Abstract
Three experiments examined the effects of drug-extinction when a drug state served as a conditional stimulus (CS) for sucrose delivery or as a positive feature for pairings between a discrete CS (e.g., 15-s light-on) and sucrose. Some conditioning models predict that drug-state will facilitate the conditional response (CR) based on an association with sucrose whether the drug is trained as a CS or as a facilitator. If so, repeated presentation of the drug state alone (drug-extinction) should decrease the CR in both situations. Nicotine (0.4 mg/kg), amphetamine (AMP, 1 mg/kg), and chlordiazepoxide (CDP, 5 mg/kg) facilitated a goal-tracking conditioned response to the discrete CS; however, AMP and CDP did not evoke reliable responding without an interposed stimulus, suggesting that associations between these drug states and sucrose are not expressed as anticipatory food-seeking (goal tracking). Repeated presentation of each drug state alone did not disrupt facilitation by nicotine, amphetamine, or CDP; suggesting that the drug states did not facilitate goal tracking based on a direct association with sucrose. This latter finding implicates a higher-order or non-associative mechanism for facilitation of anticipatory food-seeking by drug-states in this Pavlovian discrimination task.
Keywords: Addiction, Drug Abuse, Learning, Motivation, Occasion setting, Pavlovian conditioning
Introduction
Drug dependence disorders are chronic relapsing conditions that are characterized by inappropriate drug-seeking and drug-taking in the face of negative social and behavioral consequences [1]. Most current models of drug addiction take into account the well-established link between drug-associated environmental cues and drug-seeking behaviors (e.g., [3, 14, 21, 37]). Some of these models assign non-drug stimuli a central role, arguing that association with the drug distorts their value and their subsequent control over behavior becomes perseverative and intransigent [14, 37]. Unfortunately, cessation therapies that strive to break the relationship between the drug and non-drug stimulus (cue-exposure or extinction therapies) have not proven to be more successful than other treatments [12]. One reason could be that there are many associative learning phenomena which represent ‘threats to extinction’ (renewal, spontaneous recovery, reinstatement, and discriminative stimuli; see [12]). For example, extinction of nicotine-related cues in smokers (e.g., viewing lit cigarettes and ashtrays) may not account for all of the nicotine-predictive stimuli in a person’s repertoire. Conklin and Tiffany [12] and Troisi [46] argue that a number of other stimuli (contextual, sensorimotor, etc.), will retain their excitatory value and could facilitate future drug taking if untargeted by exposure therapy.
We have previously argued that Pavlovian occasion setters offer a particularly important threat to extinction and exposure therapy for addictions [5]. This argument is due in part to the ability of occasion setters to transfer their control to circumstances in which they have never previously occurred. To use an anecdotal example, the stimulus effects of alcohol (a feature) could set the occasion for a person (a target conditional stimulus, or CS), to be associated with cigarette smoking and nicotine delivery (unconditional stimulus or US). If this person were a co-worker they may be viewed in the absence of alcohol and smoking would presumably occur with more rarity. Similarly, the stimulus effects of alcohol may be present without the person, and smoking may occur less often. Both stimuli are partially associated with smoking, but alcohol sets the occasion upon which the person-CS and smoking co-occur. What if alcohol is present when another smoking-related stimulus occurs (e.g., features of a bar, lit cigarette in an ashtray, etc.)? Would alcohol increase the probability of smoking? One feature of “occasion setters” is that they are able to transfer their conditional control to novel situations [8, 18, 19, 25, 32, 33, 34, 43, 44], thus the alcohol feature may facilitate or increase the probability of goal seeking— i.e., acquiring and smoking a cigarette in this example.
Another striking property of occasion setters is that they continue to control behavior even when their associative status is altered. For example, the transfer of conditional control described in the previous paragraph is easy to explain if both the feature (alcohol) and the CS (person) are directly associated with the US (the effects of nicotine) [31]. In the presence of an alternate tobacco related CS (bar), the tobacco-seeking conditional response (CR) is stronger because there are two tobacco-related stimuli (alcohol and bar). However, direct associations between features and the US are not always needed. For example, repeated presentation of a positive feature alone (i.e., procedural extinction), should decrease its ability to strengthen the CR. However, depending on the circumstances, this extinction treatment may not decrease a feature’s ability to facilitate responding [44]. Thus, occasion setters are fluid, or functional in their control over responding. In order for a feature to be fully extinguished of facilitative control, it must be presented along with the original CS and without the US [23]. Returning to the preceding example, if our subject wanted to successfully quit smoking with an exposure treatment, then both the person-CS and alcohol would need to be presented without access to the tobacco product.
Although many discrete and contextual stimuli have served as occasion setters in previous studies, research with drug-induced contexts has been more limited. This is somewhat surprising due to the contextual nature of drug states. In contrast to discrete stimuli, a drug state turns ‘on’ and ‘off’ rather slowly, stays on for extended periods which are longer or shorter depending on pharmacology, and has multimodal biological impact. We have argued that this aspect of drug stimuli makes them particularly relevant as modulators of associative learning [5]. Drug-induced contexts can have a discriminative influence on the acquisition/expression of learning in both Pavlovian and operant conditioning paradigms (e.g., [17, 22, 38, 42, 45, 46, 48)]. In the most widely studied Pavlovian preparation, drug states set the occasion on which a novel flavor (CS, typically saccharin) will be followed by an aversive outcome (US, typically lithium chloride injection, e.g., [20, 22, 30, 40, 41]). Subjects learn to avoid the taste solution in the proper drug state. Similar to traditional occasion setting situations, discriminated avoidance of the taste does not depend on an association between the drug state and lithium [22, 41]. Moreover, rats will avoid familiar and novel tastants when they are presented in a drug state that has previously set the occasion for a saccharin-lithium pairing [40]. Skinner and colleagues [40, 41] have suggested that this transfer is analogous to that described with traditional occasion setting features [8, 19, 43].
Recent experiments from our laboratory have extended the study of drug contexts to appetitive conditioning situations and to nicotine, amphetamine, and chlordiazepoxide (CDP) drug states ([27, 28, 39]; see [24, 29] for similar studies with pigeons and opiate drugs). Briefly, in the drug state (e.g., 0.4 mg/kg nicotine injected 5-min before the session) a discrete CS (15-s stimulus light illumination) was repeatedly paired with sucrose. In the non-drug state (vehicle injected before the session) the same CS was not followed by any scheduled outcome. The CS evoked anticipatory food-seeking (i.e., goal tracking) in a manner that was specific to the drug state. Initial studies investigated the relationship between drug dose and magnitude of the conditional response [27, 28]. Maximal goal tracking was evoked at the training dose (e.g., 0.4 mg/kg nicotine, 1 mg/kg amphetamine, 5 mg/kg CDP) and decreasing each drug’s dose decreased the rate of goal tracking. Also, drugs from different pharmacological classes (i.e., stimulants and anxiolytics) did not substitute for one another [28]. Finally, anticipatory food-seeking was evoked in the drug state by both visual (i.e., light-on) and auditory (i.e., white noise-on) conditional stimuli [28].
