Abstract
This paper reviews research designed to investigate the temporal control of inhibitory responding using rats as subjects. One area of investigation has focused on the role of temporal variables in conditioned inhibition produced using Pavlov’s (1927) procedure. These studies have found that evidence of conditioned inhibition obtained by negative summation testing is strongest when the conditioned inhibitor signals the omission of the US at the same temporal location as a transfer excitor signals presentation of the US (e.g., Barnet & Miller, 1996). Similarly, retardation of acquisition of behavioral control by a previously inhibitory CS is maximal when the inhibitory CS is paired with the US at the same temporal location as the inhibitor had previously signaled US omission (Burger, Denniston, & Miller, 2001). Other lines of research designed to assess the associative structure of temporal control of inhibition (e.g., Denniston, Blaisdell, & Miller, 2004) are reviewed, as is the assessment of temporal control of inhibition produced through extinction (Denniston & Miller, 2003). These collective observations are discussed in terms of the temporal coding hypothesis (Matzel, Held, & Miller, 1988).
Timing of Omitted Events: An Analysis of Temporal Control of Inhibitory Behavior
The analysis of temporal variables in conditioned behavior has been widely studied since the pioneering work of Pavlov (1927). Pavlov’s studies of inhibition of delay revealed that following conditioned stimulus (CS)-unconditioned stimulus (US) pairings with a CS of long duration, conditioned responding was maximal during the latter parts of the CS. Pavlov’s early work both highlighted the importance of timing in conditioned behavior and provided the foundation for a thorough analysis of how animals both perceive and use temporal information. For the last 40 years, the study of animal timing has been inspired and led by Russell Church. A few of the many great contributions of Church’s work include the development of the peak procedure (Roberts & Church, 1978) and theorizing concerning internal clocks (Church, 1984) that model how animals learn about expected times of reinforcement and subsequently use this information in order to control conditioned responding. In contrast to this large literature, the present article summarizes the findings from a far smaller collection of research that has attempted to study analogous questions in inhibitory learning; specifically how animals temporally encode omitted events.
Despite the now vast literature on timing of excitatory behavior, relatively little emphasis has been placed on the temporal control of behavior indicative of conditioned inhibition. This deficit is likely due in large part to the difficulties in assessing expectations of event omission. Timing of excitatory behavior can be relatively easily assessed through measures such as the peak procedure, in which an animal is reinforced on a fixed-interval (FI) schedule of reinforcement and anticipatory responding is measured on probe trials in which the US is omitted (thereby producing a rise and fall in conditioned responding around the time at which reinforcement is expected; e.g., Roberts & Church, 1978). But assessment of inhibitory behavior is not as straightforward due to the lack of an overt response evoked by a conditioned inhibitor presented by itself (i.e., floor effects in conditioned responding). In a typical conditioned inhibition training situation using Pavlov’s procedure, a training excitor is paired with the US, except when it is compounded with the intended inhibitor (e.g., A→US / XA-noUS). The difficulty in assessing inhibitory behavior is differentiating between true inhibition (i.e., an expectation of nonreinforcement) and inattention. To overcome this difficulty, researchers (e.g., Barnet & Miller, 1996) have relied upon Rescorla’s (1969) two-test strategy for assessing timing of inhibitory behavioral control. According to Rescorla, conditioned inhibition should be assessed through both a negative summation test and a retardation test. In a negative summation test, a putative inhibitor is tested in compound with an independently established excitor (called a transfer excitor). The putative inhibitor is said to pass the summation test for inhibition if the inhibitor attenuates conditioned responding to the transfer excitor, relative to the level of responding elicited by the transfer excitor alone or in compound with a neutral stimulus. Importantly, instead of conditioned inhibition, such a decrease in responding could be due to distraction from the transfer excitor by the inhibitor (i.e., enhanced attention to the inhibitor). To control for this possibility, Rescorla recommended the use of a retardation test, in which the putative inhibitor is paired with the US, in order to assess the rate of acquisition of stimulus control of behavior relative to that of a neutral stimulus. If the rate of acquisition of conditioned responding to the previously inhibitory CS is slow relative to that of the neutral stimulus, then the stimulus is said to pass the retardation test for inhibition. However, this test is similarly open to an alternative explanation, namely decreased attention to the putative inhibitor as a consequence of repeated nonreinforcement during inhibitory training. Notably, these alternative explanations (increased attention and decreased attention, respectively) are incompatible. Hence, passage of both tests provides a fairly compelling demonstration of inhibitory learning (but see Cole, Barnet, Miller, 1997; Papini & Bitterman, 1993; Savastano, Cole, Barnet, & Miller, 1999 for further critiques of the two-test strategy).
In addition to providing a means for assessing the existence of inhibitory learning, Rescorla’s (1969) two-test strategy has also led to the study of the temporal properties and associative structure of inhibitory learning (e.g., Barnet & Miller, 1996; Denniston, Blaisdell, & Miller, 2004). This line of research has been guided by the temporal coding hypothesis (Matzel, Held, & Miller, 1988; Miller & Barnet, 1993; Savastano & Miller, 1998) which states that: 1) learning is based upon spatio-temporal contiguity; 2) animals encode the temporal relationship between events (as a temporal map; Honig, 1981); 3) animals can integrate temporal maps from different phases of training when the maps contain a common stimulus element to anchor the integration; and 4) these simple and integrated temporal maps are used to determine the form and timing of the conditioned responding. Based on the temporal coding hypothesis, Barnet and Miller hypothesized that on a summation test behavior indicative of inhibition would be strongest when the conditioned inhibitor signals the omission of the US at the same moment in time as the transfer excitor signals the occurrence of the US. These expectancies of US presentation and US omission are established during inhibition training and transfer excitor training, and can be further manipulated at or before the time of testing.
Temporal Control of Conditioned Inhibition
The first set of experiments described in the present review systematically varied five temporal relationships in order to investigate the nature of temporal coding in conditioned inhibition (see Table 1). During the procedure that Pavlov (1927) developed for conditioned inhibition training (i.e., A→US / XA-noUS), the training excitor (A) is paired with the US except when compounded with the conditioned inhibitor (X). (There are other procedures for training conditioned inhibition, but most of them lack the potential for temporal learning that Pavlov’s procedure has.) Toward investigating the representation of time in Pavlovian conditioned inhibition, both the temporal relationships between the training excitor (A) and the US (Variable 1 in Table 1) and between the inhibitor and the training excitor (X-A; Variable 2) may be manipulated. That is, during training the A-US association may be conditioned using either delay, simultaneous, or trace conditioning in order to vary the temporal expectation of the US provided by CS A. Similarly, during inhibitory training trials, presentations of X and A may be provided either simultaneously or serially in order to vary the expectation of US omission.
Table 1.
Temporal Variables in Pavlovian Conditioned Inhibition
| Training | |
| Inhibition Training | Transfer CS Training |
| A→US1 / XA2-noUS | C→US3 |
| Testing | |
| Negative Summation Test | Retardation Test |
| XC4 relative to C alone or ZC | X→US5 relative to Z→US5 |
| Potential Variables: | |
| |
Note. CS A represents the training excitor; CS X represents the conditioned inhibitor; CS C represents the transfer excitor; CS Z represents a previously neutral stimulus. Each of the potential temporal variables may be manipulated in order to assess temporal specificity of inhibitory behavioral control.
For example, Barnet and Miller (1996) provided thirsty rats with conditioned inhibition training consisting of pairings of a 5-s audiovisual stimulus with a shock US (i.e., A→US pairings) using a delay conditioning procedure, in which termination of the CS coincided with onset of the US (see top panel of Figure 1). On inhibitory training trials, CS A was presented coterminously (simultaneously) with CS X (i.e., XA-noUS). Based upon the temporal coding hypothesis, Barnet and Miller expected that CS A would become a signal for US presentation 5 s following CS onset and that, as a consequence of inhibitory training trials, CS X would become a signal for US omission 5 s following onset of CS X. This latter expectation was based upon the temporal coding hypothesis’ assertion that animals encode the temporal relationships between events and that animals can integrate these temporal maps. In other words, the expectation of US omission activated by CS X is based upon both the temporal relationship between X and A on inhibitory training trials and upon the temporal relationship between A and the US on reinforced trials. This reasoning follows from principle 3 of the temporal coding hypothesis. As depicted in Figure 1, CS X should generate an expectation of the simultaneous presentation of CS A, which was otherwise immediately followed by the US. Hence, integration of these two temporal maps (X-A and A-US) should now generate an expectation of US omission following termination of CS X (i.e., 5 s following onset of X). Notably, the expectation of US omission (or of CS A presentation) could be based upon either the time following onset or termination of the CS (Desmond & Moore, 1988). For present purposes, we will describe this expectation as being based upon timing from the onset of a stimulus because the testing procedure used by Barnet and Miller to investigate timing of inhibition does not permit differentiation of these potential timing cues.
