Abstract
Cultured skin substitutes (CSS) consisting of fibroblasts, keratinocytes, and biopolymers are an adjunctive treatment for large burns. Because CSS lack a vascular plexus, they vascularize more slowly than split-thickness autografts. Previously, CSS were prepared with dermal microvascular endothelial cells (ECs), which formed vascular analogs at a low frequency but did not contribute to increased vascularization after grafting. The present study addressed whether keratinocytes genetically modified to overexpress vascular endothelial growth factor (VEGF), an endothelial cell mitogen, could improve the persistence and organization of ECs in CSS. CSS were prepared with control or VEGF-modified keratinocytes, with (CSS + ECs) or without added ECs, and were grafted to full-thickness wounds in athymic mice. Elevated VEGF expression was detected in VEGF-modified CSS and CSS + ECs compared with controls, but no significant difference in EC density in vitro was observed. After grafting, VEGF-modified CSS and CSS + ECs showed enhanced vascularization, and organization of human ECs into multicellular structures in CSS + ECs was observed. However, VEGF overexpression did not significantly enhance the proliferation of human ECs, suggesting that other factors may be required. Improved persistence and organization of human ECs in vitro will likely be required for their participation in vascularization of CSS + ECs after grafting.
In patients with large skin injuries, their recovery is dependent on the closure of open wounds. This is often problematic in massive burns that affect a large percent of TBSA because of the lack of donor skin for autografting, which has been the historical standard of treatment. Delays in wound closure can increase the risk for infection, a major cause of burn mortality.1 Infection also is a common complication in chronic wounds. These wounds generally affect a small percent of TBSA, but they are relatively common, particularly in elderly patients. Therefore, these wounds have large medical and economic impacts.2 As with burns, a common treatment for chronic wounds historically has been split-thickness or full-thickness autologous skin grafting. Because there are often underlying diseases or deficiencies in chronic wound patients, donor site healing is often slow and painful.3 The need for timely wound closure in these patient populations has led to the development of a number of alternatives to split-thickness skin autografting. These have included several biologic wound dressings as well as temporary and permanent skin replacements.4–6
Favorable clinical outcomes have been obtained using cultured skin substitutes (CSS) as an adjunctive treatment for coverage of massive burns and chronic wounds.7–9 CSS comprise cultured keratinocytes, fibroblasts, and biopolymers. If autologous cells are used, as in the treatment of excised burn wounds, CSS function as permanent skin replacements. Upon healing, CSS perform similarly to healed split-thickness human skin grafts. However, because CSS for clinical applications contain only two cell types, deficiencies in anatomy and physiology remain.10 One obvious deficiency is the lack of a vascular plexus, which results in delayed vascularization compared with split-thickness skin autografting. The increased time required for vascularization of CSS has been found to contribute to increased susceptibility to microbial contamination and reduced engraftment.7 This limitation of CSS has been addressed indirectly through the application of dressing fluids containing nutrients and antimicrobial agents for several days after grafting.8,11–13 These dressing fluids serve to protect and nourish the cells in the grafted cultured skin during the period of ischemia and are discontinued after vascularization is achieved. However, the application of dressing fluids to CSS after grafting is expensive, labor intensive, and can cause mechanical damage that increases the risk of graft failure. Further, the use of topical antimicrobial agents to combat graft contamination may contribute to the emergence of resistant microorganisms.
In previous studies, cultured skin grafts prepared with keratinocytes genetically modified to overexpress the endothelial cell (EC) mitogen vascular endothelial growth factor (VEGF)14,15 showed increased vascularization after grafting to athymic mice.16,17 The VEGF-modified CSS displayed greater stability and increased engraftment compared with control CSS. This demonstrated the importance of vascularization in healing of grafted CSS.17 The inclusion of genetically modified cells indirectly addressed the lack of a vascular plexus in CSS by stimulating vascularization from the wound bed. In another study, CSS were prepared with human dermal microvascular ECs. These cells persisted at low numbers in the grafts and formed multicellular aggregates.18 Although this study demonstrated the transplantation of human ECs in a cultured skin model, the numbers of “vascular analogs” formed was not sufficient to enhance vascularization after grafting to athymic mice.18
Hypothetically, increased VEGF expression might enhance the persistence of human ECs in CSS or improve their organization. The present study was designed to examine the prospective benefit of VEGF overexpression in CSS containing ECs.
METHODS
Cell Culture and Preparation of Cultured Skin Substitutes
Epidermal keratinocytes, dermal fibroblasts, and dermal microvascular ECs were isolated from a single human skin sample obtained from a 42-year-old female donor undergoing reduction mammoplasty. Primary cultures of human keratinocytes and fibro-blasts were isolated as previously described19,20 with modifications to permit isolation of ECs.18 After one passage (keratinocytes and fibroblasts) or two passages (ECs), all cells were cryopreserved in the appropriate growth medium supplemented with 20% fetal bovine serum plus 10% dimethyl sulfoxide (Sigma Chemical Co., St. Louis, MO). Dermal cells were recovered from cryopreservation and expanded without additional passage for inoculation of cultured skin substitutes.
