Abstract
Little is known about the relative sensitivities of antioxidant systems in nuclei, mitochondria and cytoplasm. The present study examined the oxidation of the thiol-dependent antioxidant systems in these subcellular compartments under conditions of limited energy supply of human colonic epithelial HT-29 cells induced by depletion of glucose (Glc) and glutamine (Gln) from the culture medium. Increased oxidation of dichlorofluoroscin (DCF) indicated an increased level of reactive oxygen species (ROS). Redox western blot analysis showed oxidation of cytosolic thioredoxin-1 (Trx1) and mitochondrial thioredoxin-2 (Trx2) by 24 h, but little oxidation of nuclear Trx1. The Trx1 substrate, redox factor-1 (Ref-1), was also oxidized in cytosol but was reduced in nuclei. Protein S-glutathionylation (PrSSG), expressed as a ratio of protein thiol (PrSH), was also increased in the cytosol, while nuclear PrSSG/PrSH was not. Taken together, the data show that oxidative stress induced by depletion of Glc and Gln affects the redox states of proteins in the cytoplasm and mitochondria more than those in the nucleus. These results indicate that the nuclear compartment has better protection against oxidative stress than cytoplasm or mitochondria. These results further suggest that energy and/or substrate supply may contribute to sensitivity of mitochondrial and cytoplasmic systems to oxidative damage.
Keywords: Nuclear redox, oxidative stress, Ref-1, S-glutathionylation, thiol/disulfide
Introduction
Oxidative stress has unique effects in subcellular compartments. For instance, oxidative stress associated with the plasma membrane and cytoplasm alters cell signaling in the control of systemic blood pressure, cell proliferation and oncogenesis [1-3]. Oxidative stress in mitochondria causes mitochondrial DNA damage and stimulates apoptosis and necrosis [4,5]. Oxidative stress in nuclei inhibits binding of key transcription factors (e.g., AP1, NF-kB, Nrf2) to DNA and also causes nuclear DNA damage [6]. Consequently, an understanding of the antioxidant defense systems in the subcellular compartments is critical to developing means to protect against specific types of oxidative stress.
Nuclei, cytoplasm and mitochondria have two major thiol-dependent antioxidant systems, the thioredoxin or glutathione (GSH) systems. Redox western blot analysis [7,8] allows for the determination of the redox state of thioredoxin-1 (Trx1) in nuclei and cytosol and thioredoxin-2 (Trx2) in mitochondria. Results suggest that the major thiol-dependent antioxidant systems may have semi-autonomous functions in the subcellular compartments [7]. Although time course studies with bolus addition of hydrogen peroxide showed that nuclear and cytosolic Trx1 were oxidized similarly in a monocyte cell line [9], physiologic generation of reactive oxygen species (ROS) during EGF signaling in keratinocytes showed selective oxidation of cytosolic Trx1 without oxidation of either nuclear Trx1 or mitochondrial thioredoxin-2 (Trx2) [7]. Compartment-specific oxidation was also observed following TNF-α treatment in HeLa cells, where mitochondrial Trx2 was oxidized while cytosolic Trx1 was not [10]. Calculated redox potentials of the active site dithiol motifs under standard growth conditions in these and other cell lines show that the mitochondrial Trx2 is most reduced (approximate value, −340 mV) while nuclear Trx1 is intermediate (−300 mV) and cytosolic Trx1 is most oxidized (−280 mV) [11].
Evidence is also available to suggest that GSH has compartment-specific functions, especially in mitochondria [12,13]. Early studies showed that cell toxicity is associated with depletion of mitochondrial GSH, not cytosolic GSH [14,15]. Mitochondrial GSH concentration is slightly higher than cytosolic GSH, with secondary active transport mechanisms maintaining the mitochondrial pool [16]. The redox potential of the mitochondrial pool has been estimated to be approximately −280 mV compared to cellular values of −260 to −200 mV [17]. The nuclei have been reported to have a unique GSH pool [18] but this conclusion is controversial because analysis of the nuclear pool is subject to artifacts [19,20]. On the other hand, recent data show transcriptional regulation of c-Jun N-terminal kinase can be regulated by glutathione Stransferase [21]. These observations raise the possibility that GSH in nuclei has functions that are distinct from those in cytoplasm and mitochondria.
Cellular GSH/GSSG redox state, measured under conditions identical to those used to measure Trx1 and Trx2 redox states, show that the GSH and Trx systems function independently [22,23]. For instance, during differentiation of Caco2 cells, GSH/GSSG redox state was oxidized while Trx1 remained unchanged [23]. A study of metal toxicity also showed different responses of the GSH and Trx systems. Specifically, 10 μM Fe2+ or Cu+ induced oxidation of GSH/GSSG without oxidation of Trx1 or Trx2 while As3+ or Cd2+ induced greater oxidation of Trx2 than Trx1 and no oxidation of GSH/GSSG [22]. These responses indicate that metabolic conditions that differentially affect the thiol antioxidant systems in the subcellular compartments could have considerable importance in determining the subcellular specificity of oxidative toxicity.
Glucose (Glc) and glutamine (Gln) are major metabolic fuels in cultured mammalian cell lines. Under most conditions, glucose transport is the rate-limiting step in glucose utilization. Removal of extracellular glucose causes depletion of cellular ATP, stimulated PKC activity, translocation of PKC, phosphorylation of serine of membrane proteins and increased annexin binding, which associated with apoptosis [24]. Gln is also an important metabolic substrate for rapidly dividing cells, GSH biosynthesis, and NAD(P) cofactor biosynthesis [25]. Starvation of Gln showed energy depletion that is associated with an increased sensitivity to apoptotic stimuli and a decreased GSH concentration [26] and clinical data show that Gln administration maintains gut barrier function [27]. Consequently, gut epithelium offers a relevant tissue for study of energy deficiencies and the moderately differentiated colon cancer cell line, HT-29, provides a convenient model system for the intestinal epithelium.
The purpose of the present study was to determine whether redox systems of the cytoplasm, mitochondria and/or nuclei were selectively vulnerable to oxidation during combined Glc and Gln depletion in HT-29 cells. Measurements of redox states of proteins (Trx1, Trx2, Ref-1) in the nuclear, cytosolic, and mitochondrial fractions as well as protein S-glutathionylations show that in Glc-and Gln-deficient media, the nuclear thiol-dependent detoxification systems are selectively protected against oxidation.