The present studies sought to determine whether nicotine, amphetamine, and CDP functioned as occasion setters. These particular drugs were chosen because their dose-response and substitution profiles were already described. Subsequent studies [26] determined whether they transferred conditional control to novel situations. However, before transfer of occasion setting could be interpreted, we had to determine whether facilitated responding was the result of direct drug-US associations. For example, nicotine can serve as a conditional stimulus for sucrose (i.e., the drug-state evokes goal tracking, see [4, 47]) as well as a positive feature for CS-sucrose pairings [27, 28]. Thus, the latter may be attributed to a direct association between the drug and sucrose; facilitated goal tracking depending on presenting two conditional stimuli (the drug state and the CS [31]). Currently, no study has addressed whether drug states trained as facilitators [27, 28] increase responding because the drug is associated with the sucrose US [31], or because the drug state acquires discriminant properties that do not depend on its excitatory strength [e.g., serve as occasion setters, 43]. Given that repeated presentation of a drug feature alone (i.e., drug-extinction) attenuates drug-evoked goal tracking [4], if facilitation is based on a direct association with sucrose, then a drug extinction procedure should decrease the facilitative effect. Experiment 1 examined the effects of a drug extinction procedure after nicotine was explicitly trained as a CS (paired with sucrose) or as a positive feature/facilitator (set the occasion for CS-sucrose pairings). Experiments 2A and 2B made similar determinations for amphetamine and CDP.
Method
Subjects
Seventy-four male Sprague Dawley rats from Harlan (Indianapolis, IN) were housed individually in clear plastic tubs lined with wood-shavings in a temperature- and humidity-controlled colony. Access to food was restricted such that rats were maintained at 85% of their free-feeding weight [Mean (STD)=382 (22) g]. This target bodyweight was increased by 2 g every 28 days; water was available continuously in the home cage. Experimental sessions were conducted on consecutive days during the light portion of a 12 h light:dark cycle. All procedures were approved by the University of Nebraska-Lincoln IACUC and followed the “Principles of laboratory animal care” (NIH publication No. 85-23, revised 1985).
Apparatus
Experiments were conducted in seven standard conditioning chambers (ENV-008CT; Med Associates, Georgia VT) housed in a sound-attenuating cubicle (ENV-018). Each chamber had a Plexiglas ceiling and front and back walls with two aluminum sidewalls; all stimulus elements were attached to the aluminum walls. One side of each chamber was fitted with a liquid dipper in a receptacle (5.2 x 5.2x 3.8 cm; l x w x d); the dipper had a 0.1-ml cup attached for sucrose delivery. The receptacle was fitted with an infrared emitter/detector unit to monitor head entries. On the same wall were two white stimulus lights (100 mA), these lights were mounted above and to the side of the receptacle and served as the light CS. A speaker and amplifier unit that provided the white noise CS (70 dB) was mounted to the upper back corner of the opposite wall. A personal computer with Med Associates interface and software controlled the stimulus events and recorded dipper entries throughout each session.
Drugs
(-)-Nicotine hydrogen tartrate (Sigma, St Louis, MO) was dissolved in physiological saline, brought to a pH of 7.0 ± 0.2 with a dilute NaOH solution, and injected subcutaneously (SC) at 1 ml/kg. D-amphetamine sulfate and chlordiazepoxide hydrochloride (Sigma, St Louis, MO) were dissolved in physiological saline and injected intraperitoneally (IP) at 1 ml/kg. Nicotine doses are expressed as the base form; other drug doses are expressed as the salt form.
General Procedure
Dipper Training
In the first three sessions rats were trained to drink 26% sucrose (w/v) within 4 s. Sucrose was delivered according to a probability function with a value of 0.167 per 4 s. This function resulted in sucrose delivery on approximately 16.7% of the 4 s intervals during a 50-min session, or about 1 sucrose presentation every 6 intervals (24 s). Across three dipper training sessions the probability function was gradually decreased to 5% (1 sucrose presentation per 120 s). Robust dipper entry behavior was evident for all rats by the end of day 3.
Discrimination Training
Rats assigned to the “Drug-Feature” conditions received 8 presentations of a 15-s CS (white noise or light) during each session. On drug sessions (0.4 mg/kg nicotine, 1 mg/kg amphetamine IP, or 5 mg/kg CDP injected before the session), each CS presentation was followed immediately by 4-s access to sucrose. On saline sessions 0.9% NaCl was injected before the session and CS presentations were not followed by any outcome. Rats assigned to the “Drug-CS” conditions did not receive the white noise or light during the experiment. Rather, on drug sessions, they received 8 unsignaled presentations of the 4-s sucrose US. During saline sessions there were no scheduled events. For both Drug-Feature and Drug-CS conditions, no sucrose occurred during saline sessions, instead a 4-s “empty interval” replaced sucrose to equate data sampling across the sessions.
Discrimination Training was separated into 8-day cycles during which each rat received 4 drug and 4 saline sessions in quasi-random order. The cycles were constructed such that the same session type (drug or saline) did not occur on more than 2 consecutive days. Each session was controlled by one of 4 Med-State Notation (Med Associates, Georgia VT) programs which varied the first CS onset, US onset, and the order of inter-trial intervals (ITIs). Varying these intervals within and across trials made CS/US onset less predictable. For rats in the Drug-Feature conditions, ITIs were defined as the time from CS offset to the next CS onset (mean=154 s, range=94-214 s), the first CS occurred on average 120 s after the session began (range=75-165 s). For rats in the Drug-CS condition, ITIs were defined as the time from US offset to next US onset (mean=141 s, range=90-210 s), the first US occurred on average 120 s after the session began (range=90-150 s). The marginal interval differences between the two conditions were driven by differences between events during the session (e.g., 15 s CS vs. unsignaled US) and the manner in which programs were constructed to sample data. Unless otherwise noted, rats in each condition received 40 training sessions; 20 preceded by drug and 20 preceded by saline.
Drug Extinction
In each drug-extinction experiment, 20 sessions were included to ensure that any anticipatory food seeking evoked by the drug had been abolished. Because of the long interval between training and testing (20 days) positive control groups determined whether the drug states retained the ability to evoke or facilitate anticipatory food-seeking after this extended retention-interval. Unless otherwise noted, rats in each condition (Drug-Feature or Drug-CS) were randomly assigned to one of two extinction treatment groups [EXT (i.e., drug-extinction) or RET (i.e., retention-interval)] with the constraint that conditional responding during the training phase did not differ across groups. For rats in the Drug-Feature conditions, this assignment was further constrained by balancing of CS modality. The Drug Extinction phase consisted of 20 consecutive sessions in which no stimuli were presented (i.e., white noise, light, or sucrose). Rats in the EXT groups received drug injections before each session. Rats in the RET groups received saline injections before each session. All other parameters were identical to Discrimination Training, except that dipper entries were recorded in 30-s intervals across each session for all rats.
Post-Extinction Tests
Unless otherwise noted, the effect of drug extinction was tested in two 20-min sessions which occurred on consecutive days. For all rats, one of these sessions occurred after drug injection; the other occurred after saline injection. In each condition (Drug-Feature or Drug-CS), the test order (drug or saline) was counterbalanced for group (EXT or RET) and CS modality (white noise or light) where appropriate. For rats in the Drug-Feature condition, each test contained 3 presentations of the discrete cue from Discrimination Training. No stimuli were presented during the test sessions for rats in the Drug-CS condition.
Specific Experiments
Experiment 1
Following dipper training, rats were randomly assigned to one of 2 conditions: Nicotine-Feature, n=16 or Nicotine-CS, n=14. For both conditions nicotine (0.4 mg/kg) or saline injections were administered 5 min before each session.