Keratinocytes were modified by transduction with a replication incompetent retroviral VEGF vector, L-CVEGF-SN, which is described in detail elsewhere.16 Briefly, this vector contains the coding sequence for the 165-amino acid-secreted isoform of VEGF transcribed from a cytomegalovirus promoter/enhancer. The parent retroviral vector, pLXSN (BD Biosciences/Clontech, Palo Alto, CA), contains a long-terminal repeat promoter. Hence, target cells modified with this vector express two retroviral VEGF transcripts with identical coding sequence, from both the long terminal repeat and cytomegalo-virus promoters. The VEGF protein produced by cells infected with the L-CVEGF-SN retrovirus was shown in a previous study to be mitogenic to human microvascular ECs.16 Control virus-producing cell lines were generated using the pLXSN vector without inserted VEGF sequences. Second-passage keratinocytes were modified by coculture with growth inactivated virus-producing cells pretreated with 15 μg/ml mitomycin C (Sigma). After 5 days, the cultures were treated with 5 mM ethylenediamine tetraacetic acid to selectively remove virus-producing cells, and the keratinocytes were subcultured to expand cell populations.
CSS were prepared as previously described20,21 with modifications for the addition of ECs.18 Dermal substitutes were prepared by inoculating acellular bovine collagen-glycosaminoglycan substrates with either fibroblasts alone (5 × 105/cm2) in fibroblast growth medium20 or a mixture of fibroblasts and ECs (5 × 105 each/cm2) in a 1:1 mixture of fibroblast growth medium and EC growth medium (M131 plus Microvascular Growth Supplement; Cascade Biologics, Portland, OR) supplemented with 10 ng/ml recombinant human VEGF (R&D Systems, Minneapolis, MN). One day later, control or VEGF-modified keratinocytes were inoculated onto the dermal substrates, and the medium was changed to maturation medium for skin substitutes20 without added VEGF. A total of eight CSS (n = 2 per group), with an approximately 80 cm2 starting surface area each, were prepared (Table 1). CSS and CSS with ECs (CSS + ECs) were supported on 9-mm thick sponges (Medtronic Merocel, Mystic, CT) during cell inoculation and were lifted on stainless-steel mesh frames for subsequent culture at the air-liquid interface (14 days total, 37°C, 5% CO2) with daily changes of nutrient medium. Biopsies for light microscopy and immunohistochemistry were collected at culture days 7 and 14. At the end of the culture period, grafts were cut to 2-cm × 2-cm squares for transplantation to mice.
Table 1.
Experimental design for cultured skin substitutes
| Group | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| VEGF modified | − | + | − | + |
| Endothelial cells | − | − | + | + |
VEGF, vascular endothelial growth factor.
Grafting to Athymic Mice
Animal studies were performed with the approval of the University of Cincinnati Institutional Animal Care and Use Committee following National Institutes of Health guidelines. Control and VEGF-modified CSS and CSS + ECs were grafted to 2-cm × 2-cm full-thickness wounds on the flanks of athymic mice (n = 8 per group) as previously described.18,20,22 Briefly, each wound was prepared by cutting the wound perimeter and carefully separating and removing the skin from the underlying panniculus carnosus. Grafts with overlying nonadherent dressing (N-terface; Winfield Laboratories, Richard-son, TX) were sutured to wounds. Grafts were dressed with gauze pads coated with antibiotic ointment, the dressings were covered with OpSite (Smith & Nephew Medical, Hull, UK), and the grafted areas were bandaged with Coban (3M Medical Division, St. Paul, MN). Mice were sacrificed at 1 week or 3 weeks after surgery (n = 4 per group per time point). For mice sacrificed at 3 weeks, dressings and sutures were removed after 2 weeks. At sacrifice, multiple graft biopsies were collected for immunohistochemistry and RNA isolation. For tissue sections, biopsies were frozen in M-1 Embedding Matrix (Lipshaw, Pittsburgh, PA), or fixed in 10% buffered neutral formalin and embedded in paraffin.
Immunohistochemistry
A colorimetric immunoperoxidase procedure was used for the in vitro visualization of human ECs in CSS instead of immunofluorescent labeling because of the high levels of background fluorescence from the bovine collagen. Cryostat sections were dehydrated in methanol and fixed in acetone at −20°C. After air-drying, sections were rehydrated in phosphate-buffered saline, pH 7.6. CSS sections were incubated for 1 hour at room temperature with biotin-conjugated anti-human CD31 antibody (5 μg/ml; ID Labs, Inc., London, Ontario, Canada) followed by detection using the Vectastain® Elite ABC Universal kit and the DAB Peroxidase Substrate kit (Vector Labs, Burlingame, CA). Sections were counter-stained using Vector® Hematoxylin QS (Vector Labs).
Indirect immunofluorescence was used for species-specific antigen detection in cryostat sections of grafted CSS. Human ECs were labeled using biotin-anti-human CD31 antibody (5 μg/ml; ID Labs, Inc.), followed by detection using the Tyramide Signal Amplification Direct (Green) Kit (PerkinElmer Life Sciences, Boston, MA). Mouse endothelial cells were indirectly stained using purified rat anti-mouse CD31 antibody (12.5 μg/ml; BD PharMingen) followed by visualization with a Texas Red®-X goat anti-rat IgG antibody (10 μg/ml; Molecular Probes, Eugene, OR) for red fluorescence. All antibody incubations were performed for 1 hour at room temperature in a humidified chamber. Samples were washed with phosphate-buffered saline, and slides were coverslipped using Fluoromount-G mounting media (Southern Biotechnology Associates, Birmingham, AL). In negative controls for all antibody staining, either primary or secondary antibodies were omitted. Sections were examined using a Microphot-FXA microscope (Nikon, Melville, NY) equipped with epifluorescent illumination.
