Abstract
Although δ-catenin/neural plakophilin-related armadillo protein (NPRAP) was reported to interact with presenilin-1 (PS-1), the effects of PS-1 on δ-catenin have not been established. In this study, we report that overexpression of PS-1 inhibits the δ-catenin-induced dendrite-like morphological changes in NIH 3T3 cells and promotes δ-catenin processing and turnover. The effects of PS-1 on endogenous δ-catenin processing were confirmed in hippocampal neurons overexpressing PS-1, as well as in the transgenic mice expressing the disease-causing mutant PS-1 (M146V). In addition, disease-causing mutant PS-1 (M146V and L286V) enhanced δ-catenin processing, whereas PS-1/γ-secretase inhibitors could block the formation of processed forms of δ-catenin. Together, our findings suggest that PS-1 can affect δ-catenin-induced morphogenesis possibly through the regulation of its processing and stability.
Keywords: Alzheimer's disease, δ-Catenin/NPRAP, Presenilin/γ-secretase
Alzheimer's disease (AD) is the most common type of senile dementia and is characterized by progressive loss of memory and cognitive dysfunction [16]. In familial Alzheimer's disease (FAD), over 70 mutations have been significantly associated with presenilin-1 (PS-1) and presenilin-2 (PS-2) [7]. Multiple lines of evidence indicate that PS contributes directly to the intramembranous “γ-secretase” processing of many proteins of diverse functions, including APP [3], the developmental signaling receptor Notch1 [4], and cell adhesion molecules N- and E-cadherin [15]. Interestingly, it is reported that PS-1 interacts with a member of the armadillo/β-catenin family termed δ-catenin, a protein expressed mostly in the brain and encoded on chromosome 5 [21].
δ-Catenin, or neural plakophilin-related armadillo protein (NPRAP), is a member of the p120ctn subfamily of armadillo/β-catenin proteins, which are defined as proteins with 10 armadillo (ARM) repeats in characteristic spacing and with diverse NH2- and COOH-terminal sequences that flank the ARM repeats [18]. δ-Catenin is a nervous system-specific adherens junction protein involved in cell motility and expressed early in neuronal development [14]. The overexpression of δ-catenin can induce the branching of cellular processes in 3T3 cells and enhance dendritic morphogenesis in primary hippocampal neurons [11]. Some of the binding partners of δ-catenin include E-cadherin [14], S-SCAM [8], and PS-1 [13,19,21]. However, the consequences of PS-1 and δ-catenin interactions have not yet been illustrated.
In this report, we investigated the effects of PS-1 on δ-catenin. The co-overexpressed PS-1 significantly impaired δ-catenin-induced cellular branching in NIH 3T3 fibroblasts. We also found that PS-1 expression promoted δ-catenin processing when they were co-transfected in 3T3 cells and when PS-1 was overexpressed in hippocampal neurons, which was sensitive to PS-1/γ-secretase inhibition and was facilitated by the Alzheimer's disease-causing PS-1 mutation. Our findings suggest that one function of PS-1 expression is to promote δ-catenin processing and, thereby, affect the function of δ-catenin in cells.
Experimental procedures
Plasmid construction
Construction of full-length-δ-catenin constructs in pEGFP-C1 or pRFP-C1 has been described previously [11]. The wild-type PS-1 and mutant PS-1 in pEGFP-C1 were generated by PCR amplification of the desired PS-1 by using PS-1-specific oligonucleotides.
Generation and maintenance of transgenic mice
We generated the BAI1-AP4 promoter-M146V PS-1 transgene using the same methods as we previously reported for the construction of BAI1-AP4 promoter-lacZ transgene [12].
