Abstract
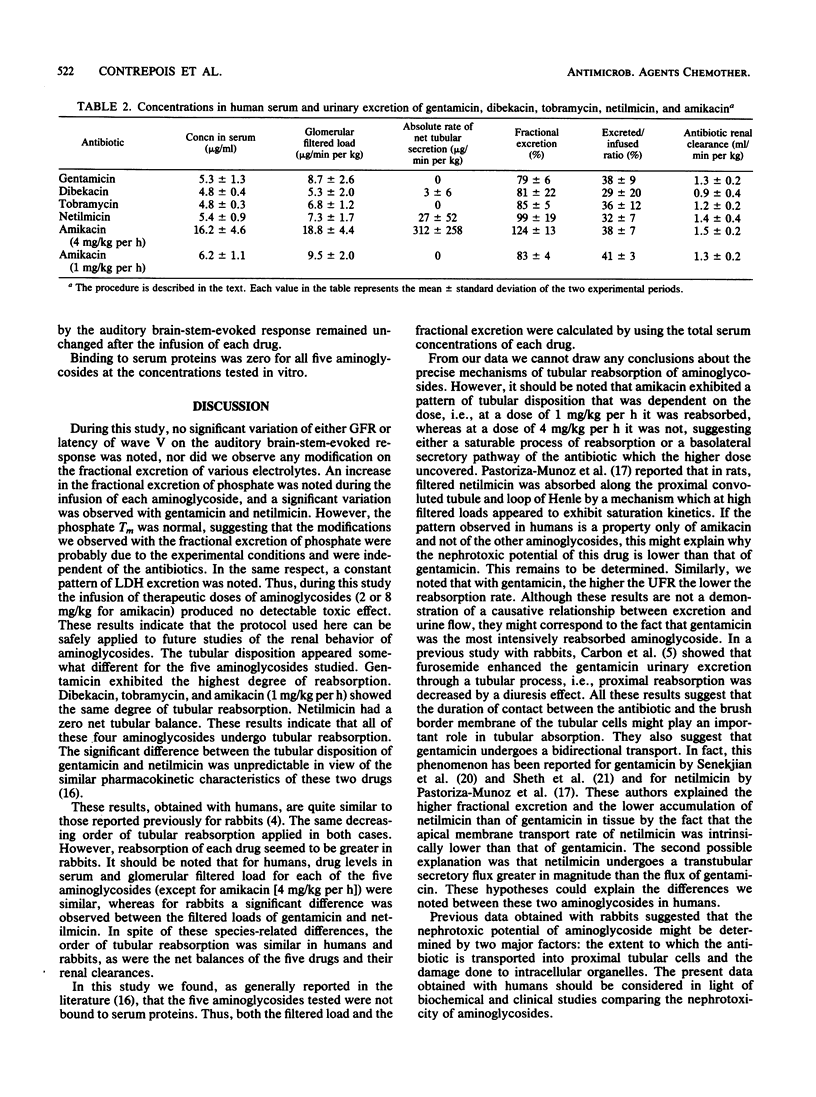
The tubular disposition of five aminoglycosides was studied in humans to establish a possible relationship between tubular reabsorption and the nephrotoxicity that has been described in the literature. Thirty-three healthy male volunteers received a continuous intravenous infusion of isotonic saline with inulin, followed 1 h later by inulin plus gentamicin, dibekacin, tobramycin, netilmicin, or amikacin (1 mg/kg per h) or amikacin (4 mg/kg per h) over a period of 2 h. Brain-stem-evoked response audiometry was performed both before and at the end of each infusion. The latency of wave V remained constant whichever antibiotic was considered. The glomerular filtration rate did not vary significantly during the infusion of each drug. The percent fractional excretion was 79 +/- 6, 81 +/- 22, 85 +/- 5, and 99 +/- 9 for gentamicin, dibekacin, tobramycin, and netilmicin, respectively, and 83 +/- 4 and 124 +/- 13 for amikacin at concentrations of 1 and 4 mg/kg per h, respectively. Net balance and renal clearance were similar for the five aminoglycosides when administered at a rate of 1 mg/kg per h. With gentamicin only, fractional excretion was correlated with the urinary flow rate. We can conclude that (i) gentamicin, generally considered the most nephrotoxic agent, had the highest degree of net reabsorption; (ii) netilmicin exhibited a net zero tubular balance; (iii) amikacin had different patterns of tubular disposition according to the dose, i.e., reabsorption at 1 mg/kg per h and secretion at 4 mg/kg per h, raising the hypothesis of a saturable process of reabsorption; and (iv) these differences in tubular reabsorption could account at least in part for the known different nephrotoxic potentials of these five aminoglycosides in humans.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett W. M. Aminoglycoside nephrotoxicity. Nephron. 1983;35(2):73–77. doi: 10.1159/000183050. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Bijvoet O. L., Morgan D. B., Fourman P. The assessment of phosphate reabsorption. Clin Chim Acta. 1969 Oct;26(1):15–24. doi: 10.1016/0009-8981(69)90280-0. [DOI] [PubMed] [Google Scholar]
- Brion N., Barge J., Godefroy I., Dromer F., Dubois C., Contrepois A., Carbon C. Gentamicin, netilmicin, dibekacin, and amikacin nephrotoxicity and its relationship to tubular reabsorption in rabbits. Antimicrob Agents Chemother. 1984 Feb;25(2):168–172. doi: 10.1128/aac.25.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbon C., Contrepois A., Vigneron A. M., Lamotte-Barrillon S. Effects of furosemide on extravascular diffusion, protein binding and urinary excretion of cephalosporins and aminoglycosides in rabbits. J Pharmacol Exp Ther. 1980 Jun;213(3):600–606. [PubMed] [Google Scholar]