Human vascular analogs in paraffin sections of grafted CSS + ECs were immunostained using an antihuman CD34 antibody (NeoMarkers, Inc., Fremont, CA). Sections were deparaffinized,23 and endogenous peroxidase was quenched by incubation in 3% hydrogen peroxide for 10 minutes. Antigen retrieval was performed using Vector® Antigen Unmasking Solution (Vector Labs). Sections were incubated overnight at 4°C with anti-human CD34 antibody (1 μg/ml), followed by detection using the Vectastain® Elite ABC Universal kit with DAB Peroxidase Substrate and Vector® Hematoxylin QS counterstain (Vector Labs).
All sections were photographed using a Spot-Jr. Digital Camera (Diagnostic Instruments, Inc., Sterling Heights, MI). Color fluorescence images were digitally converted to grayscale for publication of black and white figures.
Analysis of VEGF Expression
Expression of retroviral VEGF RNA was analyzed using Northern blot hybridization. Cells were snap-frozen in liquid nitrogen and stored at −70°C, and CSS biopsies were stored in RNAlater (Ambion, Inc., Austin, TX) at −20°C. Total cellular RNA was isolated using the RNeasy Mini Kit (QIAGEN, Santa Clarita, CA) and was separated on 1% agarose gels using NorthernMax buffers (Ambion, Inc.). Gels were transferred overnight to BrightStar™-Plus Membranes (Ambion, Inc.) using the Turboblotter system (Schleicher & Schuell, Keene, NH). The VEGF cDNA probe was a 1.89-kb EcoRI fragment from the L-CVEGF-SN plasmid16 that was biotin labeled using the BrightStar™ Psoralen-Biotin kit (Ambion, Inc.). Hybridizations were performed overnight at 68°C using PerfectHyb™ Plus buffer (Sigma), and detection of bound probe was performed using the BrightStar™ BioDetect™ kit (Ambion, Inc.).
Aliquots of culture medium from subconfluent control and VEGF-modified keratinocytes (n = 4 per group) at the time of CSS inoculation and from CSS (n = 2 per group) at 1 and 2 weeks of in vitro incubation were collected before media changes (conditioned for 24 hours by cells or CSS) and stored at −20°C until assayed. Human VEGF protein levels were determined using the Quantikine Human VEGF Immunoassay Kit (R&D Systems), and mean values are presented. Pairwise comparisons were performed by t test to determine significant differences (P < .001) between control and VEGF-modified keratinocytes or between CSS in groups 1 to 4 (Table 1). Statistical analysis was performed using SigmaStat software (SPSS Science, San Rafael, CA).
RESULTS
Higher levels of VEGF mRNA were observed in keratinocytes genetically modified by transduction with the L-CVEGF-SN retrovirus compared with control keratinocytes modified with an empty retroviral vector (Figure 1A and B). Similarly, CSS prepared with VEGF-modified keratinocytes, with or without human EC, expressed higher levels of VEGF mRNA than controls in vitro (Figure 1C). After grafting to mice, the levels of VEGF mRNA expression declined but were higher in CSS prepared with VEGF-modified cells compared with controls (Figure 1D).
Figure 1.
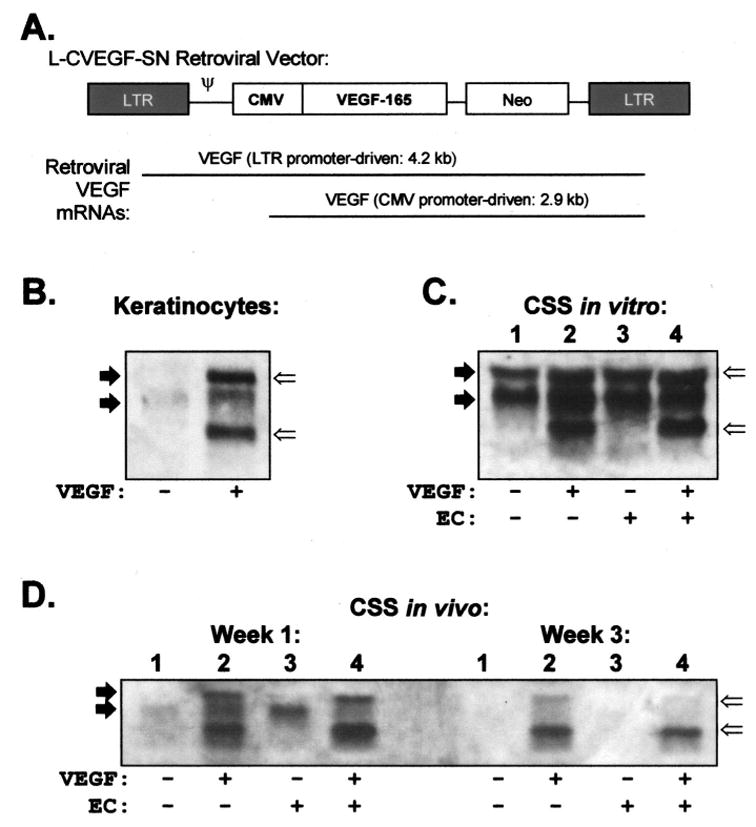
Expression of vascular endothelial growth factor (VEGF) mRNA in cells and cultured skin substitutes (CSS) is increased by genetic modification. A. Diagram of replication-incompetent retroviral vector used for genetic modification of keratinocytes.16,17 B–D. Northern blots containing 10 μg of total RNA per lane were hybridized to a human VEGF probe. B. Control (−) and VEGF-modified (+) keratinocytes. C. CSS groups 1 to 4 (Table 1) in vitro at the time of grafting to mice. D. CSS in vivo, at 1 and 3 weeks after grafting. Solid arrows indicate locations of endogenous VEGF transcripts; open arrows indicate retroviral VEGF transcripts; LTR, long terminal repeat; CMV, cytomegalovirus promoter; Neo, neomycin resistance gene; ψ, retroviral packaging signal.