Materials and Methods
Cell culture, treatment with Glc- and Gln-free medium, and cell fractionation
HT-29 cells cultured in complete media (10% FBS in DMEM) were grown to 80% confluence. Cells were then washed with HBSS twice, followed by incubation with 10% FBS in DMEM or 10% FBS in Glc and Gln free-DMEM (Cellgro). Subcellular fractions were prepared as described previously [9]. Briefly, HT-29 cells washed with PBS were pelleted by centrifugation and lysed in hypotonic lysis buffer (10 mM Hepes, pH 7.8, 10 mM KCl, 2 mM MgCl2, 0.1 mM EDTA, 0.2 mM NaF, 0.2 mM Na3VO4) with freshly added protease inhibitors (leupeptin, aprotinin, pepstatin, PMSF). For measurement of Trx1 redox state, 50 mM iodoacetic acid (IAA) was added to the lysis buffer and the pH was adjusted to pH 7.8. The suspensions were incubated on ice for 5 min, and NP-40 was added to a final concentration of 0.6%. Following centrifugation at 13,500 rpm, the pellet (nuclei) and the supernatant (cytosol) were separated. Each fraction was saved for further analyses. N-(biotinoyl)-N′-(iodoacetyl)-ethylenediamine, CellTracker (5-chloromethylfluorescein diacetate), and Hoechst dye were from Molecular Probes, and streptavidin-agarose beads, IAA, and DTNB [5,5′-Dithiobis(2-nitrobenzoic acid)] were from Sigma-Aldrich.
Examination of Trx1 and Trx2 redox states by redox western blotting
The cytoplasm and nuclear fractions of HT-29 cells were used to analyze redox forms of Trx1. Whole cell lysates were used for Trx2 analysis by redox western blot method, as previously described in detail [7], because Trx2 is only present in mitochondria. The Trx1 redox state was examined by native gel electrophoresis, blotting to nitrocellulose membrane, probing with mouse anti-human Trx1 (BD BioScience), and detection with an Alexafluor 680 anti-mouse secondary antibody. Rabbit anti-human Trx2 antibody and anti-rabbit secondary antibody were used for Trx2 detection (Molecular Probes). Bands corresponding to Trx1 and Trx2 were visualized using the Odyssey scanner (LI-COR, Lincoln, NE, USA) as outlined [7].
Examination of Ref-1 redox state
HT-29 cells treated with Glc- and Gln- free medium (0, 24, 48 h) were fractionated to prepare cytosol and nuclei. Each fraction (200 μg protein each) was incubated with biotin-labeled iodoacetamide (BIAM); (Molecular Probes), and precipitated with streptavidin-agarose. Each sample was washed 3 times, separated by SDS-PAGE, and analyzed by Western blotting probed with rabbit antibody specific to Ref-1, and Alexafluor 680 anti-rabbit secondary antibody. Bands corresponding to Ref-1 were visualized using the Odyssey scanner. To examine the total amount of Ref-1, 40 μg of protein from each sample was analyzed by SDS-PAGE, with Western blotting by the same procedures.
Glutathione analysis, redox potential (Eh) calculation, and ROS detection
GSH and GSSG were quantified by HPLC with fluorescence detection, and used to calculate the steady-state redox potential values using the Nernst equation, as described previously [28]. For ROS detection by DCF oxidation, HT-29 cells cultured in 96-well plates were incubated without or with Glc- and Gln- free media for 1 h, and DCF fluorescence was measured [29].
Analysis of protein S-glutathionylation
GSH bound to protein was measured as previously described [16]. Proteins from cytosolic and nuclear fractions were initially precipitated with cold 100% TCA [1:4 (v/v)], pellets were re-suspended in an adequate amount of 0.1 M NaOH to neutralize the acid and resolubilize the protein, and 5 mM DTT [1:1 (v/v)] was used as a reductant for 30 min. Following reduction, protein was re-precipitated with cold 10% PCA/boric acid containing internal standard [28], samples were centrifuged, and GSH and GSSG in the remaining supernatant were measured as described above.
Measurement of total protein thiols
To examine total thiols in cytoplasm and nucleus Ellman's reagent (5,5′-dithio-bis(2-nitrobenzoic acid), DTNB) was used to measure the thiol content of protein from respective cytosolic and nuclear fractions by protein precipitation (fully denatured by TCA) and resolubilization as described [16]. Sample (50 μl) was added to a cuvette containing 1 ml 0.05 M Tris/0.05 M EDTA at pH 8.3, and the A412 was measured. Ellman's reagent (25 μl, 5 mM in methanol) was then added to each cuvette, which was thoroughly mixed and then incubated at room temperature for 20 min before remeasuring A412. The increase in A412 after DTNB addition was used to determine the concentration of thiols in the sample [16]. As a secondary method to visualize thiols on proteins, peptides, intracellular thiol-containing biomolecules, and GSH in the cells, we used the thiol-reactive CellTracker probe, green CMFDA (5-chloromethylfluorescein diacetate, Molecular Probes) and fluorescence microscopy imaging. Cells exposed to Glc- and Gln- free or complete media were fixed with 0.2% glutaraldehyde, washed with PBS twice, and labeled with Celltracker. Cells were co-stained with a Hoechst dye for nuclear DNA visualization.
Statistics
Data were evaluated for statistical significance using ANOVA or Student's T-test as appropriate. P < 0.05 was considered significant.