Figure 1.
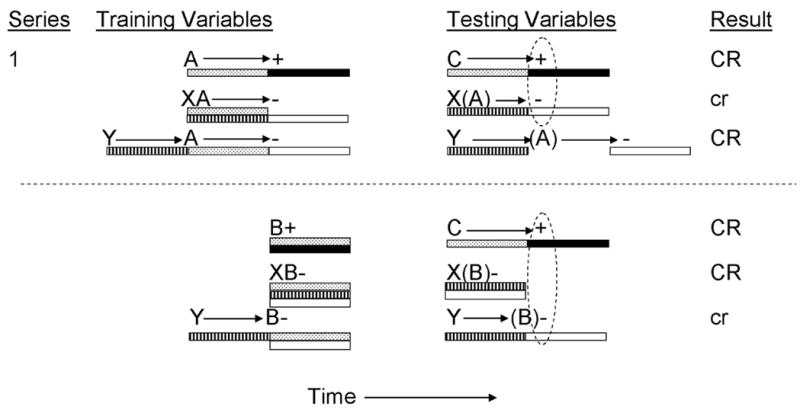
Inhibitory training provided by Barnet and Miller (1996). CSs A, B, C, X, and Y, were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. At test, animals received presentations of C, XC, or YC. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
Toward assessing the representation of omitted events, Barnet and Miller (1996) established a second conditioned inhibitor (CS Y) with a different temporal expectancy for US omission. CS Y was established as a serial inhibitor through nonreinforced presentations of Y and A, in which presentation of CS Y was followed immediately by CS A (both CSs were 5 s in duration). By manipulating Variable 2 (in this case the Y-A temporal relationship), CS Y should become a signal for US omission 10 s following its onset because CS Y was followed by CS A, which was otherwise followed by the US (see top panel of Figure 1). For the purpose of negative summation testing, Barnet and Miller trained a 5-s transfer excitor, C, using a delay conditioning procedure in which CS C was immediately followed by the US (Variable 3, the transfer excitor-US temporal interval, was held constant in this experiment). At test, the inhibitory potential of CS X was assessed through a negative summation test in which CS C was presented either alone or in simultaneous compound with CS X (holding constant Variable 4, the transfer excitor-inhibitor temporal relationship). Testing was conducted using a flooding measure in which stimuli were presented for 10 min while animals were drinking, and the time required to complete 5 cumulative seconds of drinking in the presence of the test stimuli served as the dependent measure. With this procedure, strong excitatory stimulus control (indicative of conditioned fear) is evidenced by longer times to resume drinking (denoted in Figure 1 by an uppercase ‘CR’), whereas behavior indicative of inhibition is evidenced by shorter times to resume drinking (denoted by a lowercase ‘cr’). Barnet and Miller observed strong conditioned suppression when subjects were presented with CS C alone, and attenuated conditioned suppression when subjects were presented with the XC compound (see Figure 1, top panel). This attenuation of fear is indicative of conditioned inhibition. As shown in Figure 1, CS C was anticipated to generate an expectation of US presentation 5 s following CS onset, and CS X was anticipated to generate an expectation of US omission 5 s following onset of CS X (based upon the X-A and A-US temporal relationships described above). Another group of subjects was tested with a YC simultaneous compound in order to assess the temporal specificity of inhibitory behavioral control. These subjects showed a strong conditioned response at test, indicating that CS Y failed the negative summation test for inhibition when tested with CS C. This failure was presumably a consequence of a mismatch of temporal expectancies, in that CS Y was anticipated to generate an expectancy of US omission 10 s following its onset because it was trained as a serial inhibitor, whereas CS C was anticipated to generate an expectancy of US presentation 5 s following its onset. Thus, simultaneous expectancies of US presentation (as signaled by the transfer excitor) and US omission (as signaled by the conditioned inhibitor) appear to be necessary for generating maximal inhibitory behavioral control.
In a further test of temporal control of inhibition, Barnet and Miller (1996) additionally manipulated the training excitor-US temporal relationship (Variable 1 from Table 1). During inhibition training, CS B was conditioned through simultaneous pairings of B and the US (see the bottom panel of Figure 1) and all other training procedures were analogous to those described above (i.e., X and B were presented simultaneously and Y was presented serially immediately before B). As a consequence of this training, CS B was expected to generate an expectation of US presentation at onset of CS B due to the simultaneous training procedure. This manipulation of Variable 1 should have provided different expectations of US omission based upon CSs X and Y trained with CS B. That is, CS X should have now predicted omission of the US at its onset due to the simultaneous nonreinforced presentations of X and B, whereas CS Y should have predicted US omission 5 s following its onset due to the serial presentations of CSs Y and B in which CS Y was presented 5 s before CS B. The transfer excitor C was trained with a delay procedure such that presentation of the US was expected 5 s after C’s onset. At test, attenuated conditioned suppression was observed when CS Y was compounded with CS C, but not when CS X was compounded with CS C. This opposite pattern of responding, relative to that described above when X and Y were trained with CS A, was presumably the consequence of the manipulation of the training excitor-US temporal relationship in conjunction with CS C signaling US presentation 5 s following its onset and only CS Y now signaling US omission at the same temporal location. These findings confirm that maximal inhibition tends to be observed when the inhibitor and the transfer excitor signal US omission and presentation, respectively, at the same temporal location.
In another series of experiments, Denniston, Cole, and Miller (1998) manipulated Variables 1 and 3 (top panel of Figure 2) and Variables 2 and 3 (bottom panel of Figure 2) in order to investigate the role of the transfer excitor-US temporal relationship on timing of inhibitory behavioral control. In their Experiment 1, Denniston et al. provided rats with training similar to that provided by Barnet and Miller (1996) in order to establish two conditioned inhibitors with different temporal expectancies for US omission. Inhibitor X was trained as a simultaneous inhibitor for delay training excitor (A) in order to establish X as a signal for US omission 5 s following its onset. Inhibitor Y was similarly trained as a simultaneous inhibitor, but in this instance was presented nonreinforced with trace training excitor B, which was otherwise paired with the US 5 s following termination of CS B (again, all stimuli were 5 s in duration). As a consequence of this training, CS Y was anticipated to signal US omission 10 s following its onset (due to manipulation of Variable 1). To assess these temporal expectancies of US omission, two transfer excitors were established for the purpose of negative summation testing. CS C was trained as a delay CS which was expected to signal US presentation 5 s following its onset, and CS D was trained as a trace transfer excitor, which was expected to signal US omission 10 s following its onset (a manipulation of Variable 3). At test, maximal conditioned inhibition was observed when inhibitor X was compounded with transfer excitor C, but not D, and when inhibitor Y was compounded with transfer excitor D, but not C. Denniston et al. explained this pattern of responding as being the result of inhibitor X signaling US omission 5 s following its onset which corresponds to the temporal expectancy for US presentation provided by transfer excitor C and inhibitor Y signaling US omission 10 s following its onset which corresponds to the temporal expectancy for US presentation provided by transfer excitor D. When the inhibitor and transfer excitor produced incongruent temporal expectancies for US omission and presentation, respectively, less inhibition was observed.
Figure 2.
