potential (19) although it contained an N-terminal segment similar to that of b2(84)Δ-DHFR. However, apart from the lower structural stability of the N terminus of barnase, an influence of cytosolic factors on the unfolding of the precursor cannot be completely excluded in these experiments, since the preproteins were synthesized in a radiolabeled form and imported in the presence of reticulocyte lysate. Interestingly, previous experiments using the preproteins b2(167)Δ-DHFR and b2(220)Δ-DHFR, containing the full N-terminal HBD, did not show an inhibitory effect of heme (14, 54) when they were generated by translation in reticulocyte lysate, as long as the function of the import motor was not compromised. The difference in the import behaviors of purified recombinant preproteins and those generated by in vitro translation indicates that cytosolic factors may play an important role in determining the unfolding kinetics.
Taken together, our results indicate that the contributions of the import-driving forces to the translocation and unfolding reactions in the in vivo situation might be more complex than anticipated. In accordance with the “active-motor” model for the mtHsp70 import mechanism, a cooperative action of mtHsp70 and Tim44 is responsible for the generation of the main driving force of the translocation reaction, while the membrane potential has a supportive role. Recent in vitro experiments using the purified components Ssc1, Tim44, and Mge1 seemed to suggest that the mtHsp70 system might not be capable of active force generation (27). However, in intact mitochondria, the presence of additional regulatory factors, like Pam18 and Pam16, might substantially alter the reactivities of the individual components (9, 25, 32). A newly proposed model for the mechanism of the import motor, called “entropic pulling,” postulates the generation of an active import-driving force based on thermodynamic principles and molecular geometries of the preproteins during translocation (6). According to this model, the translocation force is generated by the excluded-volume constraint between mtHsp70 and the membrane. This model also requires efficient interaction of mtHsp70 with Tim44 to obtain an efficient translocation and unfolding reaction. Irrespective of the still-controversial mechanistic details of the activity of the import motor complex, our results demonstrate its decisive role in the generation of an inward-directed translocation force, assisted by a “holding” activity exerted by the electric potential across the inner membrane.
Acknowledgments
We thank E. A. Craig and M. Meijer for the ssc1-2, ssc1-3, and tim44-8 mutants; N. Zufall for expert technical assistance; and N. Pfanner, M. van der Laan, and K. Röttgers for discussion and critically reading the manuscript.
This work was supported by the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 388, Teilprojekt A11).
Footnotes
Published ahead of print on 30 October 2006.
REFERENCES
- 1.Bauer, M. F., S. Hofmann, W. Neupert, and M. Brunner. 2000. Protein translocation into mitochondria: the role of TIM complexes. Trends Cell Biol. 10:25-31. [DOI] [PubMed] [Google Scholar]
- 2.Bauer, M. F., C. Sirrenberg, W. Neupert, and M. Brunner. 1996. Role of Tim23 as voltage sensor and presequence receptor in protein import into mitochondria. Cell 87:33-41. [DOI] [PubMed] [Google Scholar]
- 3.Bömer, U., A. C. Maarse, F. Martin, A. Geissler, A. Merlin, B. Schönfisch, M. Meijer, N. Pfanner, and J. Rassow. 1998. Separation of structural and dynamic functions of the mitochondrial translocase: Tim44 is crucial for the inner membrane import sites in translocation of tightly folded domains, but not of loosely folded preproteins. EMBO J. 17:4226-4237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chacinska, A., P. Rehling, B. Guiard, A. E. Frazier, A. Schulze-Specking, N. Pfanner, W. Voos, and C. Meisinger. 2003. Mitochondrial translocation contact sites: separation of dynamic and stabilizing elements in formation of a TOM-TIM-preprotein supercomplex. EMBO J. 22:5370-5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig, E. A., J. Kramer, J. Shilling, M. Werner-Washburne, S. Holmes, J. Kosic-Smithers, and C. M. Nicolet. 