Abstract
Fibulin-5 is an extracellular matrix glycoprotein that participates in elastogenesis. Mutations in the gene for fibulin-5 have been found to be associated with age-related macular degeneration. Little is known, however, about the expression of this gene in normal eyes or eyes with age-related macular degeneration. In this study we evaluated the expression of the fibulin-5 protein in human donor eyes and localized this protein to Bruch’s membrane and the intercapillary pillars of the choriocapillaris in normal eyes. In eyes with age-related macular degeneration, fibulin-5 was localized to pathologic basal deposits beneath the retinal pigment epithelium as well as some small drusen. These results suggest that fibulin-5 may promote extracellular deposit formation in macular degeneration.
Keywords: age-related macular degeneration, fibulins, extracellular matrix, Bruch’s membrane
Bruch’s membrane is a complex structure composed of 5 layers of extracellular matrix located between the retinal pigment epithelium (RPE) and the choriocapillaris. Bruch’s membrane plays a number of important roles in normal retinal physiology. Oxygen, glucose and growth factors derived from the choroidal vasculature must pass through this tissue to supply the photoreceptor cells and RPE, while wastes derived from these highly metabolically active cells must pass in the opposite direction to be removed by the choroidal vasculature. In addition, Bruch’s membrane is a site for RPE adhesion and also serves as a barrier to neovascularization, as ruptures in this structure are associated with abnormal angiogenesis from the choroid into the retina.
During normal aging, and especially in age-related macular degeneration (AMD), Bruch’s membrane undergoes morphological changes with extracellular deposit formation. Deposits in Bruch’s membrane include drusen and basal deposits (flat and diffuse deposits beneath the RPE that include both basal laminar and basal linear deposits, distinguished ultrastructurally) (Green and Enger, 1993).
In view of the important role of this ECM compartment in maintaining retinal health, it is not surprising that mutations in the genes encoding a number of ECM molecules result in retinal degeneration. Malattia Leventinese, also known as Doyne honeycomb retinal dystrophy, is characterized by the presence of Bruch’s membrane deposits which closely resemble drusen in AMD. A single mutation in the gene encoding fibulin-3 causes all known cases of Malattia Leventinese (Stone et al., 1999). The finding that this disease is caused by a defect in a fibulin protein focused our search for additional macular degeneration-associated fibulin gene mutations, and resulted in the identification of a number of fibulin-5 missense mutations in patients with AMD(Stone et al., 2004).
The mechanism(s) whereby fibulin-5 mutations result in AMD is not understood. One possibility is that fibulin-5 itself aggregates in drusen or other Bruch’s membrane deposits. In the current study, we sought to understand better how fibulin-5 mutations may result in Bruch’s membrane deposits in AMD by assessing the localization of fibulin-5 protein in a series of human donor eyes with and without AMD.
Human donor eyes were obtained from the Iowa Lions Eye Bank (Iowa City, IA). A superotemporal wedge spanning from the ora serrata to the fovea centralis was collected from 5 control eyes and 5 AMD eyes—as determined by chart review—including two eyes with choroidal neovascularization. Tissues were preserved in 4% formaldehyde and embedded in sucrose solution as described previously (Barthel and Raymond, 1990).
A polyclonal antibody directed against fibulin-5 was the gift of Dr. Takako Sasaki (Max-Planck Institut fur Biochemie, Martinsreid, Germany). Immunohistochemical staining of cryosections was performed as described previously (Mullins et al., 2006), using the Vector Elite ABC kit and Vector VIP substrate (Burlingame, CA). For evaluation of fibulin-5 protein by Western blot, 10μg of retinal or RPE/choroidal homogenate were separated electrophoretically, transferred to PVDF membrane and probed with the polyclonal fibulin-5 antibody (1 to 2,000 dilution). Bands were visualized using the ECL plus kit (Amersham Biosciences, Buckinghamshire, UK). The contribution of N-linked carbohydrates to the detected molecular weight of fibulin-5 was determined by deglycosylation as described previously for ICAM-1 (Mullins et al., 2006).
In normal eyes, we found fibulin-5 to be localized primarily to Bruch’s membrane (Figure 1 A). This labeling was generally along the outer aspect, i.e., external to the elastic lamina. In addition, labeling was noted in intercapillary pillars, the ECM domains that interdigitate between individual choriocapillaris lobules (Figure 1A). No significant labeling was observed in the retina, with the exception of minor labeling of some large vessels and in the internal limiting membrane and nerve fiber layer (Figure 1E).
Figure 1.