Cell lines and antibodies
Mouse NIH 3T3 cells were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL), plus 10% fetal bovine serum. PS1−/−, PS2−/−, PS1/2−/−, and PS1/2+/+ cells were kindly obtained from Dr. De Strooper. The target proteins were visualized by using antibodies to GFP or RFP (1:500 dilution, monoclonal antibody; BD Biosciences), to PS-1 (1:2000 dilution, polyclonal antibody; Sigma), and to δ-catenin (1:300 dilution, monoclonal mAbJ19; 1:1000 dilution, polyclonal rAbUBI [Upstate Biotechnology, NY]). Anti-δ-catenin indicates the use of rAbUBI unless otherwise specified.
Quantification of cellular branching phenotypes
NIH 3T3 fibroblasts were transfected with the use of LipofectAMINE PLUS reagent as described by manufacturer (Invitrogen). After transfected cells were fixed, the branching of cellular processes were scored using Universal Imaging (MetaMorph) on 4 randomly chosen fields per construct in any single experiment. The data were combined from at least three experiments, and statistical analysis was performed using t-test. Confidence level was set at 95%.
Western blotting
Cultured cells were harvested with RIPA buffer, and the protein concentration was determined using the BCA assay (Pierce). The proteins were run on an 8% Tris–Glycine gels (Novex), transferred to a hydrophobic polyvinylidene difluoride membrane (Amersham), and developed with ECL Western blotting detection reagents (Amersham). Even loading of samples were confirmed with direct blue 71 staining kits (EZ BiopacQ, Korea).
Treatments with the γ-secretase inhibitor
γ-Secretase activity of PS-1 was inhibited using the γ-secretase inhibitor III (Calbiochem). After incubating at 37 °C with 5% CO2 for 6 h, cells were treated with either the 10 μM γ-secretase inhibitor III or 300 nM WPE-III-31C. After overnight incubation, cells were transfected with the LipofectAMINE PLUS as described above. After incubating for 3 h, recovery medium with 10% FBS was added. After overnight incubation, the recovery medium was replaced with fresh culture media. The proteins were extracted for Western blot analysis, and cells were fixed for fluorescent observation.
Determination of protein half-life
To determine the half-life of δ-catenin, presenilin wild-type MEF cells (PS1/2+/+) and presenilin 1, 2 double knock-out MEF cells (PS1/2−/− ) were transfected with 0.5 μg δ-catenin, PS-1 wild-type, and PS-1 M146 V by the Lipofectamine method. After 12 h of transfection, cells were incubated with 50 μg/ml cycloheximide for the indicated times, and equal amounts of lysate in micrograms were subjected to immunoblot analysis.
Results
Overexpression of PS-1 affects δ-catenin fragment patterns and cellular branching
In order to determine the functional roles of interaction between PS-1 and δ-catenin, we initiated a study to investigate the effects of PS-1 expression on δ-catenin. Both wild-type and mutant PS-1-GFP were introduced into NIH 3T3 cells and were shown to be localized in the cytoplasm (Fig. 1A: a, b, and c). We did not observe any significant effects of overexpressed PS-1 on cell morphologies (Fig. 1A: a, b and c). In contrast, as we previously reported, δ-catenin expression induced the branching of dendrite-like processes in NIH 3T3 fibroblasts (Fig. 1A: d; arrows). The expression of exogenous PS-1 and δ-catenin was also confirmed by Western blot analyses (data not shown).
Fig. 1.

Expression of PS-1 and δ-catenin and effects of co-transfection of PS-1 and δ-catenin on the cell shape changes in NIH 3T3 cells. (A) NIH 3T3 cells were transfected with wild-type PS-1-GFP (a), mutant PS-1 (M146V, L286V)-GFP (b,c), and δ-catenin-RFP (d). The RFP labeling of δ-catenin transfected cells was converted to green fluorescence digitally to allow direct comparison of morphologies. Bar: 10 μm. (B) (a,b) NIH 3T3 cells cotransfected with EGFP and δ-catenin. (c,d) NIH 3T3 cells co-transfected with wild-type PS-1 and δ-catenin. (e,f) NIH 3T3 cells co-transfected with mutant PS-1 (M146V) and δ-catenin. (a,c,e) GFP fluorescence. (b,d,f) Anti- δ-catenin (mAbJ19) immunofluorescence. Arrows indicate cellular branching. Bar: 10 γm.