1989. SSC1, an essential member of the yeast HSP70 multigene family, encodes a mitochondrial protein. Mol. Cell. Biol. 9:3000-3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Los Rios, P., A. Ben-Zvi, O. Slutsky, A. Azem, and P. Goloubinoff. 2006. Hsp70 chaperones accelerate protein translocation and the unfolding of stable protein aggregates by entropic pulling. Proc. Natl. Acad. Sci. USA 103:6166-6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dekker, P. J. T., F. Martin, A. C. Maarse, U. Bömer, H. Müller, B. Guiard, M. Meijer, J. Rassow, and N. Pfanner. 1997. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16:5408-5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D'Silva, P., B. Schilke, W. Walter, A. Andrew, and E. Craig. 2003. J-protein co-chaperone of the mitochodrial inner membrane required for protein import into the mitochondrial matrix. Proc. Natl. Acad. Sci. USA 100:13839-13844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.D'Silva, P. R., B. Schilke, W. Walter, and E. A. Craig. 2005. Role of Pam16's degenerate J domain in protein import across the mitochondrial inner membrane. Proc. Natl. Acad. Sci. USA 102:12419-12424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eilers, M., and G. Schatz. 1986. Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature 322:228-232. [DOI] [PubMed] [Google Scholar]
- 11.Endo, T., and D. Kohda. 2002. Functions of outer membrane receptors in mitochondrial protein import. Biochim. Biophys. Acta 1592:3-14. [DOI] [PubMed] [Google Scholar]
- 12.Frazier, A. E., J. Dudek, B. Guiard, W. Voos, Y. Li, M. Lind, C. Meisinger, A. Geissler, A. Sickmann, H. E. Meyer, V. Bilanchone, M. G. Cumsky, K. N. Truscott, N. Pfanner, and P. Rehling. 2004. Pam16 has an essential role in the mitochondrial protein import motor. Nat. Struct. Mol. Biol. 11:226-233. [DOI] [PubMed] [Google Scholar]
- 13.Gambill, B. D., W. Voos, P. J. Kang, B. Miao, T. Langer, E. A. Craig, and N. Pfanner. 1993. A dual role for mitochondrial heat shock protein 70 in membrane translocation of preproteins. J. Cell Biol. 123:109-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaume, B., C. Klaus, C. Ungermann, B. Guiard, W. Neupert, and M. Brunner. 1998. Unfolding of preproteins upon import into mitochondria. EMBO J. 17:6497-6507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geissler, A., J. Rassow, N. Pfanner, and W. Voos. 2001. Mitochondrial import driving forces: enhanced trapping by matrix hsp70 stimulates translocation and reduces the membrane potential dependence of loosely folded preproteins. Mol. Cell. Biol. 21:7097-7104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herrmann, J. M., and W. Neupert. 2000. What fuels polypeptide translocation? An energetical view on mitochondrial protein sorting. Biochim. Biophys. Acta 1459:331-338. [DOI] [PubMed] [Google Scholar]
- 17.Hill, K., K. Model, M. T. Ryan, K. Dietmeier, F. Martin, R. Wagner, and N. Pfanner. 1998. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395:516-521. [DOI] [PubMed] [Google Scholar]
- 18.Huang, S., S. Murphy, and A. Matouschek. 2000. Effect of the protein import machinery at the mitochondrial surface on precursor stability. Proc. Natl. Acad. Sci. USA 97:12991-12996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang, S., K. S. Ratliff, and A. Matouschek. 2002. Protein unfolding by the mitochondrial membrane potential. Nat. Struct. Biol. 9:301-307. [DOI] [PubMed] [Google Scholar]
- 20.Huang, S., K. S. Ratliff, M. P. Schwartz, J. M. Spenner, and A. Matouschek. 1999. Mitochondria unfold precursor proteins by unraveling them from their N-termini. Nat. Struct. Biol. 6:1132-1138. [DOI] [PubMed] [Google Scholar]
- 21.Jensen, R., and C. Dunn. 2002. Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim. Biophys. Acta 1592:25-34. [DOI] [PubMed] [Google Scholar]
- 22.Kang, P. J., J. Ostermann, J. Shilling, W. Neupert, E. A. Craig, and N. Pfanner. 1990. Requirement for hsp70 in the mitochondrial matrix for translocation and folding of precursor proteins. Nature 348:137-143. [DOI] [PubMed] [Google Scholar]