Experiment 2A
Following dipper training, rats in the both groups (AMP, n=7 or CDP, n=5) underwent discrimination training in which one of the drugs served as a putative CS for sucrose (Drug-CS conditions). For the AMP group, amphetamine (1 mg/kg) or saline was injected 15-min before each session. For the CDP group, CDP (5 mg/kg) or saline was injected 15-min before each session. Discrimination Training was originally scheduled to be conducted in 5 eight-day cycles (40 sessions). However at the end of the 5 cycle, the data pattern suggested that additional training might result in a more reliable CR. Therefore, two additional training cycles were added. Extinction and test phases were not included based on the pattern of goal tracking at the end of the acquisition phase (see Results).
Experiment 2B
Following dipper training, rats in the both groups (AMP, n=16 or CDP, n=16) underwent discrimination training in which one of the drugs served as positive feature for CS-sucrose pairings (Drug-Feature conditions). Amphetamine (1 mg/kg), CDP (5 mg/kg), or saline was injected 15-min before each session. During the training phase, one AMP rat trained with the white noise CS was mistakenly switched to the light CS for 8 sessions (i.e., 1 cycle). The rat was dropped from the experiment and its data were eliminated from figures and analyses. During Discrimination Training, the experimenter (MIP) observed that rats receiving CDP and the white noise CS appeared to be making “maintained head-poke” responses during the CS. As a result, the interface equipment and programs were altered to allow collection of both frequency and duration of dipper entries. Duration data was acquired by generating a “count” for every interrupt request (10-ms resolution) that the dipper receptacle beam was broken. Beginning on session 12, dipper entry durations were recorded and converted to seconds; pre-CS durations were subtracted from CS durations to produce what will hereafter be referred to as a “duration score”. This measure was chosen because it is an analog of the elevation score (see later). During the drug extinction phase the proportion of time that the dipper receptacle beam was broken during the first 2-min of each session was calculated as an analog of the dipper entry rate score.
Dependent Measures & Data Analyses
Drug-Feature Conditions
Elevation scores were calculated as dipper entries that occurred during the 15 s CS minus dipper entries that occurred during the 15 s before CS onset [9].Average elevation scores for each session served as the main dependent measure. An elevation score of 0 indicates equal goal tracking during the CS and pre-CS periods across a session; positive elevation scores indicate more goal tracking during the CS. For Discrimination Training, three-way mixed factors analysis of variance (ANOVA) compared mean elevation scores across Drug (nicotine vs. saline), CS (light vs. white noise), and Session (1-20). For the Post-Extinction Test, a three-way mixed factors ANOVA compared mean elevation scores across Drug (nicotine vs. saline), Group (EXT vs. RET), and Trial (1-3).
Drug-CS Conditions
The main dependent measure was the number of dipper entries that occurred before the first 4-s sucrose delivery (nicotine sessions) or 4-s empty interval (saline sessions). This frequency score was converted to dipper entry rate to equate for the varying time to first US onset across sessions (see Procedures). For Discrimination Training, two-way repeated measures ANOVA compared dipper entry rate across Drug (nicotine vs. saline) and Session (1-20). For the Post-Extinction Test, two-way mixed factors ANOVA compared dipper entry rate across Drug (nicotine vs. saline) and Group (EXT vs. RET).
Drug Extinction
During the Drug Extinction phase no stimuli were presented. Therefore, the dipper entry rate from the first 2 min of each session was calculated for both conditions (Feature and CS) because this was the main dependent measure of nicotine-evoked conditional responding during Discrimination Training. Since the treatments and measures were identical across conditions during Drug Extinction, a three-way mixed factors ANOVA compared dipper entry rate for Group (EXT vs. RET), Condition (Feature vs. CS), and Session (1-20). For each phase and/or condition, significant interactions were followed up by paired or independent samples t-tests contrasting across Drug or Group, respectively. Statistical significance was set a priori at p≤0.05 (two-tailed) for all statistical analyses.
Results
Experiment 1
Discrimination Training
A stimulus paired with sucrose in the nicotine state, but not in the saline state, evoked nicotine-specific goal tracking (Figure 1A). Omnibus three-way ANOVA revealed that all main effects and interactions were significant, Fs≥4.31, ps<0.001, except for the Drug x CS interaction, F<1. More goal tracking during the white noise, relative to the light, was evident on both nicotine and saline sessions during the initial training sessions. Independent samples t-tests with Bonferroni’s correction (calculated p*20 comparisons) contrasted CS modality (white noise vs. light) for each Drug (nicotine or saline) to further investigate the Drug x CS x Session interaction. For nicotine sessions, rats trained with the white noise CS had higher mean elevation scores than rats trained with the light CS from Sessions 3-6, ts(14)≥4.16, ps≤0.04. A similar pattern was revealed across saline sessions; rats trained with the white noise CS had higher mean elevation scores than rats trained with the light CS on session 2, t(14)=3.26, p=0.04.
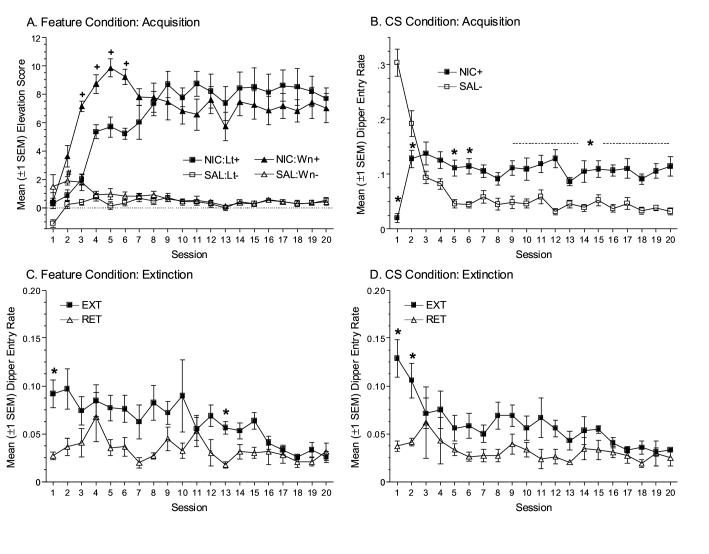
Figure 1.
(A) Mean elevation scores (±1 SEM) from discrimination training for rats in the Feature condition receiving the light or white noise target CS. (B) Mean dipper entry rates (±1 SEM) from discrimination training for rats in the CS condition. Mean dipper entry rates for EXT and RET groups during the drug extinction phase for rats in the Feature (C) and CS (D) conditions, respectively. + indicates elevation score for rats receiving the white noise CS differs significantly from rats receiving the light CS in the nicotine state, p<0.05. # indicates elevation score for rats receiving the white noise CS differs significantly from rats receiving the light CS in the saline state, p<0.05. * indicates dipper entry rate on drug session significantly differs from responding on corresponding saline session (B), or dipper entry for EXT group significantly differs from RET group (C & D), p<0.05.
The pharmacological effects of nicotine evoke a goal tracking CR when paired with intermittent sucrose presentation (CS Condition; Figure 1B). The ANOVA revealed significant main effects of Drug, F(1,247)=27.45, p<0.001, and Session, F(19,247)=9.66, p<0.001, and a significant Drug x Session interaction, F(19,247)=27.42, p<0.001. Goal tracking rate was lower on the first nicotine session relative to the first saline session, t(13)≥13.17, corrected p=0.01. On Sessions 5, 6, and 9-20, dipper entry rates on nicotine sessions were higher than respective saline sessions, ts(13)≥4.26, corrected ps≤0.02. This pattern is consistent with psychomotor suppression induced by acute nicotine treatment, followed by hyperactivity and/or acquisition of a nicotine-specific CR after chronic treatment (see Drug Extinction).
