Abstract
Previous research indicates that an age-related increase in the amplitude of the Ca2+-dependent, K+-mediated afterhyperpolarization (AHP) is related to cognitive decline. However, because the AHP is measured following completion of training, it is unclear whether the AHP amplitude is strictly dependent on biological aging or is modified by the training procedure. To address this distinction we examined the effect of environmental enrichment on the AHP amplitude. Young (5-8 months) and aged (22-24 months) male Fischer 344 rats were exposed to environmental enrichment conditions (EC) or maintained in individual cages (IC). Following 8-10 weeks of differential experience, sharp microelectrode current-clamp recordings were obtained in CA1 pyramidal neurons in hippocampal slices. Examination of the AHP demonstrated that the amplitude was significantly reduced in aged animals exposed to enriched conditions. The results indicate that the amplitude of the AHP in hippocampal pyramidal cells from aged animals is dependent on the history of experience and hippocampal activity.
Keywords: Aging, environmental enrichment, hippocampus, brain slices, electrophysiology, afterhyperpolarization
1. Introduction
Within an aging population there is considerable variability in memory, which is likely due to differences in the degree of brain senescence. In turn, the extent of brain aging is influenced by genetic and environmental factors [8]. A consistent finding across several species and laboratories is that aged animals exhibit an increase in the Ca2+-dependent, K+-mediated afterhyperpolarization (AHP) [5,20-22,26,30]. Interestingly, recent research indicates that aged animals that exhibit learning on hippocampal-dependent tasks also present with a reduction in the amplitude of the AHP relative to animals that fail to exhibit learning [25,30]. However, it is unclear whether the variability in the AHP is related to biological aging per se or may result from learning as the animal interacts with the environment. For example, one possibility is that the amplitude of the AHP is age-dependent (i.e. varies according to biological aging of the brain) and the larger AHP is observed in very old animals which exhibit impairments on hippocampal-dependent tasks [30]. Alternatively, the amplitude of the AHP may be “event-dependent”, reflecting changes brought about as a result of learning while interacting with the environment, such that the AHP of aged animals may be reduced as animals acquire the task [25]. Under this set of assumptions environmental stimuli designed to engage the hippocampus might ameliorate this form of senescent physiology.
Environmental enrichment has been shown to ameliorate age-related changes in hippocampal anatomy and biochemistry and improve cognitive function in aged animals [12,13,19,24,28]. However, little is known concerning the effects of environmental enrichment on cell excitability and senescent neurophysiology. Therefore, the current study was designed to test the hypothesis that environmental enrichment would reduce the amplitude of the AHP. As with our previous research [9,10,14], the goal of the enrichment procedure was to alter the environment in order to make available opportunities to perform the widest possible range of behaviors that depend on the hippocampus, and to limit these behaviors for animals in the control or impoverished condition. Our results demonstrate that a reduction in the AHP was observed only in aged animals exposed to environmental enrichment consistent with the idea that environmental enrichment can ameliorate senescent physiology and suggesting that the responsiveness to the enrichment procedure is modified by age.
2. Results
A total of one hundred eighteen recorded cells were acceptable according to our cell health criteria. The input resistance, resting membrane potential, and spike amplitude were not different between age groups, and no significant differences in the intrinsic membrane properties (input resistance, resting membrane potential, and spike amplitude) were observed across the enriched and isolated conditions (Table 1).
Table 1.
Biophysical properties of CA1 neurons recorded from aged/young male F 344 rats.
| Aged | IR (MΩ) | RMP | Spike Amplitude |
|---|---|---|---|
| IC (20) | 36.6 ± 2.47 | −61 ± 1.18 | 84.4 ± 1.03 |
| EC (21) | 41.8 ± 4.64 | −63 ± 1.15 | 83.1 ± 0.81 |
| Young | |||
| IC (40) | 38.9 ± 2.36 | −62 ± 0.78 | 84.0 ± 0.78 |
| EC (37) | 46.2 ± 3.49 | −65 ± 0.84 | 81.9 ± 1.31 |
Values are ± SEM. IC, individual cage, EC, enriched conditions, RMP, resting membrane potential, IR, Input resistance; RMP and spike amplitude values are in mV. Numbers in parentheses indicate number of cells for each condition.