- 23.Koehler, C. M. 2000. Protein translocation pathways of the mitochondrion. FEBS Lett. 476:27-31. [DOI] [PubMed] [Google Scholar]
- 24.Kozany, C., D. Mokranjac, M. Sichting, W. Neupert, and K. Hell. 2004. The J domain-related cochaperone Tim16 is a constituent of the mitochondrial TIM23 preprotein translocase. Nat. Struct. Mol. Biol. 11:234-241. [DOI] [PubMed] [Google Scholar]
- 25.Li, Y., J. Dudek, B. Guiard, N. Pfanner, P. Rehling, and W. Voos. 2004. The presequence translocase-associated protein import motor of mitochondria. Pam16 functions in an antagonistic manner to Pam18. J. Biol. Chem. 279:38047-38054. [DOI] [PubMed] [Google Scholar]
- 26.Lim, J. H., F. Martin, B. Guiard, N. Pfanner, and W. Voos. 2001. The mitochondrial Hsp70-dependent import system actively unfolds preproteins and shortens the lag phase of translocation. EMBO J. 20:941-950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu, Q., P. D'Silva, W. Walter, J. Marszalek, and E. A. Craig. 2003. Regulated cycling of mitochondrial Hsp70 at the protein import channel. Science 300:139-141. [DOI] [PubMed] [Google Scholar]
- 28.Liu, Q., J. Krzewska, K. Liberek, and E. A. Craig. 2000. Mitochondrial Hsp70 Ssc1: role in protein folding. J. Biol. Chem. 28:28. [DOI] [PubMed] [Google Scholar]
- 29.Martin, J., K. Mahlke, and N. Pfanner. 1991. Role of an energized inner membrane in mitochondrial protein import. J. Biol. Chem. 266:18051-18057. [PubMed] [Google Scholar]
- 30.Matouschek, A., A. Azem, K. Ratliff, B. S. Glick, K. Schmid, and G. Schatz. 1997. Active unfolding of precursor proteins during mitochondrial protein import. EMBO J. 16:6727-6736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matouschek, A., N. Pfanner, and W. Voos. 2000. Protein unfolding by mitochondria. The Hsp70 import motor. EMBO Rep. 1:404-410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mayer, M. P. 2004. Timing the catch. Nat. Struct. Mol. Biol. 11:6-8. [DOI] [PubMed] [Google Scholar]
- 33.Merlin, A., W. Voos, A. C. Maarse, M. Meijer, N. Pfanner, and J. Rassow. 1999. The J-related segment of Tim44 is essential for cell viability: a mutant Tim44 remains in the mitochondrial import site, but inefficiently recruits mtHsp70 and impairs protein translocation. J. Cell Biol. 145:961-972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mokranjac, D., M. Sichting, W. Neupert, and K. Hell. 2003. Tim14, a novel key component of the import motor of the TIM23 protein translocase of mitochondria. EMBO J. 22:4945-4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moro, F., C. Sirrenberg, H. C. Schneider, W. Neupert, and M. Brunner. 1999. The TIM17.23 preprotein translocase of mitochondria: composition and function in protein transport into the matrix. EMBO J. 18:3667-3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neupert, W., and M. Brunner. 2002. The protein import motor of mitochondria. Nat. Rev. Mol. Cell Biol. 3:555-565. [DOI] [PubMed] [Google Scholar]
- 37.Pfanner, N., and A. Chacinska. 2002. The mitochondrial import machinery: preprotein-conducting channels with binding sites for presequences. Biochim. Biophys. Acta 1592:15-24. [DOI] [PubMed] [Google Scholar]
- 38.Pfanner, N., J. Rassow, B. Guiard, T. Söllner, F. U. Hartl, and W. Neupert. 1990. Energy requirements for unfolding and membrane translocation of precursor proteins during import into mitochondria. J. Biol. Chem. 265:16324-16329. [PubMed] [Google Scholar]
- 39.Pfanner, N., and K. N. Truscott. 2002. Powering mitochondrial protein import. Nat. Struct. Biol. 9:234-236. [DOI] [PubMed] [Google Scholar]
- 40.Pfanner, N., and N. Wiedemann. 2002. Mitochondrial protein import: two membranes, three translocases. Curr. Opin. Cell Biol. 14:400-411. [DOI] [PubMed] [Google Scholar]
- 41.Rassow, J., B. Guiard, U. Wienhues, V. Herzog, F.-U. Hartl, and W. Neupert. 1989. Translocation arrest by reversible folding of a precursor protein imported into mitochondria. A means to quantitate translocation contact sites. J. Cell Biol. 109:1421-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rassow, J., F. U. Hartl, B. Guiard, N. Pfanner, and W. Neupert. 1990. Polypeptides traverse the mitochondrial envelope in an extended state. FEBS Lett. 275:190-194. [DOI] [PubMed] [Google Scholar]