Next, we transiently co-transfected NIH 3T3 fibroblasts with PS-1 tagged with GFP and δ-catenin. The branching of dendrite-like processes induced by δ-catenin was slightly reduced when cells overexpress both the pEGFP vector and δ-catenin (Fig. 1B: a and b), although this reduction was not statistically significant (Table 1). While the cells over-expressing both GFP tagged wild-type or mutant PS-1 and untagged δ-catenin (Fig 1B: c–f) resulted in the statistically significant reduction in branching when compared to cells expressing δ-catenin alone, we did not observe any significant differences between wild-type and mutant PS-1 in terms of its morphological effects on cells (Table 1).
Table 1.
The effects of PS-1 expression on δ-catenin-induced changes in 3T3 cell morphology
| Transfections | No processes | Process elongation | Cellular branching |
|---|---|---|---|
| δ-Catenin | 18.75% ± 6.99 | 44.75% ± 6.99 | 36.38% ± 0.50 |
| EGFP/δ-catenin | 46.00% ± 1.00 | 32.50% ± 0.50 | 21.00% ± 0.00 |
| PS-1WT/δ-catenin | 34.75% ± 9.20 | 51.50% ± 12.98 | 13.75% ± 6.46* |
| PS-1(M146V)/δ-catenin | 55.33% ± 17.70* | 33.00% ± 14.93 | 9.33% ± 1.20** |
Significantly different from δ-catenin, p < 0.05.
Significantly different from δ-catenin and EGFP/δ-catenin, p < 0.05.
To determine the effects of PS-1 on δ-catenin processing, the protein banding patterns of single (untagged PS-1 or GFP tagged δ-catenin) or double (untagged PS-1 and GFP tagged δ-catenin) transfected NIH 3T3 cells were analyzed by Western blot using anti-δ-catenin and GFP antibodies. When PS-1 was co-expressed with δ-catenin, the banding patterns of δ-catenin on the SDS–PAGE showed variably an additional faster migrating form indicating a possible cleavage. The cleaved form of δ-catenin was most prominent in mutant PS-1 M146V-transfected cells (Fig. 2A). The moderate cleavages were variably observed in wild-type PS-1 and mutant PS-1 L286V transfected cells. PS-1 M146V/D257A double mutants, whose critical aspar-tate 257 residue was point-mutated to alanine, showed no noticeable cleaved forms of δ-catenin, suggesting that the cleavage of δ-catenin by mutant PS-1 M146V may be mediated by PS-1 containing a γ-secretase-like activity. In these experiments, although the transfection efficiencies were very similar among all the transfections, the expression level of δ-catenin varied when wild-type or mutant PS-1 was co-expressed (Fig. 2A). The different banding patterns of δ-catenin are less likely a result of protein phosphorylation or dephosphorylation, since alkaline phosphatase treatments did not alter the δ-catenin banding profiles (Fig. 2B). Furthermore, the formation of this additional faster migrating δ-catenin species is unlikely the result of differential transcription, because only one form of δ-catenin was ectopically transfected as a full-length cDNA. These data indicate that PS-1 may have a role in the regulation of δ-catenin cleavage, and that the effects of some mutant PS-1 (such as M146V) on the cleavage of δ-catenin are significantly greater than that of wild-type PS-1.
Fig. 2.