- Daschner F. D., Just H. M., Jansen W., Lorber R. Netilmicin versus tobramycin in multi-centre studies. J Antimicrob Chemother. 1984 Jan;13 (Suppl A):37–45. doi: 10.1093/jac/13.suppl_a.37. [DOI] [PubMed] [Google Scholar]
- De Broe M. E., Paulus G. J., Verpooten G. A., Roels F., Buyssens N., Wedeen R., Van Hoof F., Tulkens P. M. Early effects of gentamicin, tobramycin, and amikacin on the human kidney. Kidney Int. 1984 Apr;25(4):643–652. doi: 10.1038/ki.1984.69. [DOI] [PubMed] [Google Scholar]
- Fong I. W., Fenton R. S., Bird R. Comparative toxicity of gentamicin versus tobramycin: a randomized prospective study. J Antimicrob Chemother. 1981 Jan;7(1):81–88. doi: 10.1093/jac/7.1.81. [DOI] [PubMed] [Google Scholar]
- Holm S. E., Hill B., Löwestad A., Maller R., Vikerfors T. A prospective, randomized study of amikacin and gentamicin in serious infections with focus on efficacy, toxicity and duration of serum levels above the MIC. J Antimicrob Chemother. 1983 Oct;12(4):393–402. doi: 10.1093/jac/12.4.393. [DOI] [PubMed] [Google Scholar]
- Hostetler K. Y., Hall L. B. Inhibition of kidney lysosomal phospholipases A and C by aminoglycoside antibiotics: possible mechanism of aminoglycoside toxicity. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1663–1667. doi: 10.1073/pnas.79.5.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahlmeter G., Dahlager J. I. Aminoglycoside toxicity - a review of clinical studies published between 1975 and 1982. J Antimicrob Chemother. 1984 Jan;13 (Suppl A):9–22. doi: 10.1093/jac/13.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- Kaloyanides G. J., Pastoriza-Munoz E. Aminoglycoside nephrotoxicity. Kidney Int. 1980 Nov;18(5):571–582. doi: 10.1038/ki.1980.175. [DOI] [PubMed] [Google Scholar]
- Marche P., Koutouzov S., Girard A. Impairment of membrane phosphoinositide metabolism by aminoglycoside antibiotics: streptomycin, amikacin, kanamycin, dibekacin, gentamicin and neomycin. J Pharmacol Exp Ther. 1983 Nov;227(2):415–420. [PubMed] [Google Scholar]
- Moore R. D., Smith C. R., Lipsky J. J., Mellits E. D., Lietman P. S. Risk factors for nephrotoxicity in patients treated with aminoglycosides. Ann Intern Med. 1984 Mar;100(3):352–357. doi: 10.7326/0003-4819-100-3-352. [DOI] [PubMed] [Google Scholar]