Consistent with higher VEGF mRNA levels, VEGF-modified keratinocytes secreted significantly more VEGF protein compared with controls (9.50 ng/ml vs 0.99 ng/ml, P ≤ .001). Levels of VEGF protein in media sampled from CSS in vitro were 2- to 5-fold higher in VEGF-modified CSS compared with controls (Figure 2). Although the levels of VEGF decreased from the first to second week in culture, there was relatively more VEGF secreted from the VEGF-modified CSS. The differences found between groups were not statistically significant because of the small number of CSS samples per group in vitro.
Figure 2.
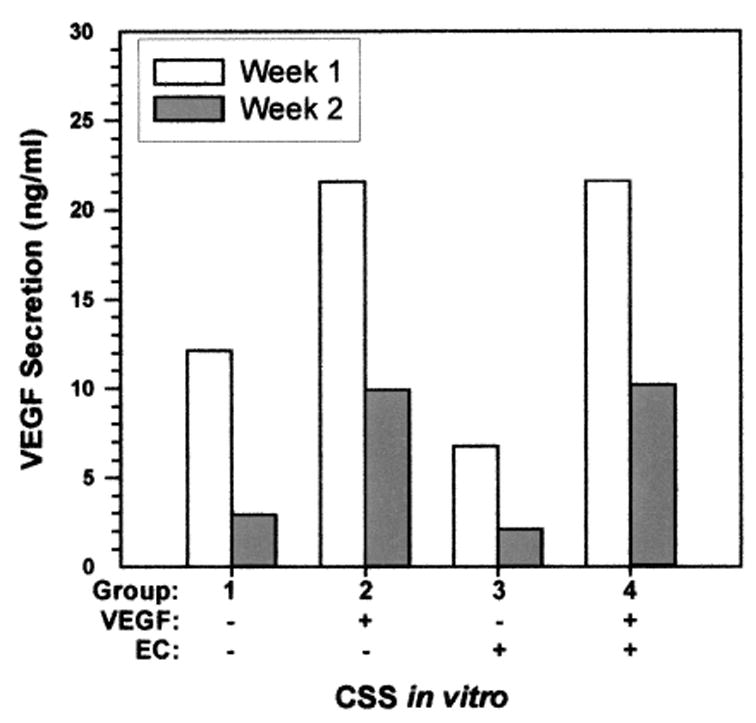
Secretion of human vascular endothelial growth factor (VEGF) protein in vitro. Cultured skin substitutes (CSS) prepared with VEGF-modified keratinocytes, prepared without (group 2) or with (group 4) endothelial cells (EC), secreted higher levels of VEGF in vitro compared with controls (groups 1 and 3).
ECs persisted in CSS + EC during culture in vitro. Human EC were localized by immunohistochemistry using an antibody against human CD31, an EC marker (Figure 3). The ECs were clustered near the top of the dermis subadjacent to the dermal–epidermal junction. Some of these clusters formed vascular analogs in vitro with putative lumens by 2 weeks. There were no significant differences in CSS + ECs prepared with VEGF-modified keratinocytes compared with controls at either 1 or 2 weeks of in vitro incubation (Figure 3).
Figure 3.
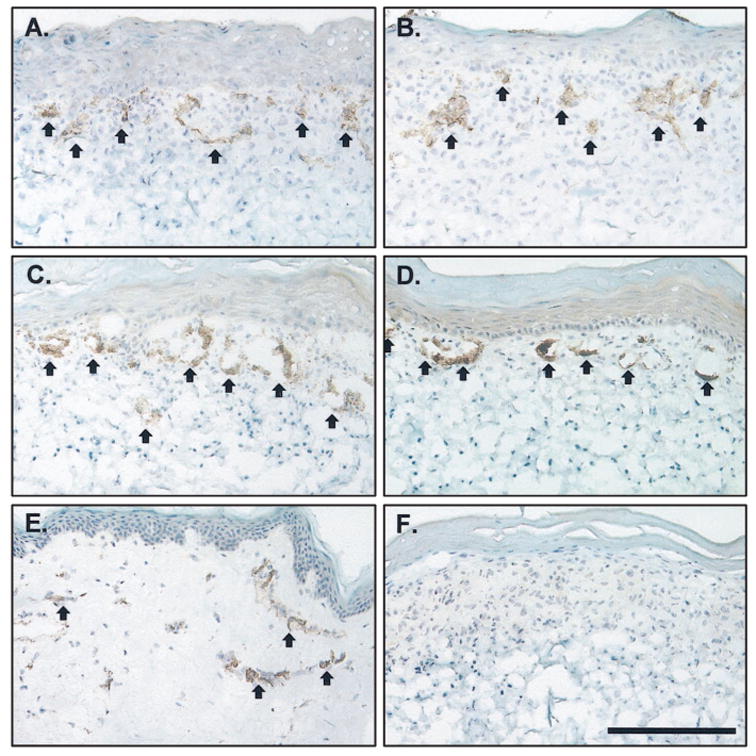
Staining of human endothelial cells in cultured skin substitutes plus endothelial cells (CSS + ECs) in vitro. Shown is immunostaining for human CD31 (arrows, dark brown staining), an EC marker. A. Control CSS + ECs (group 3), week 1 in vitro. B. VEGF-modified CSS + ECs (group 4), week 1 in vitro. C. Control CSS + ECs (group 3), week 2 in vitro. D. VEGF-modified CSS + ECs (group 4), week 2 in vitro. E. normal human skin. F. CSS without EC (group 2), negative control. The epidermis is at the top of each panel; scale bar in F (0.2 mm) is the same for all panels.