Effects of PS-1 expression on the cleavage patterns of exogenous and endogenous δ-catenin. (A) NIH 3T3 cells co-expressing untagged PS-1 (wild-type PS-1 or mutant PS-1) and δ-catenin tagged with EGFP were analyzed by Western blotting with antibodies against GFP (upper panel) and δ-catenin (lower panel). Here mutant PS-1 (M146V) shows a particularly enhanced cleavage pattern of exogenous δ-catenin. (B) PS-1 M146V induced cleavage pattern of δ-catenin was not altered by alkaline phosphatase treatment. AP: alkaline phosphatase. (C) Cleavage of endogenous δ-catenin by PS-1 M146V expression. Left panel: Cleavage of endogenous δ-catenin in PS-1 M146V transgenic mice. Total proteins were extracted with RIPA buffer from hippocampal neurons of either PS-1 M146V transgenic mice or littermate control mice, and they were analyzed by Western blotting with antibodies against δ-catenin. Right panel: cleavage of endogenous δ-catenin in cultured hippocampal neurons expressing PS-1 M146V. PS-1 M146V or vector alone was transfected into primary hippocampal neurons, and the effects of overexpressed PS-1 M146V on endogenous δ-catenin were investigated by Western blotting with antibodies against δ-catenin.
The effects of PS-1 M146V on exogenous δ-catenin processing were also confirmed on endogenous δ-catenin processing (Fig. 2C). We isolated the protein extracts from hippocampal neurons of PS-1 M146V transgenic mice and compared the banding patterns of endogenous δ-catenin with that of normal mice by Western blot analysis. As shown in Fig. 2C, left panel, we observed a prominent cleaved form of δ-catenin in PS-1 M146V transgenic mice but not in normal mice (WT). In order to confirm our observations, we transfected PS-1 M146V into cultured primary hippocampal neurons and examined the effects of overexpressed PS-1 M146V on endogenous δ-catenin. As expected, we observed an increased cleavage form of δ-catenin in PS-1 M146V transfected hippocampal neurons as compared to that of mock transfected neurons (Fig. 2C, right panel). Therefore, we propose that PS-1 overexpression promotes the cleavage of both exogenous and endogenous δ-catenin.
Cleavage of δ-catenin is sensitive to inhibitors of PS-1/γ-secretase activity
Because the expression of PS-1 M146V/D257A did not influence its banding patterns (Fig. 2A), we suspected that δ-catenin processing is affected by a γ-secretase-like activity of the PS-1 complex. As expected, the Western blot analyses clearly revealed that the banding patterns of δ-catenin were altered with increasing concentrations of γ-secretase inhibitor III. The cleaved form of δ-catenin in the M146V PS-1-transfected cells shifted toward a heavier form in a concentration-dependent manner (Fig. 3A).
Fig. 3.
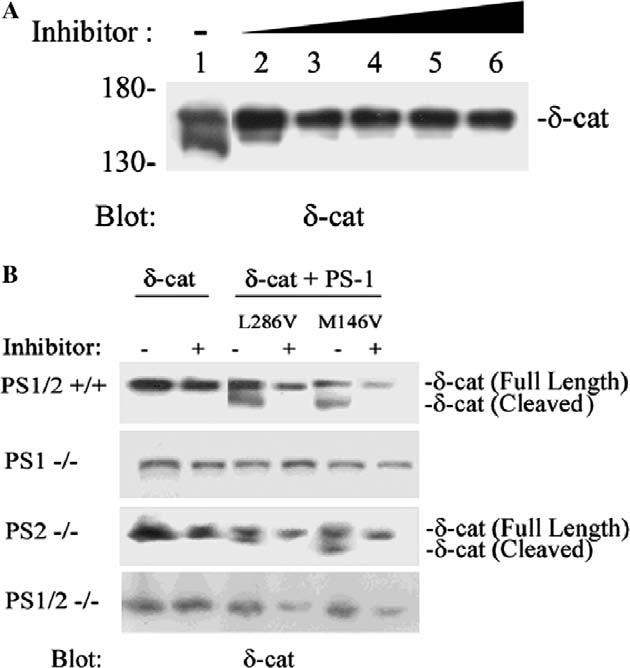
Effects of treatments with γ-secretase inhibitors on the cleavage of δ-catenin and effects of mutant PS-1 on δ-catenin in PS deficient fibroblasts. (A) δ-Catenin/PS-1 M146V transfected NIH 3T3 cells were treated with γ-secretase inhibitor III (lane 1, no treatment; lane 2, 2 μM/ml; lane 3, 4 μM/ml; lane 4, 6 μM/ml; lane 5, 8 μM/ml; lane 6, 10 μM/ml). Cell lysates were prepared for Western blotting with anti-δ-catenin antibody. (B) PS1−/−, PS2−/−, PS1/2−/−, and PS1/2+/+ cells were transiently transfected with δ-catenin or co-transfected with δ-catenin and mutant PS-1 (M146V or L286V), and some of these cells were also treated with γ-secretase inhibitor III (10 μM/ml) as indicated (+). Western blots were probed with anti-δ-catenin antibody. The full-length and the cleaved form of δ-catenin are indicated on the right.