Drug Extinction
EXT groups in both conditions initially displayed more goal tracking than RET groups; this difference decreased across Drug Extinction sessions. Omnibus ANOVA revealed significant main effects of Session, F(19,494)=6.72, p<0.001, of Group, F(1,26)=12.28, p<0.01, and a significant Group x Session interaction, F(19,494)=2.40, p<0.01. The main effects and interactions that included the Condition factor (Drug-Feature vs. Drug-CS) were not significant, Fs<1. Corrected (calculated p*20 comparisons) independent samples t-tests compared dipper entry rates from each session for Feature or CS conditions. For rats in the Feature condition, the EXT group had significantly higher dipper entry rates than the RET group on Drug Extinction sessions 1 and 13, ts(14)≥4.33, ps≤0.02. For rats in the CS condition, the EXT group had higher dipper entry rates than the RET group on Sessions 1-2, ts(12) ≥4.02, ps≤0.04. Because the nicotine-evoked goal tracking CR was extinguished after repeated nicotine-alone treatment, the CR cannot be explained by drug-induced hyperactivity (Figures 1C & 1D).
Post-Extinction Tests
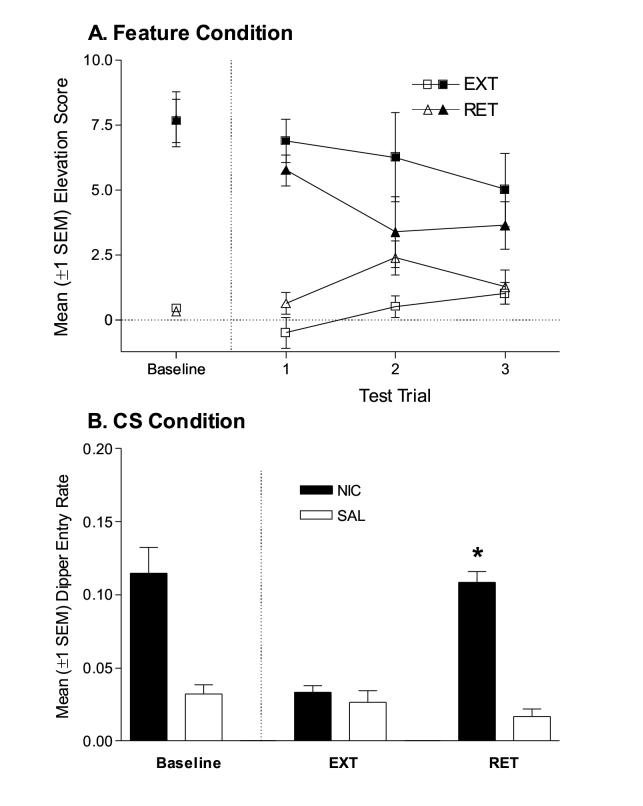
Facilitation of conditioned responding by nicotine does not depend on nicotine-evoked goal tracking (Figure 2A). The three-way ANOVA revealed a significant main effect of Drug, F(1,28)=41.84, p<0.001, a significant Drug x Group interaction, F(1,28)=4.74, p<0.01, and a significant Drug x Trial interaction, F(2,28)=3.82, p<0.01. No other main effects or interactions were significant, Fs<1. Rats in the RET group had lower elevation scores on the nicotine test and higher elevation scores on the saline test. We would not expect this pattern if nicotine was, in fact, a partially extinguished conditioned excitor in the EXT group. For the EXT group (Figure 2A), elevation scores on the nicotine session were significantly higher than the saline session for all 3 trials, ts(7) ≥2.35, ps≤0.050. For the RET group, elevation scores on the nicotine session differed from saline session scores on trials 1 and 3, ts(7)≥2.52, ps≤0.040.
Figure 2.
(A) Mean elevation scores (±1 SEM) from the post-extinction test for rats in the Feature condition. For comparative purposes, baselines are included in the left-hand portion of the graph. These data points represent mean elevation scores from the first trial of the last nicotine and saline sessions for each group from Discrimination Training. Filled symbols represent elevation scores from nicotine trials, open symbols represent elevation scores from saline test trials. (B) Mean dipper entry rates (±1 SEM) from the post-extinction test for rats in the CS condition. Baselines are included in the left-hand portion of the graph; these data points represent means for each measure from the last nicotine and saline sessions of Discrimination Training. * indicates dipper entry rate on drug session differed significantly from dipper entry rate on saline session, p<0.05.
On the Post-Extinction Tests, repeated presentation of nicotine attenuated the goal tracking CR previously evoked by nicotine; repeated saline injections did not alter the conditional response (Figure 2B). The two-way ANOVA on dipper entry rate revealed significant main effects of Group, F(1,12)=27.92, p<0.001, and of Drug, F(1,12)=56.31, p<0.001, as well as a significant Group x Drug interaction, F(1,12)=41.21, p<0.001. For the RET group, dipper entry rate was higher on the nicotine test, relative to the saline test, t(6)=10.29, p<0.001. However, dipper entry rate for the EXT group did not differ across test sessions, t<1.
Experiment 2A
Discrimination Training
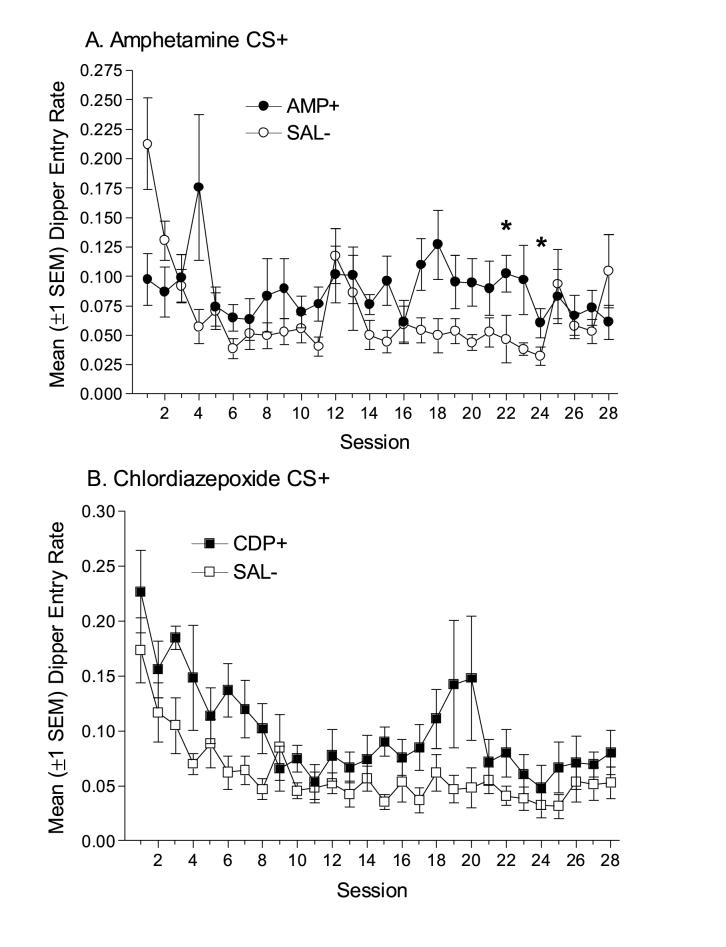
Amphetamine evoked weak and unreliable goal-tracking as the result of repeated pairing with sucrose (Figure 3A). Omnibus two-way ANOVA revealed a significant main effect of Session and a significant Drug x Session interaction, Fs(27,324)≥2.83, ps<0.001. The main effect of Drug was not significant, F(1,324)=2.05, p=0.178. In order to further explore the interaction, paired-samples t-tests contrasted dipper entry rates from amphetamine sessions with corresponding saline sessions. Notably, dipper entry rates differed only on sessions 22 and 24, ts≥2.81, corrected ps≤0.03. CDP also evoked weak and unreliable goal tracking (Figure 3B). Omnibus two-way ANOVA revealed a significant main effect of Session, F(27,81)=7.30, p<0.001. The main effect of Drug, F(1,81)=4.60, p=0.064, and the Drug x Session interaction, F(27,81)=1.08, p=0.362, were not significant. The present study suggests that under conditions in which amphetamine and CDP serve as positive features [28], and nicotine functions as a CS (Experiment 1, see also [4, 47]) CDP and amphetamine do not function as interoceptive conditional stimuli and evoke goal tracking.