Results
Depletion of glucose (Glc), glutamine (Gln), or Glc and Gln elevated ROS level in HT29 cells
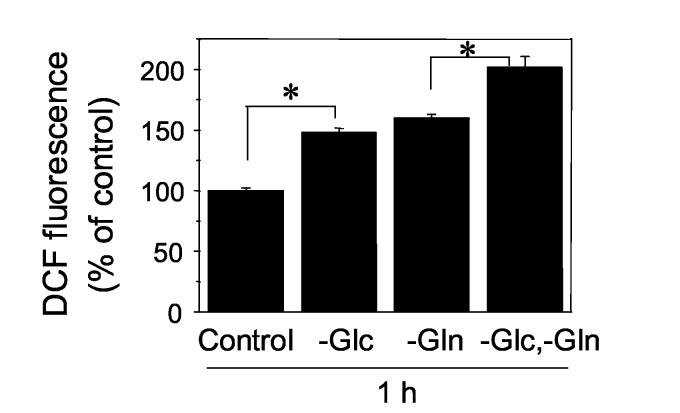
To examine the effects of depleting precursors for energy metabolism on ROS elevation, DCF fluorescence in cells was monitored after treating with complete, Glc-free, Gln-free, or Glc- and Gln-free medium for 1 h (Fig. 1). ROS level was significantly increased by deletion of these nutritients including Glc alone (-Glc: 148 ± 3.8 %), Gln alone (-Gln: 159 ± 3.1 %), or Glc and Gln (-Glc,-Gln: 201 ± 9.1 %) compared to that of control (Cont: 100 ± 2.2 %). Also, ROS level by Glc and Gln depeletion was significantly higher than that of depletion of Glc or Gln alone.
Figure 1.
Depletion of Glc, Gln, or Glc and Gln elevates ROS level in cells. DCF fluorescence as a measure of ROS detection was examined in HT29 cells exposed to complete medium (Control), Glc (-Glc), Gln (-Gln), or Glc-and Gln- (-Glc,-Gln) free medium for 1 h. Data represent the mean ± SE of 8 determinations. *P<0.05.
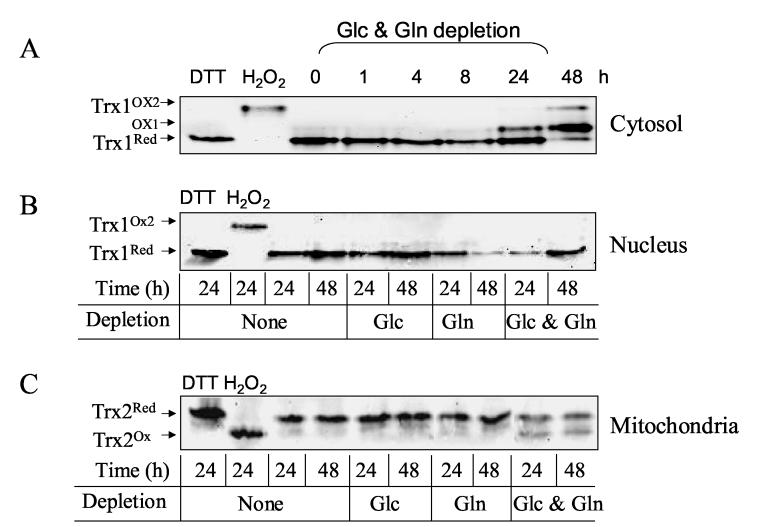
Glc- and Gln-free medium selectively oxidized cytosolic Trx1 and mitochondrial Trx2 without oxidation of nuclear Trx1
To examine thiol/disulfide redox states in the subcellular compartments, we relied upon the unique mitochondrial localization of Trx2 and the ability to examine Trx1 separately in nuclear and cytosolic fractions. Redox western blot analyses of Trx1 in the nuclear and cytosolic fractions (A and B, respectively) and mitochondrial Trx2 (C) are shown in Fig. 2. Transfer of cells to Glc- and Gln-free medium resulted in oxidation of cytosolic Trx1 (Fig. 2A, Trx1Ox1 and Trx1Ox2) and Trx2 in mitochondria (Fig. 2C, Trx2Ox) but not oxidation of nuclear Trx1 (Fig. 2B). In the blots of Trx1, both active site disulfide (Ox1) and a more extensively oxidized form (Ox2) with both active site C32, C35 and a regulator C62, C69 disulfide are visible [8]. Although there was significant increase in ROS level in cells treated with either Glc (-Glc) or Gln (-Gln)- deficient medium as shown in Fig.1, Trx oxidation was observed only in the cytoplasm and mitochondria incubated with Glc- and Gln-free medium. Oxidation of cytosolic Trx1 was clearly observed at 24 h (note Trx1Ox1) and further oxidized (Trx1Ox2) at 48 h incubation. Viability measurements showed no significant cell death at 24 h, however, by 48 h significant cell death (20%-40%) occurred. To minimize contribution of dead cells and detached cells undergoing apoptosis, cells on the plate were washed prior to nuclei preparation.
Figure 2.
Glc and Gln depletion selectively oxidized cytosolic Trx1 and mitochondrial Trx2 without oxidizing nuclear Trx1. Confluent HT29 cells cultured in complete medium, washed with PBS twice, and then exposed to Glc-, Gln-, or Glc- and Gln-free medium for indicated time. As controls, cells were treated with DTT or H2O2 for 30 min. Redox changes in cytosolic (A) and nucleus Trx1 (B), and mitochondrial Trx2 (C) were analyzed by the methods described in previous report [7].