Immunohistochemistry with species-specific anti-CD31 antibodies was used to simultaneously localize human and mouse ECs in CSS + ECs after grafting to mice. A vigorous response of mouse ECs to VEGF overexpression was observed (Figure 4). At 1 and 3 weeks after grafting to mice, greater numbers of vessels were observed in VEGF-modified CSS compared with controls (Figure 4). A similar response of mouse ECs was observed in grafted CSS + ECs (Figure 5). At 1 and 3 weeks after grafting, greater densities of mouse vessels, particularly in the upper dermis, were observed in the VEGF-modified CSS + ECs. Human ECs persisted in the CSS + ECs after grafting and, in some regions, the ECs organized into vascular analogs containing putative lumens. However, in contrast to the vessels derived from the mouse wound beds, there were no differences in the densities of human ECs in VEGF-modified CSS + ECs compared with controls. Interestingly, there appeared to be relatively fewer human ECs and vascular analogs present in regions of grafted VEGF-modified CSS + ECs that were heavily populated with mouse vessels (Figure 5).
Figure 4.
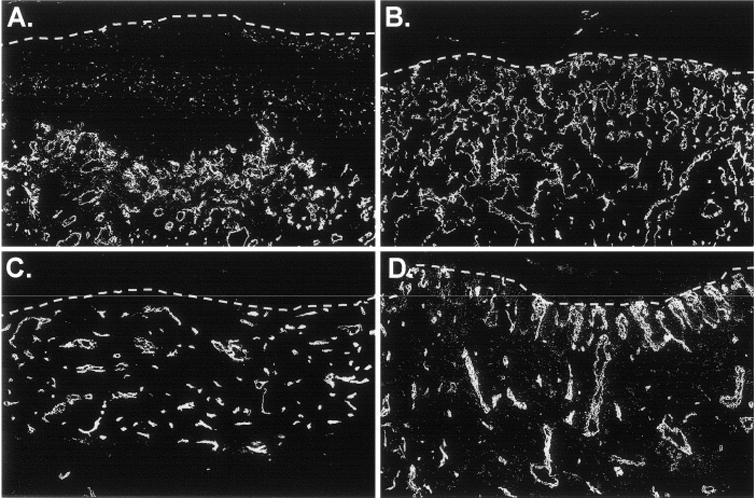
Enhanced vascularization of vascular endothelial growth factor-modified cultured skin substitutes (CSS) prepared without human endothelial cells. Mouse endothelial cells in sections of CSS from 1 week (A, B) or 3 weeks (C, D) after grafting to mice were stained with an antibody specific for mouse CD31. A and C. Control CSS (group 3). B and D. Vascular endothelial growth factor-modified CSS (group 4). Dotted white lines indicate the locations of the dermal–epidermal junctions; sections are oriented with the epidermis at the top of each panel.
Figure 5.