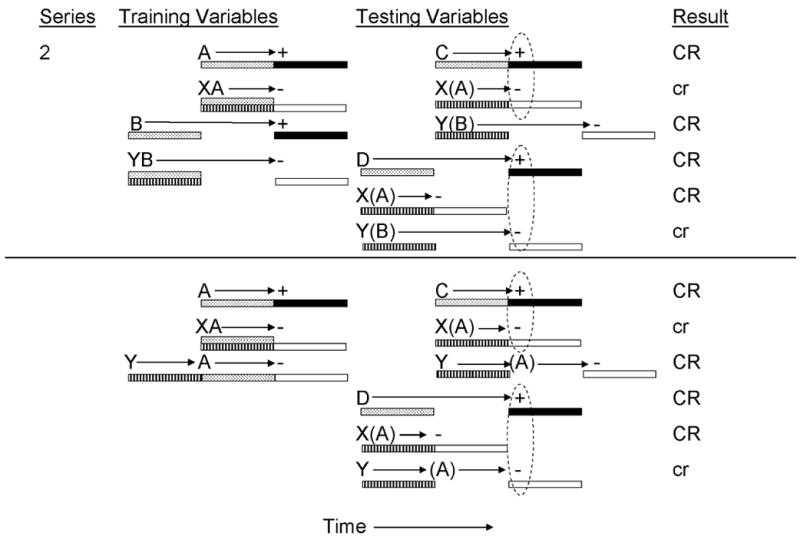
Inhibitory training provided by Denniston, Cole, & Miller, 1998). CSs A, B, C, D, X, and Y, were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. At test, animals received presentations of C, XC, YC, D, XD, or YD. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
In their Experiment 2 (see Figure 2, bottom panel), Denniston, Cole, et al. (1998) manipulated both the inhibitor-training excitor (Variable 2) and the transfer excitor-US (Variable 3) temporal relationships while holding constant the training excitor-US (Variable 1) temporal relationship. In this experiment, two conditioned inhibitors were separately established with a single delay training excitor (CS A, which was paired with the US 5 s following its onset). Inhibitor X was trained as a simultaneous inhibitor through coterminous nonreinforced presentations of X and A, whereas inhibitor Y was trained as a serial inhibitor through successive nonreinforced presentations of Y and A (see Figure 2, bottom panel). At test, the inhibitory potentials of X and Y were assessed through negative summation tests with two different transfer excitors, C and D, which were previously paired with the US 5 s following onset of C, and 10 s following onset of D (again, all stimuli were 5 s in duration, so C was a delay excitor and D was a trace excitor with a 5 s gap between termination of D and onset of the US). Negative summation testing revealed greater inhibition when inhibitor X was compounded with transfer excitor C than with transfer excitor D, and when inhibitor Y was compounded with transfer excitor D than with transfer excitor C. As depicted in Figure 2 (bottom panel), inhibitor X was predicted to signal US omission 5 s following its onset, which corresponds to the expectancy of US presentation provided by transfer excitor C, but not D, In contrast, inhibitor Y was predicted to signal US omission 10 s following its onset because it was trained in a serial conditioning procedure. This corresponds to the expectancy of US presentation provided by transfer excitor D, but not C. The results of these experiments conceptually replicated and extended those of Barnet and Miller (1996) by demonstrating that timing of inhibitory behavioral control is sensitive not only to the training excitor-US and inhibitor-training excitor temporal relationships, but also to the expectation for US presentation provided by the transfer excitor at test.
The final series of experiments designed to assess temporal control in inhibition using summation tests for conditioned inhibition explored the role of the inhibitor-transfer excitor temporal relationship at test (Variable 4; Denniston, Blaisdell, & Miller, 1998). In their Experiment 1, Denniston, Blaisdell et al., established two conditioned inhibitors with different temporal expectancies for US omission (see Figure 3a, top panel). In the absence of reinforcement inhibitor X was presented simultaneously with CS A, which was otherwise conditioned as a delay excitor, in order to generate an expectation of omission of the US 5 s following onset of inhibitor X. Inhibitor Y was separately presented with CS A, using serial nonreinforced pairings with no gap between termination of Y and onset of A in order to generate an expectation of omission of the US 10 s following onset of inhibitor Y. Manipulation of the inhibitor-training excitor temporal interval (Variable 2) was intended to assess not only temporal control of inhibition, but also transfer of serial versus simultaneous inhibitors. That is, Holland and colleagues (Holland, 1984; Holland & Lamarre, 1984; Lamarre & Holland, 1987) have found that simultaneous inhibitors readily transfer inhibitory control to independently trained excitors, whereas the transfer of serial inhibitors is restricted to other stimuli that were the target of discrimination training. Specifically, serial inhibitors appear to function as negative occasion setters (see Miller & Oberling, 1998, for a discussion) and only transfer to other targets of occasion setting training and to a lesser degree to stimuli that were partially reinforced. However, unlike Holland’s procedure, in Denniston, Blaisdell et al.’s procedure there was no gap between termination of the inhibitor and onset of the training excitor, which may be one factor that determines whether the stimulus will function as an occasion setter as opposed to a simple conditioned inhibitor.
Figure 3a.
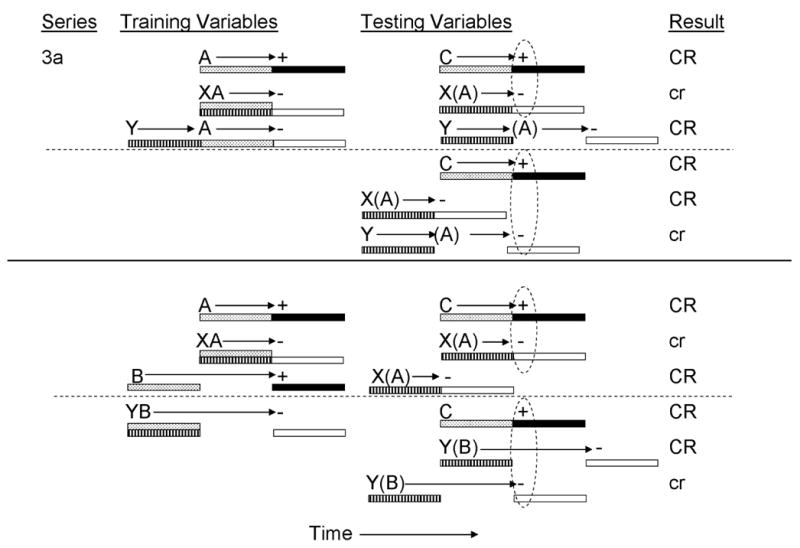
Inhibitory training provided by Denniston, Blaisdell, & Miller, 1998). CSs A, B, C, X, and Y, were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. At test, animals received either serial or simultaneous presentations of C, XC, or YC. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
For the purpose of negative summation testing, a transfer excitor, C, was established as a delay CS in order to produce an expectancy of US presentation 5 s following its onset. At test, inhibitors X and Y were separately compounded with transfer excitor C using either serial or simultaneous pairings (see Figure 3a, top). Variable 4 was manipulated in order to vary the temporal expectations for US presentation and omission at test. Simultaneous presentations of X and C were expected to produce temporally congruent expectations of US omission and presentation. In contrast, serial presentations of X and C were expected to produce a mismatch of temporal expectancies of US omission and presentation because inhibitor X was anticipated to generate an expectation of US omission 5 s following its onset whereas transfer excitor C was anticipated to generate an expectation of US presentation 5 s following its onset. By providing a serial X→C presentation, in which X was presented 5 s prior to the onset of CS C, the temporal expectation of US omission was expected to occur 5 s prior to the expectation of US presentation anticipated based on transfer excitor C. At test, reduced inhibition (i.e., stronger conditioned suppression) was observed to the serial X→C compound relative to the simultaneous XC compound which resulted in robust inhibition. An opposite pattern of responding was observed when testing was conducted with the YC serial and simultaneous compounds. At test, strong inhibition (i.e., weak conditioned suppression) was observed to the serial Y→C compound relative to the YC simultaneous compound. This pattern of results was described as being a consequence of the inhibitor Y signaling US omission 10 s following its onset, which produced a matched temporal expectation of US omission and US presentation only when presented in a serial compound with transfer excitor C at test.