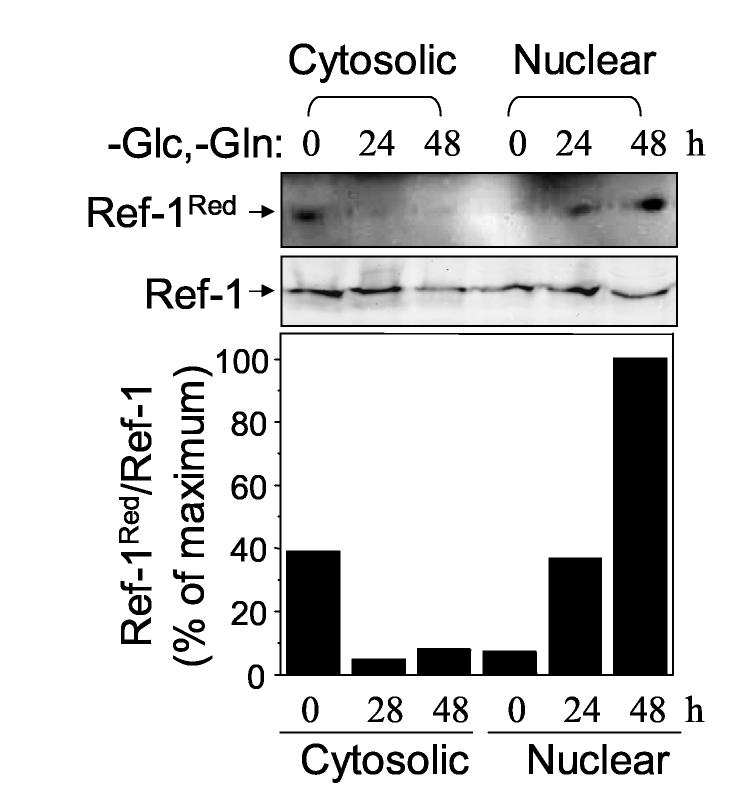
Effect of Glc and Gln removal on cytosolic and nuclear Ref-1
Redox factor-1 (Ref-1) is a substrate of Trx1 that functions in maintenance of redox state of cysteine (Cys) residues in the DNA-binding regions of transcription factors such as AP-1 and NF-κB [30]. To determine whether the difference in oxidation of Trx1 in the cytosolic and nuclear compartments had associated changes in redox state of a protein substrate, we examined Ref-1 redox state in the cytosolic and nuclear compartments following transfer of cells to Glc- and Gln-free medium. For this purpose, cytosol and nuclei were treated with BIAM to modify reduced reactive protein thiols [31]. Streptavidin beads were used to isolate proteins modified with BIAM, and subsequent electrophoresis and western blot analysis with an antibody specific to Ref-1 provided detection of the reduced forms of Ref-1. As shown in Fig. 3, transfer of cells to Glc- and Gln-free media resulted in oxidation of cytosolic Ref-1 while the Ref-1 in the nuclei was reduced (Fig. 3 Top). Total Ref-1 contents in cytosol and nuclei were unaffected by this treatment for 24 h but were diminished by 48 h treatment (Fig. 3 middle) due to loss of cells. The amount of reduced Ref-1 and the total Ref-1 present in each compartment was quantified by densitometry and the ratio of these values showed that Ref-1 was more reduced in nuclei than cytosol following Glc and Gln depletion (Fig. 3 bar graph).
Figure 3.
Effect of Glc and Gln depletion on cytosolic and nuclear Ref-1. HT29 cells treated with Glc- and Gln-free medium for indicated time (0, 24, 48 h) were analyzed for reduced form (top) of Ref-1 and total Ref-1 protein amount (middle) after cytosolic and nuclear fractionation. Bottom graph shows % of reduced Ref-1 normalized to total Ref-1 protein.
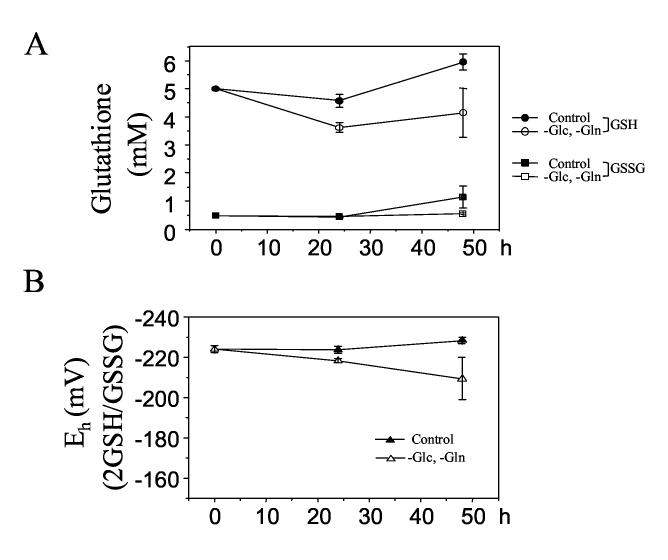
Effect of Glc and Gln depletion on cellular GSH, GSSG, and GSH/GSSG redox state (Eh)
To examine the effect of Glc and Gln depletion on the redox state of cellular GSH/GSSG, cells were exposed to complete medium or Glc- and Gln-free medium for 24 or 48 h and analyzed for GSH and GSSG (Fig. 4A). Depletion of Glc and Gln for 24 h resulted in decreased GSH (A, unfilled circle: 3.6 ± 0.2 mM) compared to the control with complete medium (A, filled circle: 4.6 ± 0.2 mM); however, GSH was increased by 48 h exposure with both control (A, filled circle: 5.9 ± 0.3 mM) and depletion (A, unfilled circle: 4.2. ± 0.3 mM) perhaps due to a cellular recovery mechanism. No significant change in GSSG level was observed following Glc and Gln removal (A, filled square; B, redox potential (Eh) of GSH/GSSG calculated by the Nernst equation). The results showed that there was no significant effect on cellular Eh of GSH/GSSG by Glc- and Gln depletion for 24 h (Depletion: −218.3 ± 1.7 mV, Cont: −223.7 ± 1.7 mV: not significantly different). A small oxidation (unfilled triangle: −209.4 ± 18.2 mV) was observed at 48 h compared to the control (filled triangle: Cont 48 h: −228.3 ± 2.9 mV); however, this oxidation was not significant when it was compared to the initial redox potential (0 h: −223.9 ± 1.8 mV).
Figure 4.
Effect of Glc and Gln depletion on HT29 cellular GSH, GSSG, and GSH/GSSG redox state (Eh). Cells treated with Glc- and Gln-free medium were analyzed for GSH and GSSG (A, filled circle and square: complete medium, empty circle and square: Glc-and Gln-free medium), and redox state (Eh) for GSH/GSSG (B, filled: complete medium, empty: Glc-and Gln-free medium) was calculated with the Nernst equation.