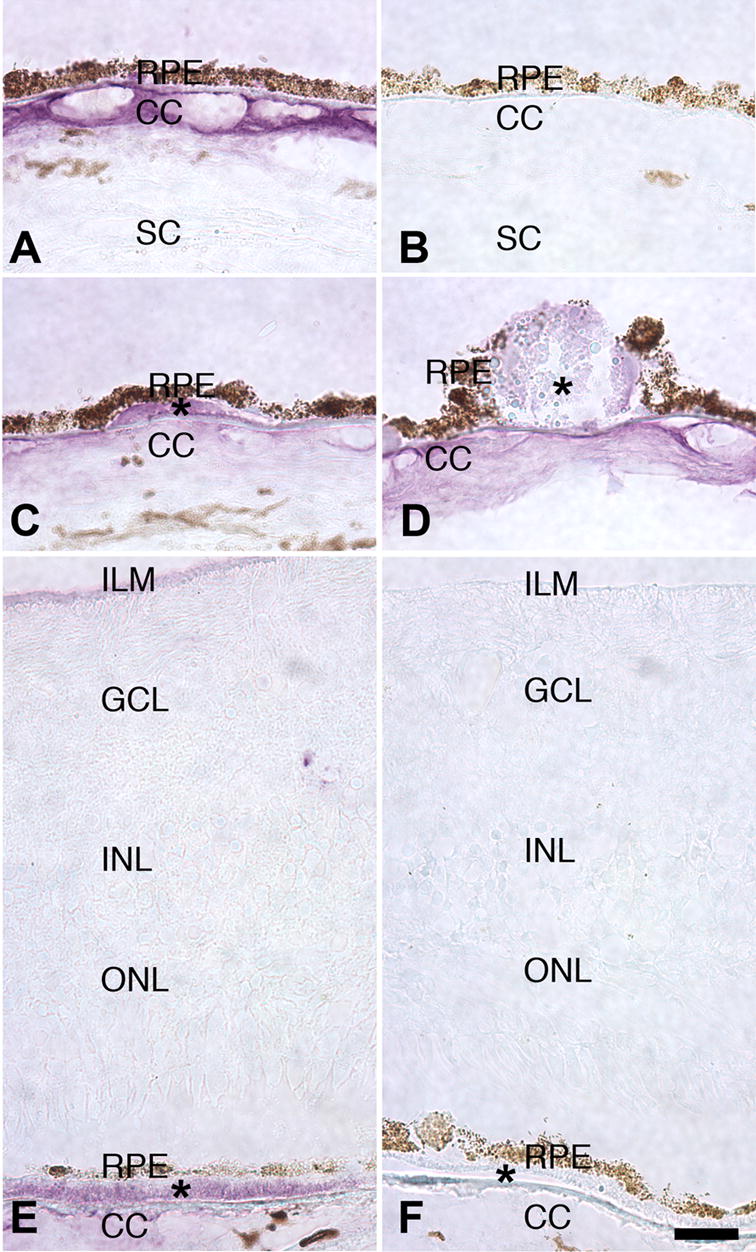
Labeling of human eyes with fibulin-5 antibody. Labeling of Bruch’s membrane and the choriocapillaris (CC) of a normal eye (A), as compared with a secondary antibody control (B). Some small drusen were immunoreactive (C, asterisk) although most drusen were unlabeled or weakly labeled (D, asterisk). In eyes with advanced AMD, diffuse basal deposits were specifically labeled (E, asterisk). Weak labeling of internal limiting membrane (ILM) was observed in some cases. Negative control antibody is depicted in F. Scale bar = 25μm. GCL, ganglion cell layer; INL, inner nuclear layer; ONL, outer nuclear layer; SC, sclera.
Interestingly, in eyes with early AMD, some labeling of Bruch’s membrane deposits was observed (Figure 1C–F). Drusen, mound-shaped deposits visible on clinical examination, showed variable reactivity, with subsets of small drusen positive (Figure 1C) but larger drusen not strongly labeled (Figure 1D).
In two eyes with advanced AMD, in which the choroidal vasculature penetrated Bruch’s membrane resulting in severe loss of vision and scarring, the fibulin-5 antibody reacted with diffuse basal deposits that lay between the RPE and choroid (Figure 1E, F).
In order to confirm that the antibodies used in these studies reacted with fibulin-5 protein, we performed Western blots of RPE/choroidal and retinal protein. The fibulin-5 protein has a predicted molecular weight of approximately 48kDa, and has 2–3 potential N-glycosylation sites. Mature fibulin-5 from human fibroblasts has a higher molecular weight (Markova et al., 2003). Western blots of retina and RPE/choroid showed a single, intense band in the RPE/choroid at approximately 76kDa and a weakly labeled band of the same molecular weight in the retina (Figure 2). The relative abundance of this band in retina and RPE/choroid is consistent with the relative intensity of labeling in these tissues in our immunohistochemical studies. Upon enzymatic deglycosylation, an additional band was observed with an apparent molecular weight of 49kDa, which is the predicted molecular weight of the native protein.