One potential concern with the preceding studies is that rather than the findings representing control of inhibition through different temporal expectancies for US omission, the results may be explained more simply through different degrees of inhibitory behavioral control (e.g., Williams, Johns, & Bindas, 2006). That is, a delay training excitor may produce an acute pattern of responding, whereas a trace training excitor may produce a more diffuse pattern of responding. Likewise, a simultaneous inhibitor established with a delay training excitor may produce an acute pattern of inhibition that may then maximally inhibit a transfer excitor that generates an acute pattern of responding (i.e., a delay transfer excitor), whereas an inhibitor established with a trace training excitor may generate a more diffuse pattern of inhibition that best inhibits a diffuse pattern of responding generated by a trace transfer CS. However, Denniston, Blaisdell et al.’s (1998) Experiment 2 (see Figure 3a, bottom panel) suggests that this alternative explanation of the previously described results is not the primary determinant of when transfer of inhibition will be observed. In their Experiment 2, Denniston, Blaisdell et al. provided rats with training intended to produce two conditioned inhibitors with different temporal expectancies for US omission. Inhibitor X was trained as a simultaneous inhibitor for delay excitor A and inhibitor Y was trained as a simultaneous inhibitor for trace excitor B. As a consequence of this training, inhibitor X was expected to signal US omission 5 s following its onset, whereas inhibitor Y was expected to signal US omission 10 s following its onset. Alternatively, inhibitor X might generate an acute pattern of inhibition as it was trained with a delay training excitor, whereas inhibitor Y might generate a more diffuse pattern of inhibition as a consequence of being trained with a trace training excitor. At test, inhibitors X and Y were tested in either serial or simultaneous compound with delay transfer CS C (a manipulation of Variable 4 in Table 1). If transfer of inhibition is a consequence of patterns of inhibition, as suggested by Williams et al., rather than temporal expectancies of US omission and presentation, then one would expect to observe inhibitory behavior control only when inhibitor X was compounded with transfer excitor C, or when inhibitor Y was compounded with another diffuse signal for US presentation (e.g., a trace transfer CS). Consistent with this analysis, inhibitor X reduced responding to transfer excitor C when presented in a simultaneous, but not a serial compound. However, inhibitor Y produced robust inhibition when presented in a serial, but not simultaneous compound with transfer excitor C. This latter pattern of results demonstrates that inhibitory behavioral control, as assessed through a summation test for conditioned inhibition, is the consequence of temporal expectancies, rather than acute vs. diffuse patterns of inhibition, because according to Williams et al. inhibitor Y should have generated a diffuse pattern of inhibition that would not be anticipated to attenuate the acute pattern of responding generated by a delay transfer CS. Instead, these results appear to be a consequence of inhibitor Y signaling US omission 10 s following its onset, which by presenting inhibitor Y 5 s prior to CS C (which signaled US presentation 5 s following its onset) produced temporally matched expectations of US omission and presentation, respectively. Presenting Y and C in a simultaneous compound produced a mismatch in temporal expectancies because the expectations of US omission would be shifted by 5 s, such that the expectation of US omission would occur 5 s following the expectation of US presentation (see Figure 3a, bottom panel).
Denniston, Blaisdell et al.’s (1998) Experiment 3 further explored this alternative account of temporal control of inhibition. In that experiment, rats received training intended to establish a single serial inhibitor for a delay trained excitor through serial compound nonreinforced presentations of inhibitor X with delay trained excitor A (see Figure 3b). At test, inhibitor X was presented in either serial or simultaneous compound with either delay transfer excitor C or trace transfer excitor D. These four test conditions were intended to manipulate the temporal expectancies of US presentation and omission generated by the transfer excitors and the conditioned inhibitor, respectively. Serial presentation of inhibitor X and delay transfer excitor C (i.e., X→A)was expected to generate maximal inhibition as a consequence of inhibitor X signaling US omission 10 s following its onset, which corresponded to the expectation of US presentation generated by transfer excitor C, provided that the inhibitor is presented 5 s prior to CS C. Likewise, simultaneous presentations of inhibitor X and trace transfer excitor D were expected to generate maximal inhibition as the expectation of US omission evoked by inhibitor X corresponded to the expectation of US presentation generated by transfer excitor D, provided that the inhibitor was presented simultaneously with CS D (which signaled US presentation 10 s following its onset). If transfer of inhibition is a consequence of patterns of inhibition, then maximal inhibition by an acute inhibitor (X) should be restricted to testing with a transfer excitor that produced an acute pattern of responding (i.e., transfer excitor C, but not D), a prediction that was not confirmed. At test, greater inhibition was observed with the X→C and XD compounds, relative to the XC and X→D compounds, thereby confirming that passage of a summation test is the consequence of temporal expectancies rather than patterns of inhibition.
Figure 3b.
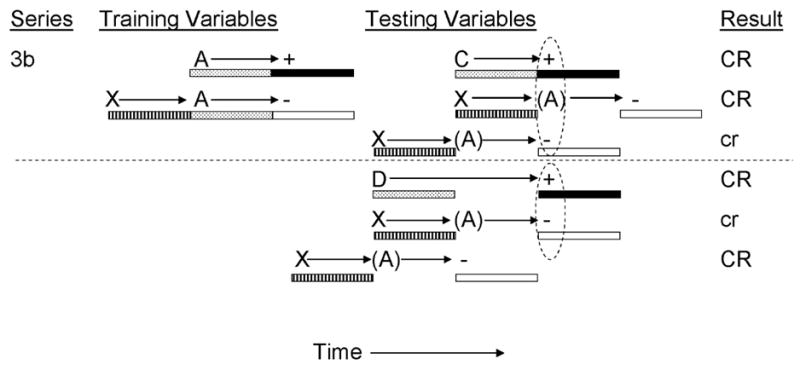
Inhibitory training provided by Denniston, Blaisdell, & Miller, 1998). CSs A, C, D, and X were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. At test, animals received either serial or simultaneous presentations of C, XC, D, or XD. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
The previously discussed studies of the timing of inhibitory behavioral control investigated the interaction of temporal expectancies generated by the conditioned inhibitor and transfer excitor at the time of negative summation testing. These studies demonstrated that behavior indicative of inhibitory behavioral control is sensitive to the temporal expectancies for US omission. However, they employed only one of the two tests recommended by Rescorla (1969) for assessing the inhibitory properties of a CS. The following series of experiments were designed to investigate whether evidence of temporal control of inhibition could be obtained with retardation tests for conditioned inhibition. Burger, Denniston, and Miller (2001) provided rats with inhibitory training in which two conditioned inhibitors were established as signals for the omission of the US at different temporal locations (see Figure 4a). Inhibitor X was trained as a simultaneous inhibitor through nonreinforced pairings with a delay training excitor (A) to establish a temporal expectancy of US omission 5 s following onset of inhibitor X. Inhibitor Y was similarly trained as a simultaneous inhibitor though nonreinforced pairings with a trace training excitor (B) in order to establish a temporal expectancy of US omission 10 s following onset of inhibitor Y. Prior to testing, inhibitors X and Y were separately paired with the US to assess retardation of acquisition of behavioral control. Different groups of subjects received retardation pairings in which an inhibitor was paired with the US using either trace or delay conditioning (a manipulation of Variable 5, see Table 1). At test, conditioned responding was assessed using a flooding measure to assess degrees of retardation of acquisition of behavioral control. Maximal inhibition (i.e., retardation) was expected when the inhibitor was paired with the US at the same temporal location as the inhibitor had previously signaled omission of the US (e.g., delay X→US and trace Y→US pairings). Results indicated a greater attenuation of conditioned responding in these groups relative to that observed to a previously neutral CS, Z, and to trace X→US or delay Y→US pairings. These findings suggest that passage of a retardation test for conditioned inhibition is influenced by the temporal expectancies of US omission and the temporal location of the US on the retardation pairings. When these temporal expectancies of reinforcement and nonreinforcement were temporally consistent, greater retardation was observed.
Figure 4a.