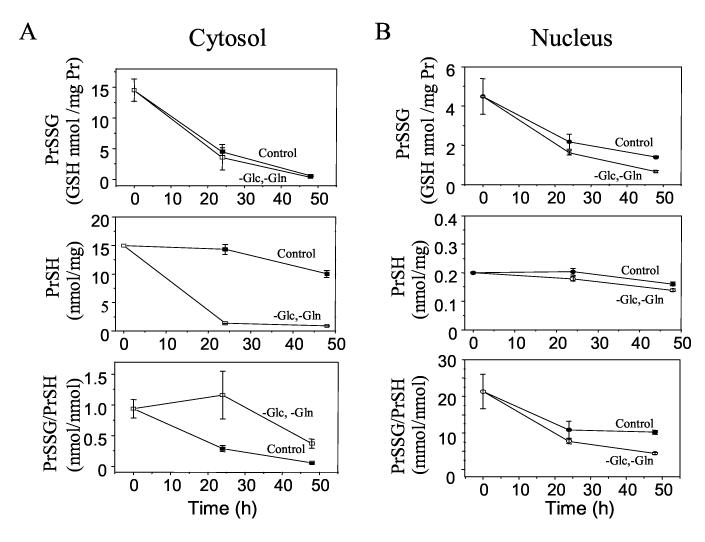
Effect of Glc and Gln depletion on protein S-glutathionylation in cytoplasm and nuclei
To measure changes in redox state of the glutathione system in cytosol and nuclei, we used protein glutathionylation (glutathione-protein mixed disulfide; PrSSG) in the respective compartments as a surrogate for GSH/GSSG redox state. Fig. 5 shows cytosolic S-glutathionylation (PrSSG, A top), protein thiol content (PrSH, A middle), cytosolic S-glutathionylation/protein thiol (PrSSG/PrSH, A bottom), nuclear S-glutathionylation (PrSSG, B top), nuclear protein thiol content (PrSH, B middle), and nuclear protein S-glutathionylation/protein thiol (PrSSG, B bottom). The protein thiol content (Fig. 5 middle, 15 nmol/mg protein) measured in colonic HT-29 cells was lower than that measured in extracts from liver tissue (50 nmol thiol/mg protein) [32]; however, these values are similar to values shown in the previous studies using colonic cancer cell lines (HT-29, Caco-2) and colon tissue [33-36].
Figure 5.
Effect of Glc and Gln depletion on cytosolic S-glutathionylation (glutathione-protein mixed disulfide; PrSSG, A top), protein thiol content (PrSH, A middle), cytosolic S-glutathionylation/Protein thiol (PrSSG/PrSH, A bottom), nuclear S-glutathionylation (PrSSG, B top), nuclear protein thiol content (PrSH, B middle), and nuclear protein Sglutathionylation/protein thiol (B bottom). As controls, cells treated with complete medium were shown as filled circle (A, cytosolic) and square (B, nucleus).
Results showed that the total amount of S-glutathionylation (PrSSG, Fig. 5 top) decreased in cytosol and nuclei independently with Glc and Gln depletion. Total protein thiols measured with DTNB (PrSH, Fig. 5 middle) showed a substantial decrease in the cytosol but not in the nuclei with the Glc- and Gln- deficient media. This observation was supported by fluorescence microscopy of cells stained with the thiol reagent, CellTracker (data not shown). Expression of S-glutathionylation (PrSSG) as a function of total protein thiols (PrSH) showed that in Glc- and Gln-deficient media, the ratio of PrSSG/PrSH was increased in cytosol (Fig. 5A bottom, 24 h control: 0.3 ± 0.04, 24 h depletion: 1.2 ± 0.4) but not in nuclei (Fig. 5B bottom, 24 h control: 10.8 ± 2.3, 24 h depletion: 7.7 ± 0.6). These results indicate a selective oxidation of cytoplasmic proteins with Glc-and Gln-free media even though the total PrSSG per mg protein did not change.
Discussion
Accumulating data show that the Trx systems in mitochondrial, nuclear and cytoplasmic compartments are not in redox equilibrium and have the character that mitochondria are most reduced, nuclei are intermediate and cytoplasm is most oxidized [7,17]. Previously, other studies with isolated nuclei showed that GSH was lost during isolation [18], and studies with an antibody to GSH indicated that the cytoplasmic GSH is considerably higher than nuclear GSH in A549 cells [37]. Thus, loss of GSH during nuclei isolation may disturb thiol/disulfide redox balance and result in artifactual oxidation of thiol/disulfide redox pool and proteins in the nuclei.
In an earlier study of Trx1 redox state in nuclei and cytoplasm [9], we found that the currently used method with inclusion of IAA in the lysis buffer prevented artifactual oxidation. In the present study, Trx1 was oxidized in the cytosolic and mitochondrial compartments but not the nuclear compartment while Trx1 was not oxidized in either compartment in the presence of substrates. Thus, the present data demonstrate that Trx1 in the nuclear compartment is resistant to oxidation. The calculated ratio of PrSSG/PrSH in the nuclear fraction compared to the cytoplasm supports this interpretation. The minimal differences in cellular GSH content without and with energy substrates further indicate that this effect is unlikely to be due to artifactual effects related to GSH oxidation or loss during fractionation. Similar results were observed in HeLa cells treated with Glc and Gln deficient media (data not shown). Thus, the data indicate that either enhanced ROS level occurs selectively in non-nuclear compartments, non-nuclear proteins are inherently more oxidizable, or nuclei contain enhanced antioxidant capability under stress conditions.
The present data do not distinguish these possibilities, but available data suggest that each of these could contribute to the differences observed. For instance, several studies have emphasized that mitochondria are a major source of ROS, and this could increase due to stress signaling mechanisms [38,39]. The present data indicate that the cytoplasm has greater protein thiol content per mg protein compared to the nuclei, and this could indicate a greater inherent oxidizability of cytoplasmic proteins. Finally, a number of studies show that Trx1 is translocated to the nuclei during stress conditions. Thus, it appears likely that multiple mechanisms contribute to differences in oxidation of the compartments during Glc and Gln deficiency.
The in vitro conditions we used were severe relative to conditions likely to occur in vivo, but systemic or tissue Glc and Gln concentrations may become limiting under some conditions. Substantial decreases in Gln occur in surgery, following severe burns, in acute trauma, and in conditions associated with AIDS and cancer [25,40-42]. Depletion of Glc is also commonly found in solid tumors due to high metabolic rates. Furthermore, depletion of essential nutrients is not seen solely under pathological conditions. For example, food deprivation or total starvation often occurs for several days in previously healthy individuals during illness, and in patients associated with medical or surgical treatments. A decrease in energy substrates also occurs during ischemia, and a contribution of anoxia with Glc and Gln deficiency could result in more severe changes. Such conditions are commonly used a model for ischemia reperfusion injury and additional studies to determine combined effects are needed.