- Morin J. P., Viotte G., Vandewalle A., Van Hoof F., Tulkens P., Fillastre J. P. Gentamicin-induced nephrotoxicity: a cell biology approach. Kidney Int. 1980 Nov;18(5):583–590. doi: 10.1038/ki.1980.176. [DOI] [PubMed] [Google Scholar]
- Pastoriza-Munoz E., Timmerman D., Kaloyanides G. J. Renal transport of netilmicin in the rat. J Pharmacol Exp Ther. 1984 Jan;228(1):65–72. [PubMed] [Google Scholar]
- Porter G. A., Bennett W. M. Nephrotoxic acute renal failure due to common drugs. Am J Physiol. 1981 Jul;241(1):F1–F8. doi: 10.1152/ajprenal.1981.241.1.F1. [DOI] [PubMed] [Google Scholar]
- SCHREINER G. E. Determination of inulin by means of resorcinol. Proc Soc Exp Biol Med. 1950 May;74(1):117–120. doi: 10.3181/00379727-74-17827. [DOI] [PubMed] [Google Scholar]
- Senekjian H. O., Knight T. F., Weinman E. J. Micropuncture study of the handling of gentamicin by the rat kidney. Kidney Int. 1981 Mar;19(3):416–423. doi: 10.1038/ki.1981.34. [DOI] [PubMed] [Google Scholar]
- Sheth A. U., Senekjian H. O., Babino H., Knight T. F., Weinman E. J. Renal handling of gentamicin by the Munich-Wistar rat. Am J Physiol. 1981 Dec;241(6):F645–F648. doi: 10.1152/ajprenal.1981.241.6.F645. [DOI] [PubMed] [Google Scholar]
- Smith C. R., Baughman K. L., Edwards C. Q., Rogers J. F., Lietman P. S. Controlled comparison of amikacin and gentamicin. N Engl J Med. 1977 Feb 17;296(7):349–353. doi: 10.1056/NEJM197702172960701. [DOI] [PubMed] [Google Scholar]
- Smith C. R., Lipsky J. J., Laskin O. L., Hellmann D. B., Mellits E. D., Longstreth J., Lietman P. S. Double-blind comparison of the nephrotoxicity and auditory toxicity of gentamicin and tobramycin. N Engl J Med. 1980 May 15;302(20):1106–1109. doi: 10.1056/NEJM198005153022002. [DOI] [PubMed] [Google Scholar]
- Tablan O. C., Reyes M. P., Rintelmann W. F., Lerner A. M. Renal and auditory toxicity of high-dose, prolonged therapy with gentamicin and tobramycin in pseudomonas endocarditis. J Infect Dis. 1984 Feb;149(2):257–263. doi: 10.1093/infdis/149.2.257. [DOI] [PubMed] [Google Scholar]
- Viotte G., Olier B., Morin J. P., Godin M. Modifications fonctionnelles, histologiques, biochimiques rénales. Etude comparative entre dibékacine, gentamicine, tobramycine, nétilmicine et amikacine. Nouv Presse Med. 1982 Nov 18;11(46):3419–3425. [PubMed] [Google Scholar]
- Whelton A., Solez K. Aminoglycoside nephrotoxicity--a tale of two transports. J Lab Clin Med. 1982 Feb;99(2):148–155. [PubMed] [Google Scholar]