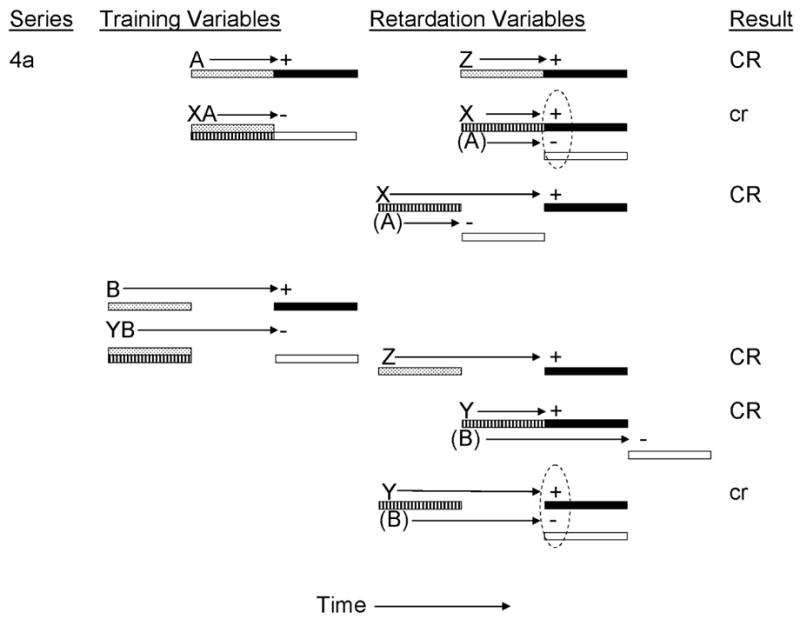
Inhibitory training provided by Burger, Dennison, & Miller, 2001). CSs A, B, X, Y, and Z were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. Prior to testing, animals received either delay or trace pairings of X, Y, or Z with the US. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
In a second experiment, Burger et al. (2001) manipulated the inhibitor-training excitor temporal relationship (Variable 2, Table 1) to assess temporal control of simultaneous and serial inhibitors using a retardation test for conditioned inhibition. In that study, rats received training to establish two conditioned inhibitors (X and Y) with a single delay excitor (CS A, see Figure 4b). Inhibitor X was trained as a simultaneous inhibitor for delay excitor A in order to produce a temporal expectancy of US omission 5 s following onset of inhibitor X, whereas inhibitor Y was trained as a serial inhibitor for delay excitor A through serial pairings of Y and A (with no gap between stimuli) in order to produce a temporal expectancy of US omission 10 s following onset of inhibitor Y. As in the previously described experiment, subjects then received X-US and Y-US retardation-test pairings in which the inhibitor-US temporal relationship was manipulated. Subjects that received delay X-US pairings, but not those receiving delay Y-US pairings, and subjects that received trace Y-US, but not those receiving trace X-US pairings, demonstrated reduced conditioned responding at test, thereby evidencing retardation of acquisition of behavioral control. Again, these results demonstrate temporal control of inhibition through retardation tests, and they additionally extend the findings to serial inhibitors.
Figure 4b.
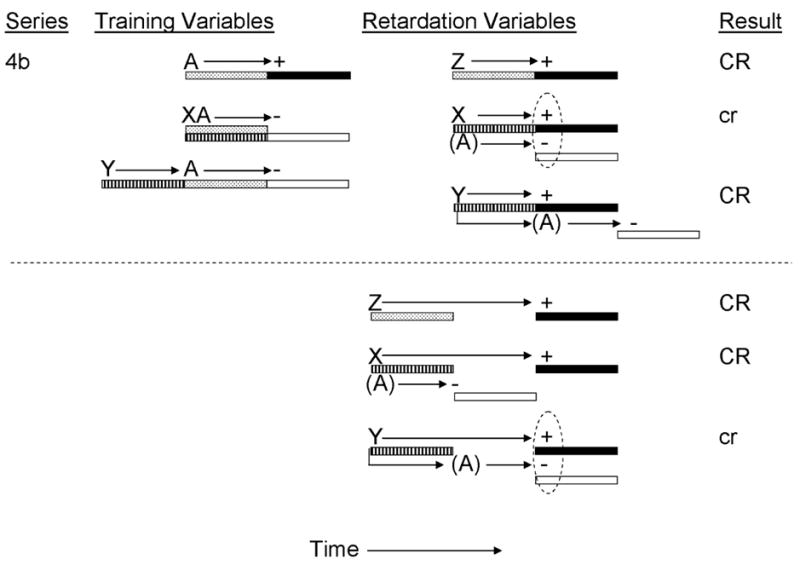
Inhibitory training provided by Burger, Dennison, & Miller, 2001). CSs A, X, Y, and Z were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. Prior to testing, animals received either delay or trace pairings of X, Y, or Z with the US. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
One potential shortcoming of the previously described series of experiments is that they used a relatively gross measure of conditioned control of behavior (i.e., conditioned suppression). Although the patterns of inhibitory behavioral control demonstrated through both the retardation and summation tests for conditioned inhibition provide seemingly compelling evidence for timing the omission of the US, the flooding test used to assess behavior indicative of inhibition does not provide a moment-by-moment measure of conditioned responding (e.g., such as that provided by the peak procedure, Roberts & Church, 1978). Toward addressing this concern, Williams et al. (2006) used a nose poke procedure with rats in which food pellets were presented during a 30-s CS and conditioned responding was assessed by the number and timing of entries into the food cup. During conditioning, two training excitors (A1 and A2) were established as signals for US presentation through presentation of the US 30 s following onset of A1 and 10 s following onset of A2. On other trials, two other stimuli (V1 and V2) were each presented nonreinforced simultaneously with A1 and A2, respectively. This training was intended to establish V1 as a signal for omission of the US 30 s following onset of V1 and V2 as a signal for omission of the US 10 s following onset of V2. For the purpose of negative summation testing, a third excitor (A3) was established through pairings of A3 with the US at both 10 and 30 s following CS onset. At test, peak responding was observed to A3 alone around the times at which the US had previously been presented (10 s and 30 s). However, when V1 was compounded with A3, reduced responding was observed 30 s, but not 10 s into the compound. Similarly, when V2 was compounded with A3, reduced responding was observed 10 s, but not 30 s into the compound. This pattern of results indicates that V1 and V2 produced maximal negative summation around the times at which their training excitors were otherwise paired with the US. This pattern of results replicates those of Miller and his colleagues (Barnet & Miller, 1996; Denniston, Blaisdell et al., 1998; Denniston, Cole et al., 1998) using a more fine grained analysis of the temporal control of inhibition made possible through the use of an appetitive conditioning preparation.
In a second experiment, Williams et al. (2006) assessed temporal specificity of inhibition using a retardation test for conditioned inhibition. Following training similar to that described above, in which V1 was established as a signal for US omission 30 s following its onset and V2 was established as a signal for US omission 10 s following its onset, retardation pairings were provided in which each stimulus was paired with the US either 10 s or 30 s following CS onset. Results indicated decreased behavioral control by V1 when the US was presented 30 s, but not 10 s, following onset of V1 and by V2 when the US was presented 10s, but not 30 s, following onset of V2. This pattern of responding during the retardation test indicates that maximal retardation is observed when the US is paired with the inhibitory CS at the same temporal location as the inhibitor signaled omission of the US, a result which replicates those of Burger et al. (2001).
Associative Structure of Temporal Control of Inhibition
The second line of experiments to be discussed in the present review investigated the mechanisms underlying temporal control of conditioned inhibition. According to the temporal coding hypothesis (Matzel et al., 1988) animals form temporal maps linking events in memory and these temporal maps can be integrated when they contain a common element. Applied to inhibitory behavioral control, Barnet and Miller’s (1996; see also Denniston, Blaisdell et al., 1998; Denniston, Cole et al., 1998) findings suggest that inhibitory behavioral control is a consequence of animals forming temporal maps in which the temporal relationships between the inhibitor and the training excitor and between the training excitor and the US are encoded during training. During a negative summation test for inhibition, presentation of the inhibitor activates these temporal maps and produces an expectancy of US omission based upon integration of the inhibitor-training excitor and the training excitor-US temporal relationships. When this expectancy of US omission corresponds to the time at which the US is expected based upon the transfer excitor, maximal inhibition is observed. Although the previously described series of experiments obtained results consistent with this view, they do not directly assess whether the temporal expectancy of US omission is based upon a direct inhibitor-US inhibitory association, or one that is mediated by the inhibitor’s training excitor (as hypothesized by Barnet & Miller).