Depletion of the two nutrients, Glc and Gln is correlated with energy depletion and a drastic decrease in the intracellular NAD/NADH and GSH levels, which, in turn, lead to an oxidative shift in most redox systems. ROS levels were increased after Glc and Gln depletion for 1 h in the HT-29 cells. The mechanism by which Glc and Gln deprivation enhanced ROS level was not identified in the current study. It could be that there was a decreased rate of ROS removal rather than an increased rate of ROS generation, resulting in an increased steady-state level of ROS. On the other hand, DCF oxidation was due to elevation of ROS generation, a major source of increased ROS is possibly mitochondria because Glc and Gln metabolic inhibition is tightly associated with mitochondrial dysfunction. Inhibiting important regulatory sites of energy/electron flow in mitochondria can result in an increase in ROS. Moreover, mitochondrial ROS production and restriction of NAD(P)H are likely to contribute to Trx2 oxidation which could be linked with stimulation of cell death mechanism involving activation of apoptosis signal-regulating kinase-1 [43].
Exogenously added oxidants cause generalized, non-specific oxidation of all cellular components; whereas, endogenously generated ROS under physiologic or pathologic conditions appear to activate discrete signal transduction pathways regulated by specific proteins [7,44]. One might expect that increased ROS level due to Glc- or Gln-deficiency would have a generalized, non-specific effect, especially since such deficiencies ultimately result in cell death. Depleting Glc or Gln alone showed a significant elevation in ROS in HT-29 cells compared with the control, but neither condition alone affected Trx1 or Trx2 redox state. Increased ROS level observed under Glc or Gln free condition was significantly less than the condition of combined Glc and Gln deprivation, suggesting that deprivation of each agent individually for 24 h may not cause an oxidative environment sufficient to alter Trx redox state in the cells.
The PrSSG data decline over time in both cytosolic and nuclear fractions and under both control and Glc and Gln deficient conditions. Although we have no explanation for this effect, the results could occur as a consequence of changing conditions during the 24 h after changing the culture medium. We previously found that extracellular cysteine/cystine redox state becomes more reduced within the first 24 h of changing culture medium [45] and that the initially oxidized extracellular cysteine/cystine redox state (approximately 0 mV) used in the present study caused an increased cellular ROS level as measured by DCF fluorescence [29]. While this could explain the observed results, experiments are needed to test this possibility.
Ref-1 is a bifunctional enzyme required for DNA repair and for maintenance of reduction of a conserved cysteine in the DNA binding region of a number of transcription factors, including AP-1, NF-κB, and HIF-1. Ref-1's DNA repair activity resides in the C-terminal portion of the protein, while the N-terminal domain is necessary for redox regulation of redox-sensitive proteins. Ref-1 is a key factor in nuclear protection against a wide variety of cellular stresses, including DNA damage and a change in oxygen tension [46]. In this study, increased oxidative stress induced by Glc and Gln depletion resulted in Ref-1 reduction in the nucleus but not in the cytoplasm. Because Trx1 is more reduced in the nucleus and Ref-1 is a substrate of Trx1, these results suggest that protection of nuclear Trx1 also protects the Ref-1 system and associated transcription factor function.
Although the nuclear protective mechanisms are not fully understood, the different antioxidant systems within the nucleus are likely to serve distinct functions. Ref-1 reduction, together with the reduction of Trx1 and the relatively less formation of S-glutathionylation in nuclei, provide an important suggestion that a more reduced nuclear environment may provide the cell with protection against oxidative stress induced by nutritional deficiency. This enhanced antioxidant function in nuclei may be a critical determinant of differential susceptibility of cells to toxicity and may also be relevant to sensitivity and resistance of cells to anticancer agents.
Acknowledgments
This work was supported by NIH grant ES011195 and ES09047. We would like to thank Siobhan Moriarty-Craige for helpful comments during preparation of the manuscript.
List of Abbreviations
- Cys
cysteine
- DCF
dichlorofluoroscin
- DTNB
5, 5′-Dithiobis(2-nitrobenzoic acid)
- Glc
glucose
- Gln
glutamine
- GSH
glutathione
- GSSG
glutathione disulfide
- IAA
iodoacetic acid
- PrSSG
protein S-glutathionylation
- Ref-1
redox factor-1
- ROS
reactive oxygen species
- Trx1
thioredoxin-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by NIH grant ES011195 and ES09047.