Increased cleavage of δ-catenin by overexpressed PS-1 in PS1/2+/+ and PS2−/− fibroblasts
To investigate the effects of PS-1 on δ-catenin in more detail, we used PS-1 deficient fibroblasts (PS1−/− ), PS-2 deficient fibroblasts (PS2−/− ), PS-1/2 deficient fibroblasts PS1/2− and wild-type Ps=1/2 containing fibroblasts (PS1/2+/+).
To confirm the results obtained in NIH 3T3 cells in which overexpressed mutant PS-1 promoted δ-catenin processing, we transiently co-transfected PS1−/−, PS2−/−, PS1/2−/−, and PS1/2+/+ cells with PS-1 M146V, L286V, and δ-catenin. The cleavage forms of δ-catenin in PS1/2+/+ cells and PS2 −/− cells were strongly influenced by mutant PS-1 M146V and L286V (Fig. 3B). Moreover, the cleavage form (Fig. 3, δ-cat cleaved) was eliminated when cells were treated with the γ-secretase inhibitor III (Fig. 3B), suggesting that the cleavage of δ-catenin was dependent on a PS-1/γ-secretase-like activity. Surprisingly, the banding patterns of δ-catenin in PS1−/− cells did not show an obvious cleavage form when compared to that of the PS1/2+/+ and PS2 −/− cells (Fig. 3B), perhaps reflecting that somehow exogenous PS-1 expression in PS1−/− cells was not sufficient to elicit a significant effect.
Increased δ-catenin turnover in PS1/2+/+ cells overexpressing δ-catenin
We further examined the turnover of δ-catenin in PS wild-type (PS1/2+/+) and PS deficient (PS1/2−/−) fibroblast cells. As shown in Fig. 4A and B, δ-catenin has a half-life around 11 h in mouse fibroblast cells. Overexpression of PS-1 (WT or M146V) in PS1/2+/+ cells reduced the half-life of δ-catenin from 11 to 8 h (Fig. 4A), whereas re-expression of PS-1 WT or PS-1 M146V in PS1/2−/− cells did not significantly alter the turnover time of δ-catenin (Fig. 4B). This again showed that exogenous PS-1 expression in PS−/− cells was not sufficient to elicit a significant effect.
Fig. 4.
Effects of PS expression on δ-catenin stability. (A) Reduction of δ-catenin immunoreactivity over time in PS1/2+/+ cells transfected with δ-catenin, δ-catenin+PS-1 WT, or δ-catenin+PS-1 M146V. (B) Reduction of δ-catenin immunoreactivity over time in PS1/2 −/− cells transfected with δ-catenin, δ-catenin+PS-1 WT, or δ-catenin+PS-1 M146V.
Discussion
Several lines of evidence suggest that δ-catenin plays an important role in synaptic plasticity and cognition. δ-Catenin−/− mice demonstrate severe learning deficits [9],and hemizygous loss of the chromosomal 5p15.2 region, where human δ-catenin gene is located, is correlated with a severe form of mental retardation known as Cri-du-Chat syndrome. To support this, overexpressed δ-catenin has shown to induce an elaborate arborization of dendrites, swellings, and enhanced mature dendritic spines [11]. Therefore, the identification of regulators affecting the δ-catenin gene expression, its protein stability, and its effects on dendrogenesis and spine formation will be important in understating its normal function and contribution to pathogenesis. Even though PS-1 has been reported to interact with δ-catenin/NPRAP [13,19,21], the functional consequences of PS-1 expression on δ-catenin have not been determined. In this report, we showed that PS-1 overexpression resulted in δ-catenin cleavage, increased its turnover, and inhibited δ-catenin-induced branching in NIH 3T3 cells.