Figure 3.
(A) Mean dipper entry rates (±1 SEM) from the Discrimination Training phase for rats in the Amphetamine group. (B) Mean dipper entry rates (±1 SEM) from the Discrimination Training phase for rats in the Chlordiazepoxide (CDP) group.
Experiment 2B
Discrimination Training
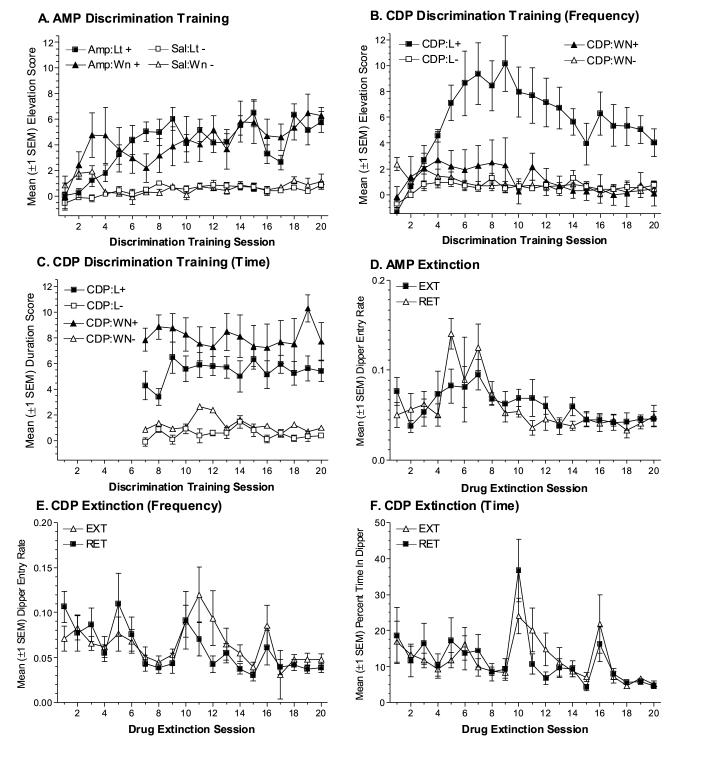
Amphetamine does not evoke anticipatory food-seeking as the result of pairings with sucrose (Experiment 2A), however under similar conditions it facilitates anticipatory food-seeking evoked by discrete auditory and visual stimuli (Figure 4A, see also [31]). The three-way ANOVA revealed significant main effects of Drug, F(1,247)=46.26, p<0.001, and Session, F(19,247)=6.55, p<0.001, as well as a Drug x Session interaction, F(19,247)=5.58, p<0.001. The main effect of CS, the Drug x CS interaction, Fs<1, and the Drug x CS x Session interaction, F(19,247)=1.44, p=0.11, were not significant. However, the CS x Session interaction was significant, F(19,247)=2.81, p<0.001.
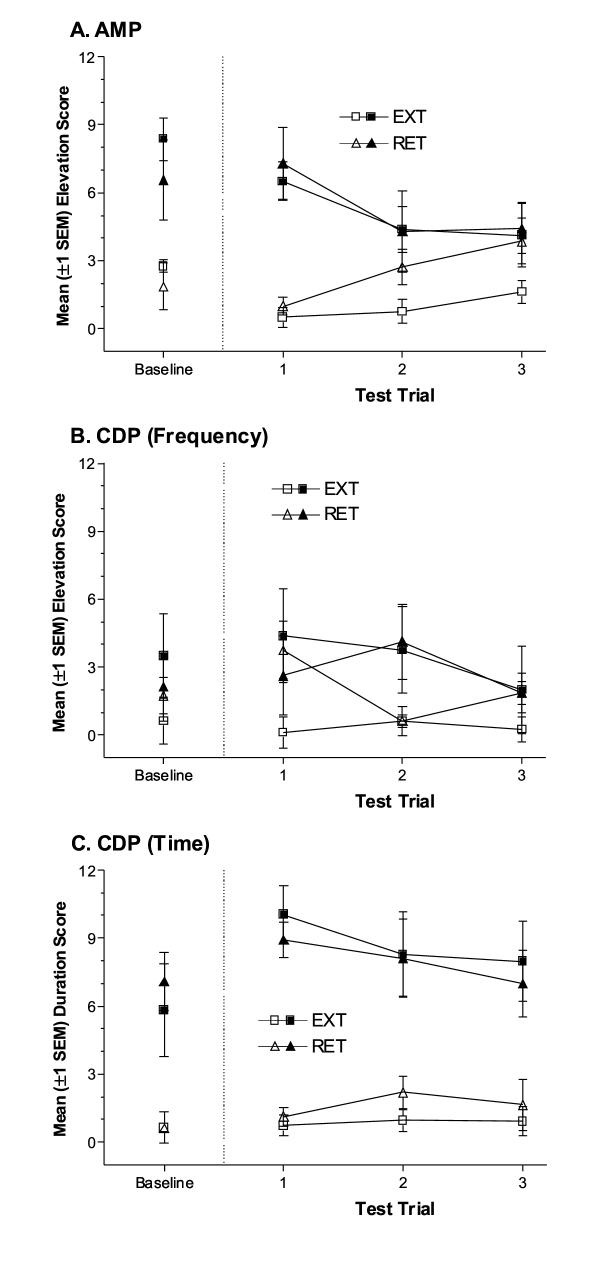
Figure 4.
(A) Mean elevation scores (±1 SEM) from the discrimination training phase for rats in the AMP condition. (B) Mean elevation scores (±1 SEM) from the discrimination training phase for rats in the CDP condition. (C) Mean duration scores (±1 SEM) from the discrimination training phase for rats in the CDP condition. (D) Mean dipper entry rates (±1 SEM) from the drug extinction phase for EXT and RET groups from the AMP condition. (E) Mean dipper entry rates (±1 SEM) from the drug extinction phase for EXT and RET groups from the CDP condition. (F) Mean dipper entry proportions (±1 SEM) from the drug extinction phase for EXT and RET groups from the CDP condition.