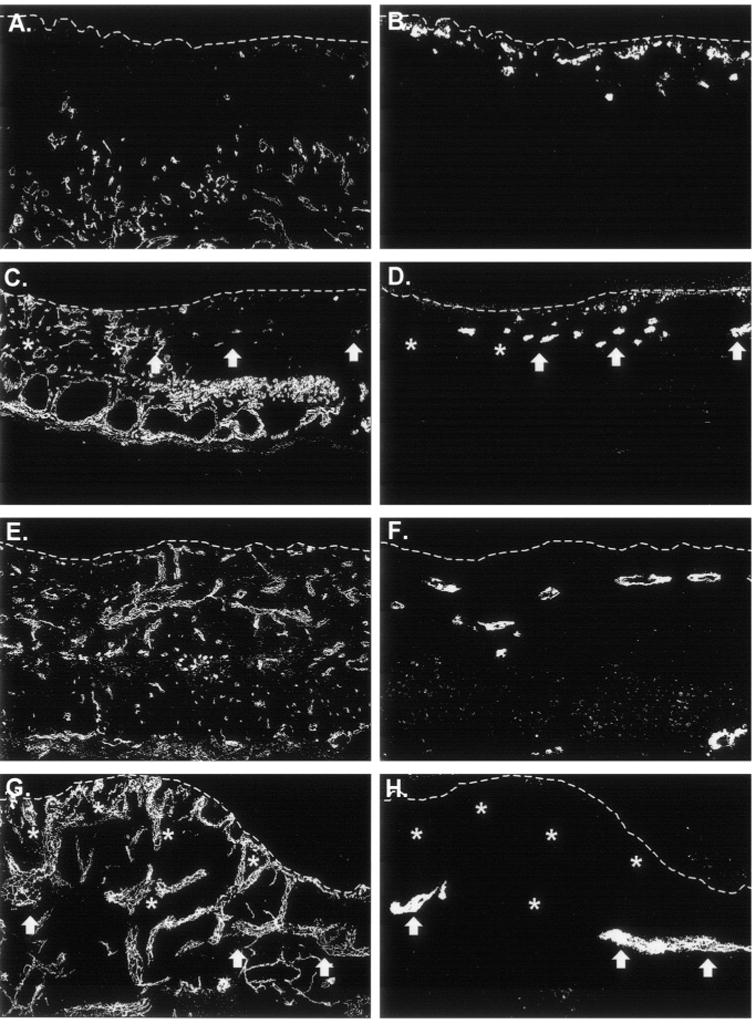
Proliferation of human endothelial cells after grafting is not enhanced in vascular endothelial growth factor (VEGF)-modified cultured skin substitutes plus endothelial cells (CSS + ECs). Sections were double-labeled with species-specific anti-CD31 antibodies to simultaneously localize mouse (A, C, E, G) and human (B, D, F, H) ECs. A and B. Control CSS + ECs (group 3) 1 week after grafting. C and D. VEGF-modified CSS + ECs (group 4) 1 week after grafting. E and F. Control CSS + ECs (group 3) 3 weeks after grafting. G and H. VEGF-modified CSS + ECs (group 4) 3 weeks after grafting. Dotted white lines indicate the locations of the dermal–epidermal junctions; sections are oriented with the epidermis at the top of each panel. In VEGF-modified CSS + ECs, relatively few human EC clusters (arrows) were observed in regions that were densely populated with mouse vessels (asterisks).
An antibody against human CD34, a protein expressed by vascular endothelium, was used to stain human vascular analogs in grafted CSS + ECs. This was performed in fixed tissue samples that were paraffin embedded and sectioned, permitting visualization of blood cells in vessels. Erythrocytes were clearly visible in many of the vessels observed in grafted CSS+ EC but not in the vascular analogs stained with the anti-human CD34 antibody (Figure 6). Therefore, perfusion of the human vascular analogs was not demonstrated.
Figure 6.
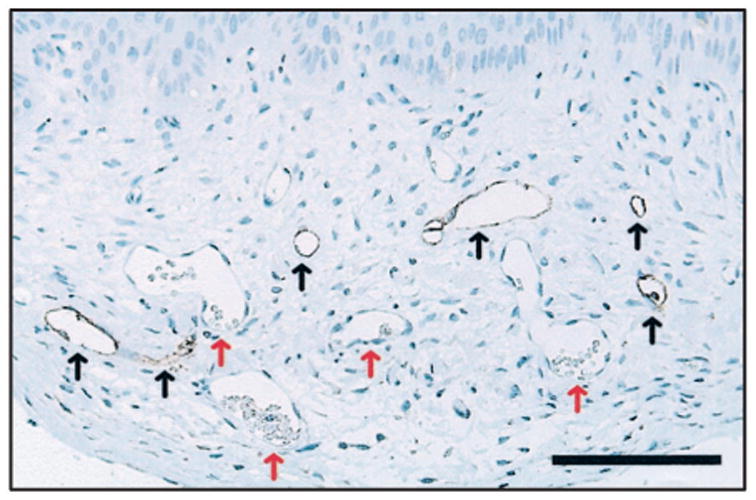
Vascular analogs in cultured skin substitutes plus endothelial cells (CSS + ECs) are not perfused by host mouse circulation. Shown is a section from vascular endothelial growth factor-modified CSS + ECs (group 4) at 3 weeks after grafting to mice. Epidermis is at the top of the panel; scale bar = 0.1 mm. Human vascular analogs (black arrows) were stained using an antibody specific for human CD34. Note that erythrocytes can be seen in unstained mouse vessels (red arrows), but not in vessels derived from transplanted human ECs.
DISCUSSION
The absence of a vascular plexus, which results in delays in vascularization, remains an important limitation of all cultured skin replacements.6 This anatomic deficiency can cause the replacements to become ischemic and nutrient deprived after grafting, contributing to secondary wound infection and increasing the risk of graft failure. The initiation of an-giogenesis in CSS in vitro could improve homology with native skin autograft and decrease the time required for vascularization after grafting. Tissue engineering of blood vessels in cultured skin requires, at a minimum, the incorporation of ECs. Previous attempts to add ECs to cultured skin models have frequently used human umbilical vein ECs, which are easily obtained from a discarded tissue source.24–26 However, these are not suitable for use in autologous-cultured skin models, such as CSS grafted to excised burn wounds. Dermal microvascular ECs, which can be simultaneously isolated along with dermal fibroblasts and epidermal keratinocytes from a single skin biopsy, can be used, but a previous study demonstrated relatively poor persistence in vitro and after grafting.18 This observation may have been caused by apoptosis of dermal ECs in the CSS, which have been engineered for optimal growth and maturation of keratinocytes and fibroblasts, and/or a low EC proliferation rate. In an attempt to increase the persistence of dermal ECs, CSS were prepared with keratinocytes that were genetically modified to over-express VEGF.