References
- 1.Kedzierska K, Bober J, Ciechanowski K, Golembiewska E, Kwiatkowska E, Nocen I, Dutkiewicz G, Chlubek D. Trace elements modify the activity of sodium transporting systems in erythrocyte membrane in patients with essential hypertension-preliminary study. Nephrol Dial Transplant. 2005;20:469–71. doi: 10.1093/ndt/gfh613. [DOI] [PubMed] [Google Scholar]
- 2.Purushotham KR, Nakagawa Y, Humphreys-Beher MG, Maeda N, Schneyer CA. Rat parotid gland acinar cell proliferation: signal transduction at the plasma membrane. Crit Rev Oral Biol Med. 1993;4:537–43. doi: 10.1177/10454411930040034001. [DOI] [PubMed] [Google Scholar]
- 3.Schmidt-Ullrich RK. Molecular targets in radiation oncology. Oncogene. 2003;22:5730–3. doi: 10.1038/sj.onc.1206662. [DOI] [PubMed] [Google Scholar]
- 4.Han D, Matsumaru K, Rettori D, Kaplowitz N. Usnic acid-induced necrosis of cultured mouse hepatocytes: inhibition of mitochondrial function and oxidative stress. Biochem Pharmacol. 2004;67:439–51. doi: 10.1016/j.bcp.2003.09.032. [DOI] [PubMed] [Google Scholar]
- 5.Hashiguchi K, Bohr VA, de Souza-Pinto NC. Oxidative stress and mitochondrial DNA repair: implications for NRTIs induced DNA damage. Mitochondrion. 2004;4:215–22. doi: 10.1016/j.mito.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 6.Galter D, Mihm S, Droge W. Distinct effects of glutathione disulphide on the nuclear transcription factor kappa B and the activator protein-1. Eur J Biochem. 1994;221:639–48. doi: 10.1111/j.1432-1033.1994.tb18776.x. [DOI] [PubMed] [Google Scholar]
- 7.Halvey PJ, Watson WH, Hansen JM, Go YM, Samali A, Jones DP. Compartmental oxidation of thiol-disulphide redox couples during epidermal growth factor signalling. Biochem J. 2005;386:215–9. doi: 10.1042/BJ20041829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Watson WH, Pohl J, Montfort WR, Stuchlik O, Reed MS, Powis G, Jones DP. Redox potential of human thioredoxin 1 and identification of a second dithiol/disulfide motif. J Biol Chem. 2003;278:33408–15. doi: 10.1074/jbc.M211107200. [DOI] [PubMed] [Google Scholar]
- 9.Watson WH, Jones DP. Oxidation of nuclear thioredoxin during oxidative stress. FEBS Lett. 2003;543:144–7. doi: 10.1016/s0014-5793(03)00430-7. [DOI] [PubMed] [Google Scholar]
- 10.Hansen JM, Zhang H, Jones DP. Mitochondrial thioredoxin-2 has a key role in determining tumor necrosis factor-alpha-induced reactive oxygen species generation, NF-kappaB activation, and apoptosis. Toxicol Sci. 2006;91:643–50. doi: 10.1093/toxsci/kfj175. [DOI] [PubMed] [Google Scholar]
- 11.Watson WH, Chen Y, Jones DP. Redox state of glutathione and thioredoxin in differentiation and apoptosis. Biofactors. 2003;17:307–14. doi: 10.1002/biof.5520170130. [DOI] [PubMed] [Google Scholar]
- 12.Lluis JM, Morales A, Blasco C, Colell A, Mari M, Garcia-Ruiz C, Fernandez-Checa JC. Critical role of mitochondrial glutathione in the survival of hepatocytes during hypoxia. J Biol Chem. 2005;280:3224–32. doi: 10.1074/jbc.M408244200. [DOI] [PubMed] [Google Scholar]
- 13.Zhao P, Kalhorn TF, Slattery JT. Selective mitochondrial glutathione depletion by ethanol enhances acetaminophen toxicity in rat liver. Hepatology. 2002;36:326–35. doi: 10.1053/jhep.2002.34943. [DOI] [PubMed] [Google Scholar]
- 14.Bonnefoi MS. Mitochondrial glutathione and methyl iodide-induced neurotoxicity in primary neural cell cultures. Neurotoxicology. 1992;13:401–12. [PubMed] [Google Scholar]
- 15.Shan X, Jones DP, Hashmi M, Anders MW. Selective depletion of mitochondrial glutathione concentrations by (R,S)-3-hydroxy-4-pentenoate potentiates oxidative cell death. Chem Res Toxicol. 1993;6:75–81. doi: 10.1021/tx00031a012. [DOI] [PubMed] [Google Scholar]
- 16.Lash LH, Jones DP. Distribution of oxidized and reduced forms of glutathione and cysteine in rat plasma. Arch Biochem Biophys. 1985;240:583–92. doi: 10.1016/0003-9861(85)90065-7. [DOI] [PubMed] [Google Scholar]
- 17.Hansen JM, Go YM, Jones DP. Nuclear and mitochondrial compartmentation of oxidative stress and redox signaling. Annu Rev Pharmacol Toxicol. 2006;46:215–34. doi: 10.1146/annurev.pharmtox.46.120604.141122. [DOI] [PubMed] [Google Scholar]
- 18.Jevtovic-Todorovic V, Guenthner TM. Depletion of a discrete nuclear glutathione pool by oxidative stress, but not by buthionine sulfoximine. Correlation with enhanced alkylating agent cytotoxicity to human melanoma cells in vitro. Biochem Pharmacol. 1992;44:1383–93. doi: 10.1016/0006-2952(92)90540-y. [DOI] [PubMed] [Google Scholar]
- 19.Cotgreave IA. Analytical developments in the assay of intra- and extracellular GSH homeostasis: specific protein S-glutathionylation, cellular GSH and mixed disulphide compartmentalisation and interstitial GSH redox balance. Biofactors. 2003;17:269–77. doi: 10.1002/biof.5520170126. [DOI] [PubMed] [Google Scholar]
- 20.Voehringer DW, McConkey DJ, McDonnell TJ, Brisbay S, Meyn RE. Bcl-2 expression causes redistribution of glutathione to the nucleus. Proc Natl Acad Sci U S A. 1998;95:2956–60. doi: 10.1073/pnas.95.6.2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adler V, et al. Regulation of JNK signaling by GSTp. Embo J. 1999;18:1321–34. doi: 10.1093/emboj/18.5.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hansen JM, Zhang H, Jones DP. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic Biol Med. 2006;40:138–45. doi: 10.1016/j.freeradbiomed.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 23.Nkabyo YS, Ziegler TR, Gu LH, Watson WH, Jones DP. Glutathione and thioredoxin redox during differentiation in human colon epithelial (Caco-2) cells. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1352–9. doi: 10.1152/ajpgi.00183.2002. [DOI] [PubMed] [Google Scholar]