The production of a cleaved form of δ-catenin by PS-1 regulation is of great interest. PS-1 is known to produce cleaved forms of many proteins, including APP, Notch, and E-cadherin [3,4,15], whose biological and/or pathological roles are under active investigation. However, all of the above substrates for PS-1/γ-secretase fulfill several criteria. First, they are all type I receptor transmembrane proteins. Second, the cleavage occurs at their intramembranous domains that involve the lipid environment. Furthermore, PS-1/γ-secretase mediated processing of proteins is sensitive to the inhibitors of γ-secretase and can be blocked when the two critical aspartate residues located between PS-1 transmembrane domains 6 and 7 are mutated. δ-Catenin does not display any predicted transmembrane domains; therefore, it does not fulfill the classical criteria for a γ-secretase substrate. Interestingly, δ-catenin cleavage in the PS-1 overexpressed NIH 3T3 cells was sensitive to PS-1/γ-secretase inhibition, and this cleavage was reduced when δ-catenin was co-expressed with PS-1 double mutant M146V/D257A. In addition, Alzheimer's disease causing mutations in PS-1 enhanced the δ-catenin cleavage. There can be two different explanations for these observations. On one hand, δ-catenin could be processed by a γ-secretase-like activity that involves PS-1. In this scenario, δ-catenin has to be in the vicinity of the PS-1/γ-secretase complex physically. Indeed, δ-catenin is enriched in the postsynaptic junction where PS-1 is also localized and δ-catenin interacts with glutamate receptors (NR2A and mGluR1α) and postsynaptic protein-95 (PSD-95) [10].On the other hand, δ-catenin may be processed by an as yet unidentified protease that is activated by PS-1/γ-secretase activity. In this case, no direct γ-secretase-like activity exists for δ-catenin.
This latter possibility seems to be supported by the data presented in this report. We found that δ-catenin in PS1 −/− and PS1/2−/− cells did not show an obvious cleavage form when compared to that of the PS1/2+/+ and PS2−/− cells (Fig. 3). The co-expression of wild-type and mutant PS-1 in PS1−/− MEF cells did not change the outcome and did not restore the cleavage of δ-catenin (data not shown). In addition, re-expression of PS-1 WT or PS-1 M146V in PS1/2−/− cells did not significantly alter the half-life of δ-catenin. However, overexpression of PS-1 (WT or M146V) reduced the half-life of δ-catenin in PS1/2+/+ cells (Fig. 4A). These data suggest that somehow the re-introduction of PS-1 alone into PS1−/− cells was not sufficient to elicit a significant effect, and some other factors that were present in the PS1/2+/+ cells also contributed to the cleavage of δ-catenin. One such factor may be E-/N-cadherin mediated cell–cell junction complex since a number of studies showed that PS-1 interacts with classical cadherins and modulates β-catenin stability [20]. Further studies will be needed to address the possible involvement of cadherin in the regulation of δ-catenin stability.
Throughout our researches reported here, mutant PS-1 showed stronger effects on the cleavage of δ-catenin than wild-type PS-1. Some of the mutant PS-1 showed different endoproteolytic processing compared to wild-type PS-1, suggesting their different roles in the cells. PS-1 M146V mutant increases the ratio of Aβ42-long species production and down-regulates the production of APP-α [1]. Furthermore, cells expressing PS-1 L286V mutant exhibit enhanced elevation of Ca2+ following exposure to Aβ and increased vulnerability to Aβ toxicity [6]. Whereas the naturally occurring FAD mutation PS-1 L286V did not interfere with Notch endoproteolysis, charged amino acid substitutions strongly inhibited NICD generation [17]. All of these published data support our observation for the differences of wild-type vs. mutant PS-1 and among mutants.