Similar to amphetamine, CDP facilitates anticipatory food-seeking only when an interposed stimulus is paired with the sucrose US. However, the topography of this goal tracking CR depends on CS modality (Figures 4B and 4C). For elevation scores (Figure 4B), the three-way ANOVA revealed that all main effects and interactions were significant, Fs≥3.02, ps≤0.017. For rats receiving the light CS, mean elevation scores were significantly higher in the CDP state relative to saline from sessions 3-20, ts(7)≥2.73, corrected ps≤0.03. However, for rats receiving the white noise CS, CDP did not appear to facilitate anticipatory food-seeking; mean elevation scores did not differ across drug states on any session, ts(7)≤1.70, ps≥0.133. Despite a lack of drug state-specific goal tracking with the elevation score measure, a drug state-specific CR was evident for rats receiving the white noise CS when the duration of dipper-entry behaviors was measured (Figure 4C). The ANOVA revealed that only the main effects of Drug, F(1,182)=59.98, p<0.001, and CS, F(1,14)=5.19, p=0.04, were significant. This “maintained headpoke” conditional response was unsurprising given previous studies of goal tracking CRs evoked by auditory CSs [9, 15]. What was surprising was that the topography of conditional responding interacted with drug state. Goal tracking frequency during auditory and visual cues did not differ when rats were under the influence of psychomotor stimulants (AMP group, see also Feature condition, Experiment 1). However, they did differ when rats were under the influence of psychomotor depressants (CDP group). Future studies will be required to determine how and whether stimulants prevented the “maintained headpoke” topography or whether CDP may have exaggerated it.
Drug Extinction
During Drug Extinction, goal tracking evoked by drug (EXT groups) and saline (RET groups) did not differ, confirming that amphetamine and CDP do not evoke a conditional goal tracking response under the parameters employed in these studies. Mean dipper entry rates for the AMP condition are presented in Figure 4D. The two-way ANOVA revealed a significant main effect of Session, F(19,247)=4.79, p<0.001. The main effect of Group and the Group x Session interaction were not significant, Fs≤1.27, ps≥0.29. Mean dipper entry data for the CDP condition are presented in Figures 4E and 4F. The two-way ANOVA for dipper entry rates (Figure 4E) revealed a significant main effect of Session, F(19,266)=3.82, p<0.001. The main effect of Group, F<1, and the Group x Session interaction, F(19,266)=1.29, p=0.19, were not significant. For dipper proportion (Figure 4F), there was a main effect of Session, F(19,266)=6.07, p<0.001. The main effect of Group and the Group x Session interaction were not significant, Fs<1.
Post-Extinction Tests
On the Post-Extinction Test, both EXT and RET groups displayed more goal tracking during the CS on the drug session relative to the saline session (Figures 5A & 5C). Thus, repeated presentation of the drug feature alone did not abolish its ability to facilitate anticipatory food-seeking. The three-way ANOVA for the AMP condition revealed a significant main effect of Drug, F(1,26)=17.76, p<0.001, and a significant Drug x Trial interaction, F(2,26)=8.31, p<0.01. No other main effects or interactions were significant, Fs≤1.49, ps≥0.24.
Figure 5.
(A) Mean elevation scores (±1 SEM) for EXT and RET groups from the post-extinction test for rats in the AMP condition of Experiment 2B. (B) Mean elevation scores (±1 SEM) for EXT and RET groups from the post-extinction test for rats in the CDP condition. (C) Mean duration scores (±1 SEM) for EXT and RET groups from the post-extinction test for rats in the CDP condition. Filled symbols represent elevation or duration scores from drug test trials, open symbols represent elevation or duration scores from saline test trials. Baselines are included in the left-hand portion of each panel; these data points represent mean elevation scores from the first trial of the last drug and saline sessions from the Discrimination Training phase.
For the CDP condition, the three-way ANOVA on elevation scores revealed that none of the main effects or interactions were significant, Fs≤3.39, ps≥0.09. For duration scores, there was a significant main effect of Drug, F(1,28)=48.64, p<0.001; with no other main effects or interactions being significant, Fs≤1.59, ps≥0.22.
Discussion
One reason for investigating drug-states as occasion setters is their contextual nature. Drug states are relatively enduring and therefore may be ‘on’ in the presence of many different contexts, stimuli, and responses (any of which may be rewarding or aversive). This ubiquity may bias them toward facilitative and/or inhibitory control over behavior by providing information about relationships between contexts, stimuli, and responses/reinforcement. In the present studies, the stimulus effects of nicotine, amphetamine, and CDP are experienced for long periods without sucrose delivery (e.g., inter-trial intervals and any drug effect lasting after the session). Thus, our working hypothesis has been that the drug states should facilitate goal tracking in a manner that is independent of a direct association with sucrose. The present studies support this hypothesis, the drug states facilitated goal-tracking behavior without having to be directly associated with the goal stimulus. In Experiment 1, the interoceptive effects of nicotine reliably evoked a goal-tracking response. Although the drug-extinction treatment attenuated goal tracking that was directly evoked by nicotine, it had no effect on nicotine’s ability to facilitate goal tracking when a discrete CS was presented in the nicotine-state. Although the psychomotor stimulant effects of nicotine may have contributed to drug-evoked goal tracking, extinction in the nicotine state eliminates this possibility. In Experiments 2A and 2B, there was no evidence that amphetamine and CDP directly evoked a reliable goal tracking response. Experiment 2A confirms that both drug states continue to facilitate responding to the CS even after any latent excitatory influence had been abolished.
A second important finding that emerged during these studies was a different topography of goal tracking evoked by the white noise CS in the CDP state, relative to other drug state-CS combinations. Although this finding is somewhat surprising, a different topography does not change the interpretation of the studies; if rats anticipate sucrose delivery, then they should spend more time in the proximity of the goal area. By extension, they would be expected to make dipper entries more frequently and with greater duration. Rats receiving CDP-white noise combinations expressed a goal tracking CR that was not affected by CDP-extinction (Experiment 2B); although the form of the CR was unexpected. We originally chose to measure frequency based on previous studies [9], as well as preliminary studies which suggested this measure adequately captured white noise-evoked goal tracking in both drug and no-drug contexts [28; unpublished data]. However, other studies have shown that auditory stimuli paired with sweet tastes tend to evoke a ‘maintained head-poke’ whereas visual stimuli tend to evoke a higher frequency head-poke [10, 16]. Farwell and Ayres [16] described more frequent goal tracking with a visual CS as the result of both goal- and sign-tracking. Rats alternated between goal seeking and ‘checking’ a light CS to determine its status (i.e., on or off). They argued that the auditory CS did not evoke any ‘checking’ because it was detectable when the subjects’ heads were in the receptacle. Related to this issue, Cleland and Davey [11] found that auditory cues evoke more goal tracking, relative to visual cues, when paired with food rewards. They argued that more sign tracking to the visual stimulus occurred because rats were better able to localize its source. Finally, goal tracking topography may also be affected by use of a discrete US (4-s dipper access) [16; present studies]. A temporally discrete US would presumably make goal seeking temporally explicit and lead to CR topographies that maximize the amount of time spent in the dipper during CS-offset/US-onset periods.
If auditory stimuli encourage a ‘maintained headpoke’, then why was this topography specific to the CDP drug state in the present studies? As previously argued, many of the parameters of these studies should bias rats toward a sustained CR when auditory cues are used. In addition, a depressant drug that reduces operant response-rate [15, 36] might be expected to decrease dipper entry rate. If there were no interference with goal tracking, then the sustained response topography would be expected. Similarly, drugs that increase response rate and do not interfere with goal seeking [i.e., nicotine, 42] might be expected to increase the frequency of dipper entries, precluding the bias. Although this argument hinges on a preexisting and undetected bias for a sustained response to the auditory cue, this bias is not without precedent [16]. In fact, alternative accounts seem even less parsimonious. For example, one could argue that the CDP-white noise combination predisposed rats to make drug-sucrose associations, and therefore rats in this condition simply spent more time in the dipper receptacle. However, some of the many problems with this argument include the discriminant nature of the duration score measure (more goal tracking was still specific to the CS) and the lack of drug-evoked goal tracking after acquisition (during drug-extinction). Further testing is required to determine if there was an unobserved bias toward the sustained CR. If the psychomotor suppressant effects of CDP exposed this bias, then other psychomotor suppressants would be expected to induce the same topography of goal tracking with an auditory CS.