To investigate the associative structure of temporal control of inhibition, Denniston et al. (2004) provided rats with conditioned inhibition training in which a simultaneous inhibitor was established with a delay training excitor (all CSs were 5 s in duration and the US was a brief, mild footshock, see Figure 5a). This training was intended to establish inhibitor X as a signal for US omission 5 s following X’s onset. Prior to negative summation testing, some subjects received further training with the training excitor, A, in order to manipulate the temporal expectancy of US omission (control subjects received equivalent training with a previously neutral stimulus, B, not shown in Figure 5a). That is, if the temporal expectancy of US omission provided by inhibitor X is mediated by both the X-A and the A-US temporal relationships that prevailed at test, then changes in the A-US temporal relationship implemented following completion of inhibition training should produce a corresponding shift in temporal control of inhibition. Alternatively, if temporal control of inhibition is based upon a direct X-noUS association, then posttraining manipulation of the A-US temporal relationship should have no effect. Following temporal shifting treatment in which CS A received trace pairings with a 5-s gap between termination of A and onset of the US, all subjects received negative summation testing with one of two transfer excitors. One transfer excitor, C, was trained as a delay excitor so that the US was expected 5 s after its onset, whereas a second transfer excitor, D, was trained as a delay excitor so that the US was expected 10 s after its onset. Subjects in the No-Shift control group displayed maximal inhibitory behavioral control when inhibitor X was compounded with a delay, but not a trace, transfer excitor. This finding replicated those of the previously described studies of timing of inhibitory behavioral control in that inhibitor X was expected to signal omission of the US 5 s following its onset which corresponds to the time at which transfer excitor C, the delay excitor, signaled US presentation. By contrast, subjects that received further training with training excitor A demonstrated maximal inhibition when inhibitor X was compounded with trace transfer excitor D, but not delay transfer excitor C. This finding suggests that the expectation of the omission of the US is mediated by the inhibitor’s training excitor at the time of testing in that changes in the temporal expectancy of the US after completion of inhibition training (based upon CS A) produced a corresponding shift in the temporal expectancy of the omission of the US. In other words, shifting the A-US temporal relationship from delay to trace allowed the simultaneous inhibitor to attenuate responding to the trace, but not delay transfer excitor. In the absence of this manipulation, the opposite pattern of results was obtained.
Figure 5a.
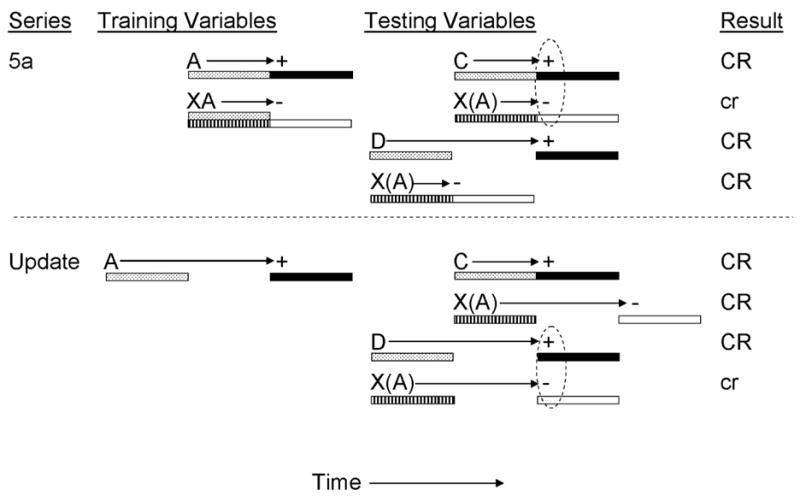
Inhibitory training provided by Denniston, Blaisdell & Miller, 2004). CSs A, C, D, and X were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. Prior to testing, animals received additional training with CS A (or a previously neutral stimulus, B) in which the CS was conditioned as a trace excitor. At test, animals received presentations of C, XC, D, or XD. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
In a second study, Denniston et al. (2004) investigated the effect of shortening the A-US temporal relationship from delay to simultaneous. As in the previous experiment, a single inhibitor was established though nonreinforced pairings of inhibitor X with training excitor A (which was paired with a 5-s footshock US), but in this experiment inhibitor X was trained as a serial inhibitor in order to generate an expectancy of omission of the US 10 s following onset of inhibitor X (see Figure 5b). Following inhibitory training, some subjects received further training with training excitor A (or a previously neutral stimulus, B) in which the temporal relationship of A was shifted from delay to simultaneous (i.e., the CS-US interval was shortened by 5 s). As a consequence of this manipulation, it was expected that the temporal expectancy of US omission based upon inhibitor X would be similarly shortened by 5 s (i.e., X should now signal US omission 5 s following its onset). At test, subjects lacking the A-US update training demonstrated maximal inhibition when inhibitor X was compounded with a trace transfer excitor D (which signaled US presentation 10 s following its onset), but not a delay transfer excitor C (which signaled US presentation 5 s followings its onset). By contrast, subjects that received posttraining temporal shifting of the A-US association demonstrated strong inhibition when inhibitor X was compounded with the delay, but not the trace transfer excitors. These results support the view that temporal control of inhibition is dependent upon both the inhibitor-training excitor and the training excitor-US temporal relationships in effect at the time of testing. In other words, the temporal expectancy of US omission appears to be mediated by its training excitor. Furthermore, these findings are consistent with the temporal coding hypothesis’ assertion that animals form temporal maps linking events in memory and that these temporal maps can be integrated when they have common associates. For example, presentation of inhibitor X activates a temporal map containing training excitor A (in the absence of reinforcement), which in turn activates a representation of the US not occurring at a specific moment in time. When this temporal expectancy of the absence of the US is temporally consistent with the temporal expectancy of the US activated by the transfer excitor, maximal inhibition is observed.
Figure 5b.
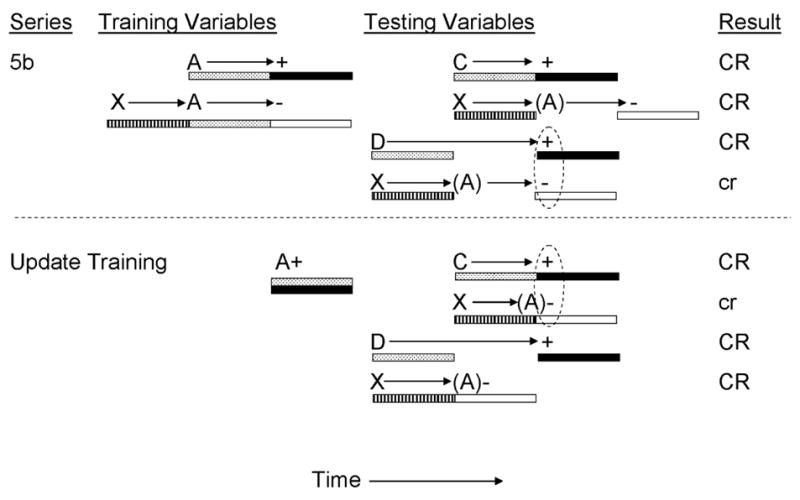
Inhibitory training provided by Denniston, Blaisdell & Miller, 2004). CSs A, C, D, and X were 5-s audiovisual stimuli; ‘+’ denotes a footshock; ‘−’ denotes nonreinforcement. Stippled bars indicate presentation of an excitatory CS; filled bars depict presentation of the US; striped bars depict presentation of the conditioned inhibitor; and open bars depict expectation of the omission of the US. Prior to testing, animals received additional training with CS A (or a previously neutral stimulus, B) in which the CS was conditioned as a simultaneous excitor. At test, animals received presentations of C, XC, D, or XD. Stimuli in parentheses are hypothetical neural activations of the training excitors and their associated USs. Dashed ovals represent simultaneous activation of the US by the transfer excitor and the omission of the US by the conditioned inhibitor. Upper case CR’s denote the observation at test of strong conditioned behavior and lower case cr’s denote weak conditioned behavior.