Figure 2.
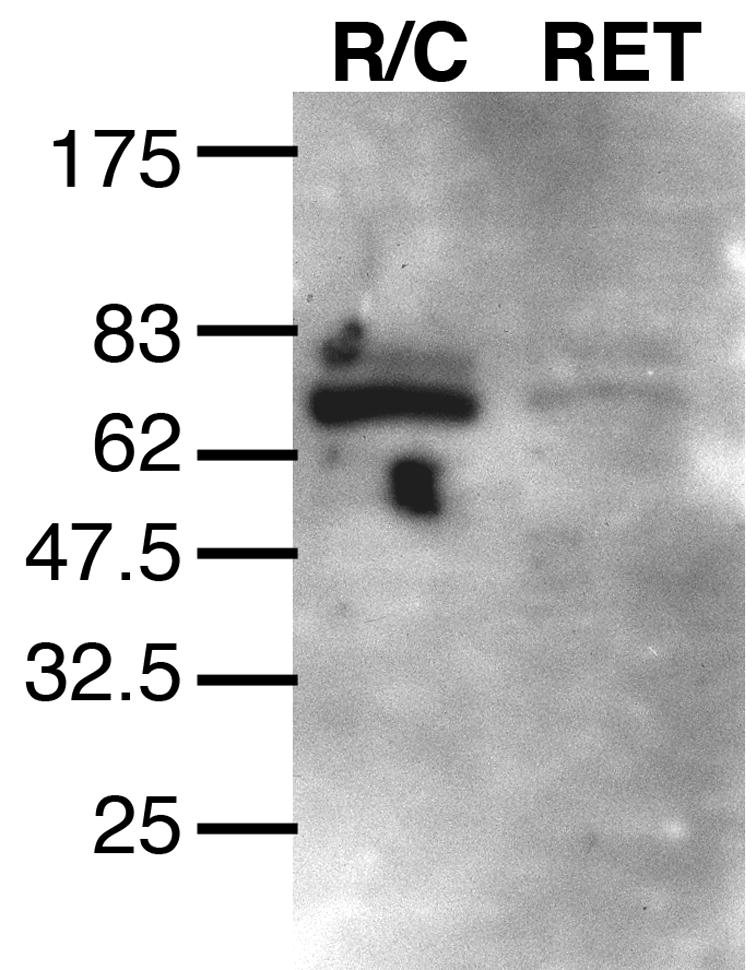
Detection of fibulin-5 protein in RPE-choroid (R/C) and neural retina (RET) protein homogenate from a human donor eye. Note the single major band with an apparent molecular weight of 77kDa. Deglycosylation results in a 49kDa band (data not shown).
Fibulin-5 missense mutations are responsible for a small subset of AMD (Stone et al., 2004). In light of the interdependency of the retina, RPE and choriocapillaris, mutations in genes responsible for maintaining the health of any of these layers could in principle lead to AMD. However, Bruch’s membrane is of particular interest because it is the site at which lesions associated with AMD develop, including drusen, basal laminar deposits and basal linear deposits(Green and Enger, 1993). The localization of fibulin-5 to Bruch’s membrane demonstrates that this glycoprotein is present at the site of deposit formation and, significantly, is also localized to basal deposits in eyes with AMD (Figure 1E). This finding suggests that fibulin-5 may participate in basal deposit formation in AMD. Interestingly, the distribution of fibulin-5 was more widespread than that of elastin, which appears on immunohistochemistry as a narrow band (see for example, Chong et al., 2005).
Fibulin-5 has diverse molecular interactions that, when disrupted, could plausibly lead to macular degeneration. For example, data from fibulin-5 deficient mice reveal that this protein binds elastin and is necessary for normal elastogenesis (Yanagisawa et al., 2002). Disruption of normal elastic lamina formation in Bruch’s membrane during development could lead to greater susceptibility of this structure to deposit formation and/or neovascularization later in life. Fibulin-5 also binds to extracellular superoxide dismutase (Nguyen et al., 2004) and mutations in fibulin-5 that inhibit this interaction may lead to localized increased oxidative damage. Notably, oxidative injury of the RPE has been proposed as an important pathogenic mechanism in AMD (Winkler et al., 1999). Finally, fibulin-5 was recently shown to interact with vascular endothelial growth factor (VEGF) (Albig and Schiemann, 2004). The primary cause of vision loss in AMD is choroidal neovascularization, which is characterized by abnormal angiogenesis of the choroidal vasculature through Bruch’s membrane. This neovascularization is mediated by VEGF, and it is therefore intriguing that some fibulin-5 missense mutations may alter the regulation of VEGF by fibulin-5, either by reduced fibulin-5 secretion (Lotery et al., 2006) or by reduced affinity of fibulin-5 and VEGF, leading to unregulated blood vessel growth.