A second unexpected finding was that nicotine directly evoked goal tracking, but amphetamine and CDP did not. Although the study that directly tested whether these drugs would evoke goal tracking (Experiment 2A) had a relatively small sample size, the finding was corroborated by Experiment 2B in which the drug state did not evoke goal tracking during the extinction phase. Although we cannot determine whether associations were made between the drug states and sucrose, we can reasonably conclude that such associations did not contribute to the goal tracking CR. This conclusion prompts a number of interesting questions about why amphetamine and CDP do not directly evoke goal seeking responses, whereas nicotine did evoke anticipatory food seeking behavior that was susceptible to extinction. Do the effects of these two drugs overshadow the sucrose reward or interfere with the goal tracking response? Unlikely, given that these accounts also make the prediction that amphetamine and CDP would not facilitate CRs evoked by a discrete stimulus (cf. Experiment 2B). In other unpublished studies we have manipulated various parameters including the use of less reinforcing drug states (e.g., caffeine), different drug doses (e.g., 0.5 mg/kg amphetamine), and more frequent sucrose deliveries (e.g., 36 deliveries in 20 min). At this time, nicotine is the only drug state which reliably evokes anticipatory food seeking [4, 47]. Future studies should determine what features of nicotine make it relatively unique in this regard, or determine the necessary conditions to establish other drugs as interoceptive CSs. For example, do rats fail to make associations between these other drug states and the sucrose US? Is a longer session necessary due to pharmacokinetic or pharmacodynamic reasons? Why are these drugs able to facilitate responding to discrete cues despite their inability to directly evoke anticipatory food seeking?
In the present studies nicotine, amphetamine, and CDP facilitated goal tracking and this facilitation did not depend on direct associations between the drug state and the goal stimulus. Preliminary evidence [26] suggests that these drug states will also transfer their facilitative control over responding to novel situations. Together, these two findings suggest that the drug states may influence goal tracking via hierarchical or non-associative processes, similar to other Pavlovian occasion setters [44]. Returning to our earlier example, a drug state that previously set the occasion for pairings between a discrete stimulus and smoking could also facilitate conditional responses (craving, tobacco seeking, etc.) when a new smoking-related stimulus occurs in that context. Moreover, extinguishing the drug context would not be sufficient to eliminate its facilitative control. This offers a complex set of challenges to exposure therapies [5]. Evidence from the associative learning literature suggests that eliminating facilitation by occasion setters requires the original feature-CS combination to be extinguished [23]. Although such extensive exposure may not be realistic in the clinical setting, both the recipients and providers of these treatments should be wary of ‘drug contexts’ and how they may influence the meaning of stimuli and responses as hindrances to treatment or inducers of relapse.
Acknowledgements
This research was partially supported by NIH (DA11893) and the University of Nebraska-Lincoln Research Council. The experiments partially fulfilled the doctoral degree requirements of the first author, who was supported by NIH grant DA16179A. RA Bevins was partially supported by DA018114 while preparing this manuscript. We thank Cody Brooks for his thoughtful comments on a previous version of this manuscript.
References
- [1].American Psychiatric Association . American Psychiatric Association. Fourth Edition. Washington, DC: 2000. Diagnostic and statistical manual of mental disorders. Third Revision (DSM IV-TR) [Google Scholar]
- [2].Appel JB, West WB, Rolandi WG, Alici T, Pechersky K. Increasing the selectivity of drug discrimination procedures. Pharmacol Biochem Behav. 1999;64:353–358. doi: 10.1016/s0091-3057(99)00089-1. [DOI] [PubMed] [Google Scholar]
- [3].Balfour DJ, Wright AE, Benwell ME, Birrel CE. The putative role of extra-synaptic mesolimbic dopamine in the neurobiology of nicotine dependence. Behav Brain Res. 2000;113:73–83. doi: 10.1016/s0166-4328(00)00202-3. [DOI] [PubMed] [Google Scholar]
- [4].Besheer J, Palmatier MI, Metschke DM, Bevins RA. Nicotine as a signal for the presence or absence of sucrose reward: a Pavlovian drug appetitive conditioning preparation in rats. Psychopharmacology. 2004;172:108–117. doi: 10.1007/s00213-003-1621-9. [DOI] [PubMed] [Google Scholar]
- [5].Bevins RA, Palmatier MI. Extending the role of associative learning processes in nicotine addiction. Behav Cog Neurosci Rev. 2004;3:143–158. doi: 10.1177/1534582304272005. [DOI] [PubMed] [Google Scholar]
- [6].Boakes RA. Performance on learning to associate a stimulus with positive reinforcement. In: Davis H, Hurwitz HMB, editors. Operant-Pavlovian interactions. Lawrence Erlbaum Associates; Hillsdale, New Jersey: 1977. pp. 67–101. [Google Scholar]
- [7].Bouton ME, King DA. Effect of context on performance to conditioned stimuli with mixed histories of reinforcement and nonreinforcement. J Exp Psych: Anim Behav Proc. 1983;12:4–15. [Google Scholar]
- [8].Bouton ME, Swartzenturber D. Analysis of the associative and occasion-setting properties of contexts participating in a Pavlovian discrimination. J Exp Psych: Anim Behav Proc. 1986;12:333–350. [Google Scholar]
- [9].Brooks DC, Bouton ME. A retrieval cue for extinction attenuates response recovery (renewal) caused by a return to the conditioning context. J Exp Psych: Anim Behav Proc. 1994;20:366–379. [Google Scholar]
- [10].Carrigan PF, Benedict JO, Ayres JJ. A comparison of lever press and head-poke discriminated Sidman avoidance. Behav Res Meth Inst. 1972;4:301–303. [Google Scholar]
- [11].Cleland GG, Davey GC. Autoshaping in the rat: The effects of localizable visual and auditory signals for food. J Exp Anal Behav. 1983;40:47–56. doi: 10.1901/jeab.1983.40-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Conklin CA, Tiffany ST. Applying extinction research and theory to cue-exposure addiction treatments. Addiction. 2002;97:155–67. doi: 10.1046/j.1360-0443.2002.00014.x. [DOI] [PubMed] [Google Scholar]
- [13].Connelly JF, Connelly JM, Phifer R. Disruption of state-dependent learning (memory retrieval) by emotionally-important stimuli. Psychopharmacologia. 1975;41:139–143. doi: 10.1007/BF00421071. [DOI] [PubMed] [Google Scholar]
- [14].Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- [15].Evenden JL, Robbins TW. The effects of d-amphetamine, chlordiazepoxide and alpha-flupenthixol on food-reinforced tracking of a visual stimulus by rats. Psychopharmacology. 1985;81:361–366. doi: 10.1007/BF00428202. [DOI] [PubMed] [Google Scholar]