- 43.Ryan, M. T., W. Voos, and N. Pfanner. 2001. Assaying protein import into mitochondria. Methods Cell Biol. 65:189-215. [DOI] [PubMed] [Google Scholar]
- 44.Schägger, H. 2001. Blue-native gels to isolate protein complexes from mitochondria. Methods Cell Biol. 65:231-244. [DOI] [PubMed] [Google Scholar]
- 45.Schülke, N., N. B. Sepuri, D. M. Gordon, S. Saxena, A. Dancis, and D. Pain. 1999. A multisubunit complex of outer and inner mitochondrial membrane protein translocases stabilized in vivo by translocation intermediates. J. Biol. Chem. 274:22847-22854. [DOI] [PubMed] [Google Scholar]
- 46.Shariff, K., S. Ghosal, and A. Matouschek. 2004. The force exerted by the membrane potential during protein import into the mitochondrial matrix. Biophys. J. 86:3647-3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stan, T., U. Ahting, M. Dembowski, K. P. Künkele, S. Nussberger, W. Neupert, and D. Rapaport. 2000. Recognition of preproteins by the isolated TOM complex of mitochondria. EMBO J. 19:4895-4902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Strub, A., J. H. Lim, N. Pfanner, and W. Voos. 2000. The mitochondrial protein import motor. Biol. Chem. 381:943-949. [DOI] [PubMed] [Google Scholar]
- 49.Truscott, K. N., P. Kovermann, A. Geissler, A. Merlin, M. Meijer, A. J. M. Driessen, J. Rassow, N. Pfanner, and R. Wagner. 2001. A presequence- and voltage-sensitive channel of the mitochondrial preprotein translocase formed by Tim23. Nat. Struct. Biol. 8:1074-1082. [DOI] [PubMed] [Google Scholar]
- 50.Truscott, K. N., W. Voos, A. E. Frazier, M. Lind, Y. Li, A. Geissler, J. Dudek, H. Müller, A. Sickmann, H. E. Meyer, C. Meisinger, B. Guiard, P. Rehling, and N. Pfanner. 2003. A J-protein is an essential subunit of the presequence translocase associated protein import motor of mitochondria. J. Cell Biol. 163:707-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ungermann, C., W. Neupert, and D. M. Cyr. 1994. The role of hsp70 in conferring unidirectionality on protein translocation. Science 266:1250-1253. [DOI] [PubMed] [Google Scholar]
- 52.Voisine, C., E. A. Craig, N. Zufall, O. von Ahsen, N. Pfanner, and W. Voos. 1999. The protein import motor of mitochondria: unfolding and trapping of preproteins are distinct and separable functions of matrix Hsp70. Cell 97:565-574. [DOI] [PubMed] [Google Scholar]
- 53.von Ahsen, O., J. H. Lim, P. Caspers, F. Martin, H. J. Schönfeld, J. Rassow, and N. Pfanner. 2000. Cyclophilin-promoted folding of mouse dihydrofolate reductase does not include the slow conversion of the late-folding intermediate to the active enzyme. J. Mol. Biol. 297:809-818. [DOI] [PubMed] [Google Scholar]
- 54.Voos, W., B. D. Gambill, B. Guiard, N. Pfanner, and E. A. Craig. 1993. Presequence and mature part of preproteins strongly influence the dependence of mitochondrial protein import on heat shock protein 70 in the matrix. J. Cell Biol. 123:119-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Voos, W., and N. Pfanner. 2001. The role of chaperone proteins in the import and assembly of proteins in mitochondria and chloroplasts. Front. Mol. Biol. 37:61-89. [Google Scholar]
- 56.Voos, W., O. von Ahsen, H. Müller, B. Guiard, J. Rassow, and N. Pfanner. 1996. Differential requirement for the mitochondrial Hsp70-Tim44 complex in unfolding and translocation of preproteins. EMBO J. 15:2668-2677. [PMC free article] [PubMed] [Google Scholar]
- 57.Wienhues, U., K. Becker, M. Schleyer, B. Guiard, M. Tropschug, A. L. Horwich, N. Pfanner, and W. Neupert. 1991. Protein folding causes an arrest of preprotein translocation into mitochondria in vivo. J. Cell Biol. 115:1601-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wilcox, A. J., J. Choy, C. Bustamante, and A. Matouschek. 2005. Effect of protein structure on mitochondrial import. Proc. Natl. Acad. Sci. USA 102:15435-15440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xia, Z.-X., and F. S. Mathews. 1990. Molecular structure of flavocytochrome b2 at 2.4 Å resolution. J. Mol. Biol. 212:837-863. [DOI] [PubMed] [Google Scholar]