The results reported here support previous studies demonstrating enhanced vascularization of VEGF-modified CSS.16 Retroviral transduction with the L-CVEGF-SN vector led to increased VEGF mRNA expression and protein secretion in keratinocytes and in CSS. VEGF-modified CSS showed increased dermal staining for the EC marker CD31 after grafting, indicating enhanced vascularization and demonstrating the bioactivity of VEGF secreted by the modified CSS. Interestingly, although increased VEGF expression was demonstrated in CSS + ECs, no increase in EC persistence or organization was observed. In both control and VEGF-modified CSS + ECs, ECs were localized to the upper dermis, adjacent to the dermal–epidermal junction. No significant differences were observed in CD31 staining between control and VEGF-modified CSS + ECs in vitro. The absence of a response of human ECs to VEGF overexpression in CSS + ECs suggests that other factors, such as additional growth factors or extracellular matrix components, may be required for increased persistence, proliferation, and organization of human dermal ECs in cultured skin grafts.
After grafting to mice, human ECs persisted in both control and VEGF-modified CSS. In contrast to the increase in mouse ECs observed in VEGF-modified CSS + ECs, there was no increase in human ECs in response to increased VEGF production. In fact, in regions of grafted CSS that were heavily populated by mouse vessels, there was a relative deficiency of human vascular analogs compared with control CSS. In other regions of grafted VEGF-modified CSS + ECs that contained a lower density of mouse vessels, human vascular analogs were identified at frequencies similar to control CSS + ECs. These observations suggest that there may have been competition between human and mouse ECs for VEGF secreted by the epidermis. Outgrowth of the mouse vessels from the wound bed may have been favored over the organization of aggregated human ECs in the grafted dermal substitute.
The inability to identify blood cells in human vascular analogs in grafted control and VEGF-modified CSS + ECs suggests that they were not perfused by host circulation. Thus, these vascular analogs could not contribute to improved vascularization of grafted CSS. Other investigators have demonstrated that the mechanism of early skin graft vascularization is inosculation, the anastomosis of capillaries in the wound bed to the ends of graft vessels.27 It was hypothesized that the presence of a capillary network would permit skin substitutes to vascularize like a native skin graft.27 Although transplantation of ECs in CSS has been accomplished, it is likely that improved persistence and organization of human ECs in vitro will be required for these cells to participate in enhanced vascularization of CSS after grafting. The present study indicates that increased expression of VEGF is insufficient to support in vitro angiogenesis in CSS + EC and that additional modifications or improvements to the model will be necessary to reach this goal.
Acknowledgments
We thank Jodi Miller and Chris Lloyd for reagent preparation, Gail Macke for the preparation of histological sections, and Andrew Supp for technical assistance.
Footnotes
Presented at the meeting of the American Burn Association, Miami Beach, Florida, April 1 to 4, 2003.
Supported by Research Grant #8680 from the Shriners Hospitals for Children.
References
- 1.Berthod F, Damour O. In vitro reconstructed skin models for wound coverage in deep burns. Br J Dermatol. 1997;136:809–16. doi: 10.1111/j.1365-2133.1997.tb03917.x. [DOI] [PubMed] [Google Scholar]
- 2.Falanga V. Chronic wounds: pathophysical and experimental considerations. J Invest Dermatol. 1993;100:721–5. doi: 10.1111/1523-1747.ep12472373. [DOI] [PubMed] [Google Scholar]
- 3.Phillips TJ, Gilchrest BA. Cultured epidermal grafts in the treatment of leg ulcers. Adv Dermatol. 1990;5:33–48. [PubMed] [Google Scholar]
- 4.Balasubramani M, Kumar TR, Babu M. Skin substitutes: a review. Burns. 2001;27:534–44. doi: 10.1016/s0305-4179(01)00018-3. [DOI] [PubMed] [Google Scholar]
- 5.Bellow YM, Falabella AF. Use of skin substitutes in dermatology. Dermatol Clin. 2001;19:555–61. doi: 10.1016/s0733-8635(05)70295-3. [DOI] [PubMed] [Google Scholar]
- 6.Boyce ST, Supp DM. Skin substitutes: theoretical and developmental considerations. In: McCauley RL, editor. Functional and aesthetic reconstruction of burn patients. New York: Marcel Dekker; In press. [Google Scholar]
- 7.Boyce ST, Goretsky MJ, Greenhalgh DG, Kagan RJ, Rieman MT, Warden GD. Comparative assessment of cultured skin substitutes and native skin autograft for treatment of full-thickness burns. Ann Surg. 1995;222:743–52. doi: 10.1097/00000658-199512000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boyce ST, Kagan RJ, Yakuboff KP, et al. Cultured skin substitutes reduce donor skin harvesting for closure of excised, full-thickness burns. Ann Surg. 2002;235:269–79. doi: 10.1097/00000658-200202000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyce ST, Glatter R, Kitzmiller WJ. Treatment of chronic wounds with cultured cells and biopolymers. Wounds. 1995;7:24–9. [PubMed] [Google Scholar]