δ-Catenin is highly expressed in the dendrites of the mature brain [10]. The induction of branching of dendrite-like processes by overexpressed δ-catenin suggests its role in dendrogenesis and brain functions [11].It is worth noting that PS-1 is detected in neuronal processes and filopodia-like structures of growth cones in a primary culture of rat hippocampal neurons where δ-catenin is also localized [2]. Previous studies demonstrated that the inhibition of PS-1/γ-secretase increases neurite outgrowth in NT2N cells [5]. Our data showed that the overexpression of PS-1 affects δ-catenin-induced cellular branching in NIH 3T3 cells (Table 1). Taken together, these findings are consistent with the notion that the direct binding of PS-1 to δ-catenin and the effects of overexpressed PS-1 on δ-catenin may play important roles in neuronal function. However, the downstream effects of increased δ-catenin cleavage forms require further investigation to define its exact biological and pathological role in neurons.
Acknowledgments
We thank Drs. J. Choi, B. De Strooper, W. Franke, K.S. Kosik, R. Paffenholz, and M. Wolfe for providing reagents. We are also indebted to Drs. Y.H. Chen, and D. Im for technical advice and assistance. This study was supported in part by Korea Health 21 R&D Project of Ministry of Health and Welfare A040042 (K.K.), the American Cancer Society IRG5-89812 (Q.L.), and the US Department of Public Health/National Institute on Aging AG026630 (Q.L.).
References
- 1.Ancolio K, Marambaud P, Dauch P, Checler F F. Alpha-secretase-derived product of beta-amyloid precursor protein is decreased by presenilin 1 mutations linked to familial Alzheimer's disease. J. Neurochem. 1997;69:2494–2499. doi: 10.1046/j.1471-4159.1997.69062494.x. [DOI] [PubMed] [Google Scholar]
- 2.Busciglio J, Hartmann H, Lorenzo A, Wong C, Baumann K, Sommer B, Staufenbiel M, Yankner BA. Neuronal localization of presenilin-1 and association with amyloid plaques and neurofibrillary tangles in Alzheimer's disease. J. Neurosci. 1997;17:5101–5107. doi: 10.1523/JNEUROSCI.17-13-05101.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 4.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent gamma-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 5.Figueroa DJ, Morris JA, Ma L, Kandpal G, Chen E, Li YM, Austin CP. Presenilin-dependent gamma-secretase activity modulates neurite outgrowth. Neurobiol. Dis. 2002;9:49–60. doi: 10.1006/nbdi.2001.0447. [DOI] [PubMed] [Google Scholar]
- 6.Guo Q, Furukawa K, Sopher BL, Pham DG, Xie J, Robinson N, Martin GM, Mattson MP. Alzheimer's PS-1 mutation perturbs calcium homeostasis and sensitizes PC12 cells to death induced by amyloid beta-peptide. Neuroreport. 1996;8:379–383. doi: 10.1097/00001756-199612200-00074. [DOI] [PubMed] [Google Scholar]
- 7.Hutton M, Perez-Tur J, Hardy J. Genetics of Alzheimer's disease. Essays Biochem. 1998;33:117–131. doi: 10.1042/bse0330117. [DOI] [PubMed] [Google Scholar]
- 8.Ide N, Hata Y, Deguchi M, Hirao K, Yao I, Takai Y. Interaction of S-SCAM with neural plakophilin-related Armadillo-repeat protein/delta-catenin. Biochem. Biophys. Res. Commun. 1999;256:456–461. doi: 10.1006/bbrc.1999.0364. [DOI] [PubMed] [Google Scholar]