- [16].Farwell BJ, Ayres JJ. Stimulus-reinforcer and response-reinforcer relations in the control of conditioned appetitive headpoking (goal tracking) in rats. Learn Motiv. 1979;10:295–312. [Google Scholar]
- [17].Heishman SJ, Henningfield JE. Discriminative stimulus effects of d-amphetamine, methylphenidate, and diazepam in humans. Psychopharmacology. 1991;103:436–442. doi: 10.1007/BF02244241. [DOI] [PubMed] [Google Scholar]
- [18].Holland PC. Element pretraining influences the content of appetitive serial compound conditioning in rats. J Exp Psych: Anim Behav Proc. 1985;11:367–387. [PubMed] [Google Scholar]
- [19].Holland PC. Feature extinction enhances transfer of occasion setting. Anim Learn Behav. 1989;17:269–279. [Google Scholar]
- [20].Jarbe TU, Lamb RJ. Effects of lithium dose (UCS) on the acquisition and extinction of a discriminated morphine aversion: tests with morphine and delta9-THC. Behav Pharmacol. 1999;10:349–58. doi: 10.1097/00008877-199907000-00002. [DOI] [PubMed] [Google Scholar]
- [21].Koob GF, LeMoal M. Plasticity of reward neurocircuitry and the ‘dark side’ of drug addiction. Nat Neurosci. 2001;8:1442–1444. doi: 10.1038/nn1105-1442. [DOI] [PubMed] [Google Scholar]
- [22].Martin GM, Gans M, Van der Kooy D. Discriminative properties of morphine that modulate associations between tastes and lithium chloride. J Exp Psych: Anim Behav Proc. 1990;16:56–68. [PubMed] [Google Scholar]
- [23].Miller RR, Oberling P. Analogies between occasion setting and Pavlovian conditioning. In: Holland PC, Schmajuk NA, editors. Occasion setting: Associative learning and cognition in animals. American Psychological Association; Washington, DC, US: 1998. pp. 3–35. [Google Scholar]
- [24].Miller MA, Parker BK, Keely JP, Johnson JL, Schaal DW. Searching for evidence of transfer between drug facilitators. Learn Motiv. 2002;33:197–229. [Google Scholar]
- [25].Nakajima S. Transfer testing after serial feature-ambiguous discrimination in Pavlovian keypeck conditioning. Anim Learn Behav. 1997;25:413–426. [Google Scholar]
- [26].Palmatier MI, Bevins RA. Transfer of facilitation by drug states. Behav Brain Res. doi: 10.1016/j.bbr.2006.10.015. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palmatier MI, Peterson JL, Wilkinson JL, Bevins RA. Nicotine serves as a feature-positive modulator of Pavlovian appetitive conditioning in rats. Behav Pharmacol. 2004;15:183–194. [PubMed] [Google Scholar]
- [28].Palmatier MI, Wilkinson JL, Metschke DM, Bevins RA. Stimulus properties of nicotine, amphetamine, and chlordiazepoxide as positive features in a Pavlovian appetitive discrimination task in rats. Neuropsychopharmacol. 2005;30:731–741. doi: 10.1038/sj.npp.1300629. [DOI] [PubMed] [Google Scholar]
- [29].Parker DK, Schaal DW, Miller M. Pharmacol Biochem Behav. 1994;49:955–960. doi: 10.1016/0091-3057(94)90249-6. [DOI] [PubMed] [Google Scholar]
- [30].Quertemont E. Discriminative stimulus effects of ethanol with a conditioned taste aversion procedure: lack of acetaldehyde substitution. Behav Pharmacol. 2003;14:343–350. doi: 10.1097/01.fbp.0000082130.08343.47. [DOI] [PubMed] [Google Scholar]
- [31].Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: Variations in the effectiveness of reinforcement and non-reinforcement. In: Black AH, Prokasy WF, editors. Classical Conditioning II: Current Research and Theory. Appleton Century Crofts; New York: 1972. pp. 64–99. [Google Scholar]
- [32].Rescorla RA. Extinction of facilitation. J Exp Psych: Anim Behav Proc. 1986a;12:16–24. [Google Scholar]
- [33].Rescorla RA. Facilitation and excitation. J Exp Psych: Anim Behav Proc. 1986b;12:325–332. [Google Scholar]
- [34].Rescorla RA. Combinations of modulators trained with the same and different target stimuli. Anim Learn Behav. 1991;29:281–290. [Google Scholar]
- [35].Revusky S, Coombes S, Pohl RW. Drug states as discriminative stimuli in a flavor-aversion learning experiment. J Comp Physiol Psych. 1982;96:200–211. doi: 10.1037/h0077870. [DOI] [PubMed] [Google Scholar]
- [36].Rijnders HJ, Jarbe TU, Slangen JL. Extinction and reacquisition of differential responding in rats trained to discriminate between chlordiazepoxide and saline. Psychopharmacology. 1990;102:404–410. doi: 10.1007/BF02244111. [DOI] [PubMed] [Google Scholar]
- [37].Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- [38].Rowan GA, Lucki I. Discriminative stimulus properties of the benzodiazepine receptor antagonist flumazenil. Psychopharmacology. 1992;107:103–112. doi: 10.1007/BF02244973. [DOI] [PubMed] [Google Scholar]
- [39].Sanderson SD, Cheruku SR, Padmanilayam MP, Vennerstrom JL, Thiele GM, Palmatier MI, Bevins RA. Immunization to nicotine with a peptide-based vaccine composed of a conformationally biased agonist of C5a as a molecular adjuvant. International Immunopharmacology. 2003;3:137–146. doi: 10.1016/s1567-5769(02)00260-6. [DOI] [PubMed] [Google Scholar]
- [40].Skinner DM, Goddard MJ, Holland PC. What can nontraditional features tell us about conditioning and occasion setting? In: Holland PC, Schmajuk NA, editors. Occasion setting: Associative learning and cognition in animals. American Psychological Association; Washington, DC: 1998. pp. 113–124. [Google Scholar]
- [41].Skinner DM. Modulation of taste aversions by a pentobarbital drug state: An assessment of its transfer properties. Learn Motiv. 2000;31:381–401. [Google Scholar]
- [42].Stolerman IP, Chandler CJ, Garcha HS, Newton JM. Selective antagonism of behavioural effects of nicotine by dihydro-beta-erythroidine in rats. Psychopharmacology. 1997;129:390–397. doi: 10.1007/s002130050205. [DOI] [PubMed] [Google Scholar]
- [43].Swartzentruber D. Transfer of contextual control across similarly trained conditioned stimuli. Anim Learn Behav. 1993;21:14–22. [Google Scholar]
- [44].Swartzentruber D. Modulatory mechanisms in Pavlovian conditioning. Anim Learn Behav. 1995;23:123–143. [Google Scholar]
- [45].Troisi JR., II Spontaneous recovery during, but not following, extinction of the discriminative stimulus effects of nicotine in rats: Reinstatement of stimulus control. Psych Record. 2003;53:579–592. [Google Scholar]
- [46].Troisi JR., II Nicotine vs. ethanol discrimination: Extinction and spontaneous recovery of responding. Integr Phys & Behav Sci. 2003;38:104–123. doi: 10.1007/BF02688829. [DOI] [PubMed] [Google Scholar]
- [47].Wilkinson JL, Murray JE, Li C, Wiltgen SM, Penrod RD, Berg SA, Bevins RA. Interoceptive Pavlovian conditioning with nicotine as the conditional stimulus varies as a function of number of conditioning trials and unpaired sucrose deliveries. Behav Pharm. 2006;17:161–172. doi: 10.1097/01.fbp.0000197456.63150.cd. [DOI] [PubMed] [Google Scholar]
- [48].Zarcone TJ, Ator NA. Drug discrimination: Stimulus control during repeated testing in extinction. Journal of the Experimental Analysis of Behavior. 2000;74:283–294. doi: 10.1901/jeab.2000.74-283. [DOI] [PMC free article] [PubMed] [Google Scholar]