In summary, fibulin-5 is expressed in the aging eye and is localized to Bruch’s membrane and basal deposits in human eyes. Further studies will reveal how this distribution, combined with emerging data on the function of this protein, can lead to AMD.
Footnotes
Funded in part by the Howard Hughes Medical Institute (EMS), NEI grant EY-014563 (RFM), NEI Grant EY-016822 (EMS), and a center grant from the Foundation Fighting Blindness.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albig AR, Schiemann WP. Fibulin-5 antagonizes vascular endothelial growth factor (VEGF) signaling and angiogenic sprouting by endothelial cells. DNA Cell Biol. 2004;23:367–379. doi: 10.1089/104454904323145254. [DOI] [PubMed] [Google Scholar]
- Barthel L, Raymond P. Improved method for obtaining 3-microns cryosections for immunocytochemistry. J Histochem Cytochem. 1990;38:1383–1388. doi: 10.1177/38.9.2201738. [DOI] [PubMed] [Google Scholar]
- Chong N, Keonin J, Luthert P, Frennesson C, Weingeist D, Wolfe R, Mullins R, Hageman G. Decreased thickness and integrity of the macular elastic layer of Bruch's membrane correspond to distribution of lesions associated with AMD. Am J Pathol. 2005;166:241–251. doi: 10.1016/S0002-9440(10)62248-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green W, Enger C. Age-related macular degeneration histopathologic studies. Ophthalmology. 1993;100:1519–1535. doi: 10.1016/s0161-6420(93)31466-1. [DOI] [PubMed] [Google Scholar]
- Lotery AJ, Baas D, Ridley C, Jones RP, Klaver CC, Stone E, Nakamura T, Luff A, Griffiths H, Wang T, et al. Reduced secretion of fibulin 5 in age-related macular degeneration and cutis laxa. Hum Mutat. 2006;27:568–574. doi: 10.1002/humu.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markova D, Zou Y, Ringpfeil F, Sasaki T, Kostka G, Timpl R, Uitto J, Chu ML. Genetic heterogeneity of cutis laxa: a heterozygous tandem duplication within the fibulin-5 (FBLN5) gene. Am J Hum Genet. 2003;72:998–1004. doi: 10.1086/373940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins R, Skeie J, Malone E, Kuehn M. Macular and peripheral distribution of ICAM-1 in the human choriocapillaris and retina. Mol Vis. 2006;12:224–235. [PubMed] [Google Scholar]
- Nguyen AD, Itoh S, Jeney V, Yanagisawa H, Fujimoto M, Ushio-Fukai M, Fukai T. Fibulin-5 is a novel binding protein for extracellular superoxide dismutase. Circ Res. 2004;95:1067–1074. doi: 10.1161/01.RES.0000149568.85071.FB. [DOI] [PubMed] [Google Scholar]
- Stone E, Braun T, Russell S, Kuehn M, Lotery A, Moore P, Eastman C, Casavant T, Sheffield V. Missense variations in the fibulin 5 gene and age-related macular degeneration. N Engl J Med. 2004;351:346–353. doi: 10.1056/NEJMoa040833. [DOI] [PubMed] [Google Scholar]
- Stone E, Lotery A, Munier F, Heon E, Piguet B, Guymer R, Vandenburgh K, Cousin P, Nishimura D, Swiderski R, et al. A single EFEMP1 mutation associated with both Malattia Leventinese and Doyne honeycomb retinal dystrophy. Nature Genetics. 1999;22:199–202. doi: 10.1038/9722. [DOI] [PubMed] [Google Scholar]
- Winkler B, Boulton M, Gottsch J, Sternberg P. Oxidative damage and age-related macular degeneration. Molecular Vision. 1999;5:32. [PMC free article] [PubMed] [Google Scholar]
- Yanagisawa H, Davis EC, Starcher BC, Ouchi T, Yanagisawa M, Richardson JA, Olson EN. Fibulin-5 is an elastin-binding protein essential for elastic fibre development in vivo. Nature. 2002;415:168–171. doi: 10.1038/415168a. [DOI] [PubMed] [Google Scholar]