It is important to note that similar additional reinforcement of the training excitor A without any change in the temporal location of the US can result in enhancement of Pavlovian conditioned inhibition (Amundson, Wheeler, & Miller, 2005). Presumably, in the studies by Denniston et al. (2004) additional reinforcement of the training excitor decreased behavior indicative of inhibition because the temporal relationship between A and the US was altered. This suggests that, as others have noted (e.g., Lysle & Fowler, 1985), behavior indicative on inhibition depends on both the associative strength of training excitor A and the temporal information, provided through inhibitor X, of the training excitor’s temporal relationship with the US.
Temporal Control of Inhibition Produced Through Extinction
The prior discussion focused on conditioned inhibition produced using Pavlov’s procedure. This was done because temporal relationships between stimuli are more clearly defined within Pavlov’s procedure than with most other procedures for inducing conditioned inhibition (e.g., explicitly unpaired inhibition, differential inhibition, and backward inhibition). However, experimental extinction offers the possibility of producing conditioned inhibition in a situation in which all stimuli have clear temporal relationships. Many mechanisms have been proposed to underlie the phenomenon of experimental extinction. For example, Pavlov (1927) viewed the loss of responding to previously conditioned CS following nonreinforced exposure to the CS as being due to the acquisition of an inhibitory association. However, despite numerous attempts to demonstrate that an extinguished CS possesses net inhibitory strength (e.g., Bouton & Swartzentruber, 1989; Hendry, 1982; Konorski & Szwejkowska, 1950, 1952; Macrae & Kehoe, 1999; Reberg, 1972), most prior studies have found either positive summation by an extinguished CS when compounded with a transfer excitor (e.g., Hendry; Reberg) or facilitated reacquisition during a retardation test for conditioned inhibition (Konorski & Szwejkowska). Although these studies have failed to obtain evidence that an extinguished CS can pass the traditional tests for conditioned inhibition, this does not necessarily indicate that inhibition is not involved in the loss of responding indicative of extinction.
Based upon Pavlov’s (1927) view of extinction, one might expect inhibition to accrue only until the strength of the inhibitory association matches that of the previously acquired excitatory association, thereby leading to a cessation of responding. Therefore, following extinction treatment it is not surprising that an extinguished CS fails both retardation and summation tests for conditioned inhibition, as the inhibitory and excitatory associations should offset eachother, thereby leading to no net inhibitory effect. However, recent research has found that an extinguished CS can pass both summation and retardation tests for conditioned inhibition, provided that a sufficiently large number of extinction trials are provided (Calton, Mitchell, & Schachtman, 1996; Hart, Bourne, & Schachtman, 1995; Schachtman, Threlkeld, & Meyer, 2000). One potential explanation for the effect of massive extinction apparently resulting in net inhibition is that massive extinction may lead to superior retrieval of the inhibitory association over the excitatory association (Denniston & Miller, 2003). However, it is also possible that after massive extinction some loss of excitation occurs, thereby allowing the inhibitory potential to be more readily expressed. In either case, that a massively extinguished CS can pass both summation and retardation tests for conditioned inhibition raises the question as to whether this form of inhibition can display the same temporal characteristics as traditional conditioned inhibitors.
Research on the temporal properties of extinguished stimuli has revealed that animals can separate the decisions of whether and when to respond to a CS, in a manner similar to that observed with the acquisition of conditioned responding (Ohyama, Gibbon, Deich, & Balsam, 1999). For example, Ohyama et al. used an autoshaping procedure with ring doves (Streptopelia risoria) in which subjects received exposure to a 4, 8, or 16-s keylight followed by access to food. On nonreinforced probe trials, peak responding was observed around that time at which food had normally been presented (i.e., 4, 8, or 16 s). In a second experiment, similar training to that described above was provided with the addition of an extinction phase in which subjects were exposed to 40-s nonreinforced presentations of the keylight CS. During extinction, conditioned responding steadily decreased; however, peak responding was maintained near the time at which the US had previously been presented. Ohyama et al. concluded that these results support the view that the decision of whether to respond is independent from the decision of when to respond. In other words, the timing of the conditioned response during extinction is independent from the absolute level of conditioned responding (see also Drew, Yang, Ohyama, & Balsam, 2004).
Given these two separate lines of research: one investigating whether an extinguished CS can pass the traditional tests for conditioned inhibition (e.g., Calton et al., 1996; Hart et al., 1995; Schachtman et al., 2000); and a second investigating the timing of responding during extinction, Denniston and Miller (2003) investigated whether an extinguished CS could exhibit temporal control of behavior indicative of inhibition in a manner analogous to the studies previously described (e.g., Barnet & Miller, 1996). In their Experiment 1, rats received conditioning with two excitors, X and Y, which were conditioned as delay and trace CSs, respectively, through pairings with a brief, mild footshock either immediately or 5 s following termination of the CS. Following acquisition treatment, subjects received massive extinction treatment of X and Y (1000 nonreinforced presentations of each stimulus, across groups). At test, the inhibitory potential of the extinguished CS was assessed through a negative summation test in which the ability of X and Y to attenuate responding to each of two transfer excitors, one delay and one trace, was assessed. Results indicated that the extinguished delay excitor, X, maximally inhibited the delay, but not trace transfer excitor and that the extinguished trace excitor, Y, maximally inhibited the trace, but not delay transfer excitor. In their Experiment 2, Denniston and Miller assessed the potential of these extinguished CSs to pass a retardation test for conditioned inhibition. Following analogous training to that described above, extinguished CSs X and Y were each paired with the US in order to assess temporal control of inhibition. During the retardation pairings, the temporal location of the US was manipulated such that it was presented either at the same or different temporal location as it was previously expected based on pre-extinction reinforced training. For example, subjects that had received extinction of CS X, the delay excitor, now received either delay or trace X-US retardation pairings. Similarly, subjects that had received extinction of CS Y, the trace excitor, received either delay or trace Y-US retardation pairings. Results indicated greater retardation when the US was presented at the same temporal location as it had been previously presented during acquisition training. That is, greater retardation was observed when the extinguished delay excitor was retrained as a delay, but not a trace CS, and when the extinguished trace excitor was retrained as a trace, but not a delay CS. These findings suggest that an extinguished CS, at least with massive extinction treatment, can pass both summation and retardation tests for conditioned inhibition provided that the temporal expectancy of US omission coincides with the temporal expectancy of US presentation provided by either the transfer excitor (in a summation test) or the CS-US pairings (in a retardation test). More generally, these results extend the observations of temporal control of inhibition to inhibition produced through extinction.
Summary
The previously described lines of investigation were designed to assess the nature of temporal control of inhibitory responding. The first series of experiments reviewed the timing of inhibitory behavioral control though both summation and retardation tests for conditioned inhibition established using Pavlov’s (1927) procedure. These experiments demonstrated that inhibitory behavioral control is influenced by temporal expectancies of nonreinforcement, which are based upon the temporal relationships between the inhibitor and the training excitor and between the training excitor and the US (e.g., Denniston et al., 2004). Such findings are consistent with the temporal coding hypothesis (Matzel et al., 1988; Savastano & Miller, 1998), which posits that animals encode the temporal relationship between events as a temporal map and can integrate these temporal maps when the maps include common stimuli. Applied to inhibitory behavioral control, presentation of the conditioned inhibitor activates a representation of the training excitor (without reinforcement) which in turn activates a representation of absence of the US. These representations include the temporal relationships between events and generate a temporal expectancy of the omission of the US based upon these temporal maps. Notably, the findings of Miller and colleagues have been replicated using an appetitive nose-poke procedure which provided a second-by-second analysis of inhibitory behavioral control (Williams et al., 2006).
The second line of experiments described in the present review were designed to explore the nature of inhibition involved in extinction (Denniston & Miller, 2003). This series of experiments found that an excitor subjected to massive extinction treatment was capable of passing both summation and retardation tests for conditioned inhibition. Of greater interest, temporal control of inhibition produced through extinction was influenced by temporal expectancies of nonreinforcement. This effect mirrored that observed with more traditional inhibitors and suggests that an extinguished CS can pass a summation test for conditioned inhibition when the transfer excitor signals US presentation at the same temporal location as the extinguished CS previously signaled US presentation. Hence, extinction appears to result in an expectation of nonreinforcement at a particular point in time, which is consistent with the observations of Balsam and colleagues (Drew et al., 2004; Ohyama et al., 1999) who found continued temporal control of conditioned responding despite nonreinforcement of a CS. The results of Denniston and Miller suggest that this temporal information is maintained and that the decision of how to respond (i.e., either excitatory or inhibitory) is influenced by the magnitude and nature of training (i.e., extinction).