- 24.Klarl BA, et al. Protein kinase C mediates erythrocyte “programmed cell death” following glucose depletion. Am J Physiol Cell Physiol. 2006;290:C244–53. doi: 10.1152/ajpcell.00283.2005. [DOI] [PubMed] [Google Scholar]
- 25.Ziegler TR, Evans ME, Fernandez-Estivariz C, Jones DP. Trophic and cytoprotective nutrition for intestinal adaptation, mucosal repair, and barrier function. Annu Rev Nutr. 2003;23:229–61. doi: 10.1146/annurev.nutr.23.011702.073036. [DOI] [PubMed] [Google Scholar]
- 26.Paquette JC, Guerin PJ, Gauthier ER. Rapid induction of the intrinsic apoptotic pathway by L-glutamine starvation. J Cell Physiol. 2005;202:912–21. doi: 10.1002/jcp.20194. [DOI] [PubMed] [Google Scholar]
- 27.Manhart N, Vierlinger K, Spittler A, Bergmeister H, Sautner T, Roth E. Oral feeding with glutamine prevents lymphocyte and glutathione depletion of Peyer's patches in endotoxemic mice. Ann Surg. 2001;234:92–7. doi: 10.1097/00000658-200107000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones DP. Redox potential of GSH/GSSG couple: assay and biological significance. Methods Enzymol. 2002;348:93–112. doi: 10.1016/s0076-6879(02)48630-2. [DOI] [PubMed] [Google Scholar]
- 29.Go YM, Jones DP. Intracellular proatherogenic events and cell adhesion modulated by extracellular thiol/disulfide redox state. Circulation. 2005;111:2973–80. doi: 10.1161/CIRCULATIONAHA.104.515155. [DOI] [PubMed] [Google Scholar]
- 30.Flaherty DM, Monick MM, Hunninghake GW. AP endonucleases and the many functions of Ref-1. Am J Respir Cell Mol Biol. 2001;25:664–7. doi: 10.1165/ajrcmb.25.6.f220. [DOI] [PubMed] [Google Scholar]
- 31.Kim JR, et al. Oxidation of thioredoxin reductase in HeLa cells stimulated with tumor necrosis factor-alpha. FEBS Lett. 2004;567:189–96. doi: 10.1016/j.febslet.2004.04.055. [DOI] [PubMed] [Google Scholar]
- 32.Natarajan SK, Thomas S, Ramamoorthy P, Basivireddy J, Pulimood AB, Ramachandran A, Balasubramanian KA. Oxidative stress in the development of liver cirrhosis: a comparison of two different experimental models. J Gastroenterol Hepatol. 2006;21:947–57. doi: 10.1111/j.1440-1746.2006.04231.x. [DOI] [PubMed] [Google Scholar]
- 33.Evans ME, Jones DP, Ziegler TR. Glutamine prevents cytokine-induced apoptosis in human colonic epithelial cells. J Nutr. 2003;133:3065–71. doi: 10.1093/jn/133.10.3065. [DOI] [PubMed] [Google Scholar]
- 34.Li XM, Metzger G, Filipski E, Boughattas N, Lemaigre G, Hecquet B, Filipski J, Levi F. Pharmacologic modulation of reduced glutathione circadian rhythms with buthionine sulfoximine: relationship with cisplatin toxicity in mice. Toxicol Appl Pharmacol. 1997;143:281–90. doi: 10.1006/taap.1996.8088. [DOI] [PubMed] [Google Scholar]
- 35.Morgenstern I, Raijmakers MT, Peters WH, Hoensch H, Kirch W. Homocysteine, cysteine, and glutathione in human colonic mucosa: elevated levels of homocysteine in patients with inflammatory bowel disease. Dig Dis Sci. 2003;48:2083–90. doi: 10.1023/a:1026338812708. [DOI] [PubMed] [Google Scholar]
- 36.Nkabyo YS, Gu LH, Jones DP, Ziegler TR. Thiol/disulfide redox status is oxidized in plasma and small intestinal and colonic mucosa of rats with inadequate sulfur amino acid intake. J Nutr. 2006;136:1242–8. doi: 10.1093/jn/136.5.1242. [DOI] [PubMed] [Google Scholar]
- 37.Briviba K, Fraser G, Sies H, Ketterer B. Distribution of the monochlorobimane-glutathione conjugate between nucleus and cytosol in isolated hepatocytes. Biochem J. 1993;294(Pt 3):631–3. doi: 10.1042/bj2940631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benhar M, Engelberg D, Levitzki A. ROS, stress-activated kinases and stress signaling in cancer. EMBO Rep. 2002;3:420–5. doi: 10.1093/embo-reports/kvf094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Elliott NA, Volkert MR. Stress induction and mitochondrial localization of Oxr1 proteins in yeast and humans. Mol Cell Biol. 2004;24:3180–7. doi: 10.1128/MCB.24.8.3180-3187.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Calder PC, Yaqoob P. Glutamine and the immune system. Amino Acids. 1999;17:227–41. doi: 10.1007/BF01366922. [DOI] [PubMed] [Google Scholar]
- 41.Dechelotte P, et al. L-alanyl-L-glutamine dipeptide-supplemented total parenteral nutrition reduces infectious complications and glucose intolerance in critically ill patients: the French controlled, randomized, double-blind, multicenter study. Crit Care Med. 2006;34:598–604. doi: 10.1097/01.CCM.0000201004.30750.D1. [DOI] [PubMed] [Google Scholar]
- 42.Decker GM. Glutamine: indicated in cancer care? Clin J Oncol Nurs. 2002;6:112–5. doi: 10.1188/02.CJON.112-115. [DOI] [PubMed] [Google Scholar]
- 43.Zhang R, Al-Lamki R, Bai L, Streb JW, Miano JM, Bradley J, Min W. Thioredoxin-2 inhibits mitochondria-located ASK1-mediated apoptosis in a JNK-independent manner. Circ Res. 2004;94:1483–91. doi: 10.1161/01.RES.0000130525.37646.a7. [DOI] [PubMed] [Google Scholar]
- 44.Svegliati S, et al. Platelet-derived growth factor and reactive oxygen species (ROS) regulate Ras protein levels in primary human fibroblasts via ERK1/2. Amplification of ROS and Ras in systemic sclerosis fibroblasts. J Biol Chem. 2005;280:36474–82. doi: 10.1074/jbc.M502851200. [DOI] [PubMed] [Google Scholar]
- 45.Jiang S, Moriarty-Craige SE, Orr M, Cai J, Sternberg P, Jr., Jones DP. Oxidant-induced apoptosis in human retinal pigment epithelial cells: dependence on extracellular redox state. Invest Ophthalmol Vis Sci. 2005;46:1054–61. doi: 10.1167/iovs.04-0949. [DOI] [PubMed] [Google Scholar]
- 46.Hall JL, Wang X, Van A, Zhao Y, Gibbons GH. Overexpression of Ref-1 inhibits hypoxia and tumor necrosis factor-induced endothelial cell apoptosis through nuclear factor-kappab-independent and -dependent pathways. Circ Res. 2001;88:1247–53. doi: 10.1161/hh1201.091796. [DOI] [PubMed] [Google Scholar]