- 10.Harriger MD, Warden GD, Greenhalgh DG, Kagan RJ, Boyce ST. Pigmentation and microanatomy of skin regenerated from composite grafts of cultured cells and biopolymers applied to full-thickness burn wounds. Transplantation. 1995;59:702–7. doi: 10.1097/00007890-199503150-00011. [DOI] [PubMed] [Google Scholar]
- 11.Boyce ST, Supp AP, Harriger MD, Greenhalgh DG, Warden GD. Topical nutrients promote engraftment and inhibit wound contraction of cultured skin substitutes in athymic mice. J Invest Dermatol. 1995;104:345–9. doi: 10.1111/1523-1747.ep12665374. [DOI] [PubMed] [Google Scholar]
- 12.Boyce ST, Harriger MD, Supp AP, Warden GD, Holder IA. Effective management of microbial contamination in cultured skin substitutes after grafting to athymic mice. Wound Rep Reg. 1997;5:191–7. doi: 10.1046/j.1524-475X.1997.50212.x. [DOI] [PubMed] [Google Scholar]
- 13.Boyce ST, Kagan RJ, Meyer NA, Yakuboff KP, Warden GD. The 1999 Clinical Research Award. Cultured skin substitutes combined with Integra to replace native skin autograft and allograft for closure of full-thickness burns. J Burn Care Rehabil. 1999;20:453–61. doi: 10.1097/00004630-199920060-00006. [DOI] [PubMed] [Google Scholar]
- 14.Conn G, Soderman DD, Schaeffer M-T, Wile M, Hatcher VB, Thomas KA. Purification of a glycoprotein vascular endothelial cell mitogen from a rat glioma-derived cell line. Proc Natl Acad Sci USA. 1990;87:1323–7. doi: 10.1073/pnas.87.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Detmar M, Yeo KT, Nagy JA, et al. Keratinocyte-derived vascular permeability factor (vascular endothelial growth factor) is a potent mitogen for dermal microvascular endothelial cells. J Invest Dermatol. 1995;105:44–50. doi: 10.1111/1523-1747.ep12312542. [DOI] [PubMed] [Google Scholar]
- 16.Supp DM, Supp AP, Bell SM, Boyce ST. Enhanced vascularization of cultured skin substitutes genetically modified to overexpress vascular endothelial growth factor. J Invest Dermatol. 2000;114:5–13. doi: 10.1046/j.1523-1747.2000.00824.x. [DOI] [PubMed] [Google Scholar]
- 17.Supp DM, Boyce ST. Overexpression of vascular endothelial growth factor accelerates early vascularization and improves healing of genetically modified cultured skin substitutes. J Burn Care Rehabil. 2002;23:10–20. doi: 10.1097/00004630-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Supp DM, Wilson-Landy K, Boyce ST. Human dermal microvascular endothelial cells form vascular analogs in cultured skin substitutes after grafting to athymic mice. FASEB J. 2002;16:797–804. doi: 10.1096/fj.01-0868com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boyce ST, Ham RG. Cultivation, frozen storage, and clonal growth of normal human epidermal keratinocytes in serum-free media. J Tissue Cult Meth. 1985;9:83–93. [Google Scholar]
- 20.Boyce ST. Methods for serum-free culture of keratinocytes and transplantation of collagen-GAG based composite grafts. In: Morgan JR, Yarmush M, editors. Methods in tissue engineering. Totowa, NJ: Humana Press, Inc; 1998. pp. 365–89. [Google Scholar]
- 21.Swope VB, Supp AP, Greenhalgh DG, Warden GD, Boyce ST. Expression of insulin-like growth factor-I by cultured skin substitutes does not replace the physiologic requirement for insulin in vitro. J Invest Dermatol. 2001;116:650–7. doi: 10.1046/j.1523-1747.2001.01325.x. [DOI] [PubMed] [Google Scholar]
- 22.Boyce ST, Foreman TJ, English KB, et al. Skin wound closure in athymic mice with cultured human cells, biopolymers, and growth factors. Surgery. 1991;110:866–76. [PubMed] [Google Scholar]
- 23.Osborn M, Isenberg S. Immunocytochemistry of frozen and of paraffin tissue sections. In: Celis JE, editor. Cell biology: a laboratory handbook. San Diego, CA: Academic Press; 1998. pp. 486–92. [Google Scholar]
- 24.L’Heureux N, Germain L, Labbe R, Auger FA. In vitro construction of a human blood vessel from cultured vascular cells: a morphologic study. J Vasc Surg. 1993;17:499–509. doi: 10.1067/mva.1993.38251. [DOI] [PubMed] [Google Scholar]
- 25.Black AF, Berthod F, L’Heureux N, Germain L, Auger FA. In vitro reconstruction of a human capillary-like network in a tissue-engineered skin equivalent. FASEB J. 1998;12:1331–40. doi: 10.1096/fasebj.12.13.1331. [DOI] [PubMed] [Google Scholar]
- 26.Hudon V, Berthod F, Black AF, Damour O, Germain L, Auger F. A tissue-engineered endothelialized dermis to study the modulation of angiogenic and angiostatic molecules on capillary-like tube formation in vitro. Br J Dermatol. 2003;148:1094–104. doi: 10.1046/j.1365-2133.2003.05298.x. [DOI] [PubMed] [Google Scholar]
- 27.Young DM, Greulich KM, Weier HG. Species-specific in situ hybridization with fluorochrome-labeled DNA probes to study vascularization of human skin grafts on athymic mice. J Burn Care Rehab. 1996;17:305–10. doi: 10.1097/00004630-199607000-00005. [DOI] [PubMed] [Google Scholar]