Collectively, these studies point to temporal attributes being included in what is learned during inhibitory learning that results from either Pavlov’s (1927) procedure or extinction of a simple excitor. Through assessment of inhibition using the two-test strategy (Rescorla, 1969), much has been learned about the nature of inhibitory conditioning. These studies demonstrate temporal control of inhibitory conditioned responding that is analogous to that observed in excitatory behavioral control, a finding complementary to those of Russ Church’s highly productive career.
Footnotes
Support for this research was provided by National Institute of Mental Health Grant 33881. We thank Bridget McConnell, Gonzalo P. Urcelay, Koji Urushihara, Daniel S. Wheeler, and James Witnauer for comments on an earlier version of this manuscript. For information concerning this research, contact James C. Denniston at dennistonjc@appstate.edu.
References
- Amundson JC, Wheeler DS, Miller RR. Enhancement of Pavlovian conditioned inhibition achieved by posttraining inflation of the training excitor. Learning and Motivation. 2005;36:331–352. doi: 10.1016/j.lmot.2004.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnet RC, Miller RR. Temporal encoding as a determinant of inhibitory control. Learning and Motivation. 1996;27:73–91. [Google Scholar]
- Bouton ME, Swartzentruber DE. Slow reacquisition following extinction: Context, encoding, and retrieval mechanisms. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:43–53. [Google Scholar]
- Burger D, Denniston JC, Miller RR. Temporal coding in condition inhibition: Retardation tests. Animal Learning & Behavior. 2001;29:281–290. [Google Scholar]
- Calton JL, Mitchell KG, Schachtman TR. Conditioned inhibition produced by extinction of a conditioned stimulus. Learning and Motivation. 1996;27:335–361. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Cole RP, Barnet RC, Miller RR. An evaluation of conditioned inhibition as defined by Rescorla’s two-test strategy. Learning and Motivation. 1997;28:323–341. [Google Scholar]
- Church RM. Properties of the internal clock. In J. Gibbon & L. G. Allan (Eds.). Timing and time perception. Annals of the New York Academy of Sciences. 1984;423:566–582. doi: 10.1111/j.1749-6632.1984.tb23459.x. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Blaisdell AP, Miller RR. Temporal coding affects transfer of serial and simultaneous inhibitors. Animal Learning & Behavior. 1998;26:336–350. [Google Scholar]
- Denniston JC, Blaisdell AP, Miller RR. Temporal control in conditioned inhibition: Analysis of associative structure of inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:190–202. doi: 10.1037/0097-7403.30.3.190. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Cole RP, Miller RR. The role of temporal variables in the transfer of conditioned inhibition. Journal of Experimental Psychology: Animal Behavior Processes. 1998;24:200–214. doi: 10.1037//0097-7403.24.2.200. [DOI] [PubMed] [Google Scholar]
- Denniston JC, Miller RR. The role of temporal variables in inhibition produced through extinction. Learning & Behavior. 2003;31:35–48. doi: 10.3758/bf03195969. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Moore JW. Adaptive timing in neural networks: The conditioned response. Biological Cybernetics. 1988;58:405–415. doi: 10.1007/BF00361347. [DOI] [PubMed] [Google Scholar]
- Drew MR, Yang C, Ohyama T, Balsam PD. Temporal specificity of extinction in autoshaping. Journal of Experimental Psychology: Animal Behavior Processes. 2004;30:163–176. doi: 10.1037/0097-7403.30.3.163. [DOI] [PubMed] [Google Scholar]
- Hart JA, Bourne MJ, Schachtman TR. Slow reacquisition of a conditioned taste aversion. Animal Learning & Behavior. 1995;23:297–303. [Google Scholar]
- Hendry JS. Summation and undetected excitation following extinction of the CER. Animal Learning & Behavior. 1982;10:476–482. [Google Scholar]
- Holland PC. Differential effects of reinforcement of an inhibitory feature after serial and simultaneous feature negative discrimination training. Journal of Experimental Psychology: Animal Behavior Processes. 1984;10:461–475. [PubMed] [Google Scholar]
- Holland PC, Lamarre J. Transfer of inhibition after serial and simultaneous feature negative discrimination training. Learning and Motivation. 1984;15:219–243. [Google Scholar]
- Honig WK. Working memory and the temporal map. In: Spear NE, Miller RR, editors. Information processing in animals: Memory mechanisms. Hillsdale, NJ: Erlbaum; 1981. pp. 167–197. [Google Scholar]
- Konorski J, Szwejkowska G. Chronic extinction and restoration of conditioned reflexes. I. Extinction against the excitatory background. Acta Biologiae Experimentalis. 1950;15:155–170. [Google Scholar]
- Konorski J, Szwejkowska G. Chronic extinction and restoration of conditioned reflexes. III. Defensive motor reflexes. Acta Biologiae Experimentalis. 1952;16:91–94. [Google Scholar]
- Lamarre J, Holland PC. Transfer of inhibition after serial feature negative discrimination training. Learning and Motivation. 1987;18:319–342. [Google Scholar]
- Lysle DT, Fowler H. Inhibition as a “slave” process: Deactivation of conditioned inhibition through extinction of conditioned excitation. Journal of Experimental Psychology: Animal Behavior Processes. 1985;11:71–94. doi: 10.1037//0097-7403.11.1.71. [DOI] [PubMed] [Google Scholar]
- Macrae M, Kehoe EJ. Savings after extinction in conditioning of the rabbit’s nictitating membrane response. Psychobiology. 1999;27:85–94. [Google Scholar]
- Matzel LD, Held FP, Miller RR. Reexamination of simultaneous and backward conditioning: Implications for contiguity theory. Learning and Motivation. 1988;19:317–344. [Google Scholar]
- Miller RR, Barnet RC. The role of time in elementary associations. Current Directions in Psychological Science. 1993;2:106–111. [Google Scholar]
- Miller RR, Oberling P. Analogies between occasion setting and Pavlovian conditioning. In: Schmajuk NA, Holland PC, editors. Occasion setting: Associative learning and cognition in animals. Washington, DC: American Psychological Association; 1998. pp. 3–35. [Google Scholar]
- Ohyama T, Gibbon J, Deich JD, Balsam PD. Temporal control during maintenance and extinction of conditioned keypecking in ring doves. Animal Learning & Behavior. 1999;27:89–98. [Google Scholar]
- Papini MR, Bitterman ME. The two-test strategy in the study of inhibitory conditioning. Journal of Experimental Psychology: Animal Behavior Processes. 1993;19:342–352. doi: 10.1037//0097-7403.19.4.342. [DOI] [PubMed] [Google Scholar]
- Pavlov IP. Conditioned reflexes. London: Oxford University Press; 1927. [Google Scholar]
- Reberg D. Compound tests for excitation in early acquisition and after prolonged extinction of conditioned suppression. Learning and Motivation. 1972;3:246–258. [Google Scholar]
- Rescorla RA. Pavlovian conditioned inhibition. Psychological Bulletin. 1969;72:77–94. [Google Scholar]
- Roberts S, Church RM. Control of an internal clock. Journal of Experimental Psychology: Animal Behavior Processes. 1978;4:318–337. [Google Scholar]
- Savastano HI, Cole RP, Barnet RC, Miller RR. Reconsidering conditioned inhibition. Learning and Motivation. 1999;30:101–127. [Google Scholar]
- Savastano HI, Miller RR. Time as content in Pavlovian conditioning. Behavioural Processes. 1998;44:147–162. doi: 10.1016/s0376-6357(98)00046-1. [DOI] [PubMed] [Google Scholar]
- Schachtman TR, Threlkeld R, Meyer K. Retention of conditioned inhibition produced by extinction. Learning and Motivation. 2000;31:283–300. doi: 10.1006/lmot.1996.0020. [DOI] [PubMed] [Google Scholar]
- Williams DA, Johns KW, Bindas M. Timing during inhibitory conditioning. 2006. Manuscript submitted for publication. [DOI] [PubMed] [Google Scholar]