- 9.Israely I, Costa RM, Xie CW, Silva AJ, Kosik KS, Liu X. Deletion of the neuron-specific protein delta-catenin leads to severe cognitive and synaptic dysfunction. Curr. Biol. 2004;14:1657–1663. doi: 10.1016/j.cub.2004.08.065. [DOI] [PubMed] [Google Scholar]
- 10.Jones SB, Lanford GW, Chen YH, Moribito M, Kim K, Lu Q. Glutamate-induced delta-catenin redistribution and dissociation from postsynaptic receptor complexes. Neuroscience. 2002;115:1009–1021. doi: 10.1016/s0306-4522(02)00532-8. [DOI] [PubMed] [Google Scholar]
- 11.Kim K, Sirota A, Chen YH, Jones SB, Dudek R, Lanford GW, Thakore C, Lu Q. Dendrite-like process formation and cytoskeletal remodeling regulated by delta-catenin expression. Exp. Cell Res. 2002;275:171–184. doi: 10.1006/excr.2002.5503. [DOI] [PubMed] [Google Scholar]
- 12.Kim MY, Ahn KY, Lee SM, Koh JT, Chun BJ, Bae CS, Lee KS, Kim KK. The promoter of brain-specific angiogenesis inhibitor 1-associated protein 4 drives developmentally targeted transgene expression mainly in adult cerebral cortex and hippocampus. FEBS Lett. 2004;566:87–94. doi: 10.1016/j.febslet.2004.03.106. [DOI] [PubMed] [Google Scholar]
- 13.Levesque G, Yu G, Nishimura M, Zhang DM, Levesque L, Yu H, Xu D, Liang Y, Rogaeva E, Ikeda M, Duthie M, Murgolo N, Wang L, VanderVere P, Bayne ML, Strader CD, Rommens JM, Fraser PE, George-Hyslop P. Presenilins interact with armadillo proteins including neural-specific plakophilin-related protein and beta-catenin. J. Neurochem. 1999;72:999–1008. doi: 10.1046/j.1471-4159.1999.0720999.x. [DOI] [PubMed] [Google Scholar]
- 14.Lu Q, Paredes M, Medina M, Zhou J, Cavallo R, Peifer M, Orecchio L, Kosik KS. Delta-catenin an adhesive junction-associated protein which promotes cell scattering. J. Cell Biol. 1999;144:519–532. doi: 10.1083/jcb.144.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moehlmann T, Winkler E, Xia X, Edbauer D, Murrell J, Capell A, Kaether C, Zheng H, Ghetti B, Haass C, Steiner H. Presenilin-1 mutations of leucine 166 equally affect the generation of the Notch and APP intracellular domains independent of their effect on Abeta 42 production. Proc. Natl. Acad. Sci. USA. 2002;99:8025–8030. doi: 10.1073/pnas.112686799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Paffenholz R, Franke WW. Identification and localization of a neurally expressed member of the plakoglobin/armadillo multigene family. Differentiation. 1997;61:293–304. doi: 10.1046/j.1432-0436.1997.6150293.x. [DOI] [PubMed] [Google Scholar]
- 19.Tanahashi H, Tabira T. Isolation of human delta-catenin and its binding specificity with presenilin 1. Neuroreport. 1999;10:563–568. doi: 10.1097/00001756-199902250-00022. [DOI] [PubMed] [Google Scholar]
- 20.Zhang Z, Hartmann H, Do VM, Abramowski D, Sturchler-Pierrat C, Staufenbiel M, Sommer B, van de Wetering M, Clevers H, Saftig P, De Strooper B, He X, Yankner BA. Destabilization of beta-catenin by mutations in presenilin-1 potentiates neuronal apoptosis. Nature. 1998;395:698–702. doi: 10.1038/27208. [DOI] [PubMed] [Google Scholar]
- 21.Zhou J, Liyanage U, Medina M, Ho C, Simmons AD, Lovett M, Kosik KS. Presenilin 1 interaction in the brain with a novel member of the Armadillo family. Neuroreport. 1997;8:2085–2090. doi: 10.1097/00001756-199705260-00054. [DOI] [PubMed] [Google Scholar]