Figure 1 illustrates the effect of age and treatment condition on the amplitude of the AHP. For each cell, the average of five to ten AHPs elicited by a burst of 4 action potentials was calculated and used for statistical analysis. A two-way analysis of variance (ANOVA) on the AHP averages indicated a significant age x treatment interaction [F(1, 114) 13.36, p < 0.005]. Subsequent ANOVAs within each treatment group confirmed a significant [F(1, 58) 10.89, p < 0.005] increase in the AHP for aged (6.43 ± 0.49 mV, n = 20), relative to young (4.72 ± 0.27 mV, n = 40) rats exposed to the IC condition (Fig 1A & C). Interestingly, no age difference was observed for animals exposed to environmental enrichment (Fig 1B & C). However, there was a tendency (p = 0.057) for a decrease in the AHP of aged animals (4.06 ± 0.55 mV, n = 21) relative to young (5.17 ± 0.30 mV, n = 37) following 8-10 weeks of environmental enrichment. An ANOVA within each age group indicated a significant [F(1,39) 10.27, p < 0.005] effect of differential experience only in aged animals due to a reduction in the AHP observed for the EC animals.
Figure 1.
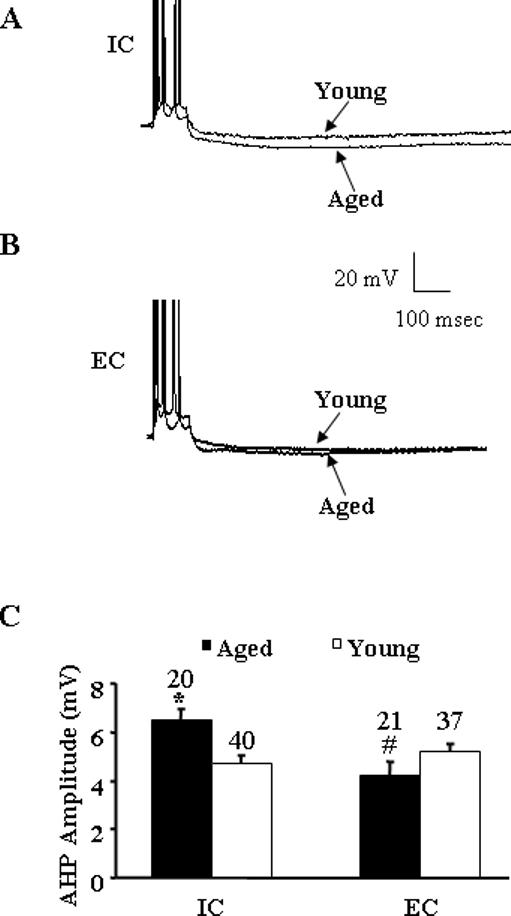
A: Representative voltage records from CA1 pyramidal cells from aged and young rats exposed to individual cages (IC). The AHP is evoked after a train of four action potentials, elicited by a 100-ms pulse of depolarizing current. Both cells were held at −62 mV. Note that action potentials are truncated to better illustrate the AHPs. B: Representative voltage records from CA1 pyramidal cells from aged and young rats exposed to environmental enrichment conditions (EC). Again the AHP is evoked after a train of four action potentials elicited by a 100-ms pulse of depolarizing current and the cells were held at −61 mV. C: Mean AHP amplitude (mV) recorded in neurons of aged (filled bars) and young (open bars) rats exposed to IC and EC. Asterisk indicates a significant increase (P < 0.005) in AHP amplitude in aged rats compared to young rats in IC group. Pound sign indicates a significant decrease (P < 0.005) in AHP amplitude in EC aged compared to IC aged rats. Number above each bar indicates number of cells recorded in each group.
3. Discussion
The current study demonstrates that 8-10 weeks of environmental enrichment is associated with a significant reduction in the AHP amplitude for older rats. In contrast, an age-related increase in the AHP was limited to control animals that did not receive environmental enrichment. The results indicate that the AHP amplitude is not simply a function of aging, rather the amplitude is sensitive to the history of experience.
Environmental enrichment is designed to provide animals opportunities to perform a wide range of activities, including social, physical, and cognitive behaviors. The opposite is true for the impoverished environments, which limit the animal's use of natural skills and behaviors. Furthermore, the hippocampus is activated by social interactions [3], exposure to novel environments, [31] and the spatial rearrangement of familiar objects in the environment [1]. In considering what aspect of environmental enrichment might underlie the reduction in the AHP, it is important to note that this study re-affirms the well documented age-related enhancement in the AHP amplitude [5,20-22,26,30]. In most of the previous studies, animals were group housed. Thus, the reduction in the AHP is not likely due to pair housing and availability of social interactions per se.
While motor activity was not specifically measured in the current study, it is possible that the enriched animals engaged in more exercise since they were permitted to explore a novel environment each day. Thus, variability in the AHP amplitude may relate to the level of exercise, which can reduce some biological markers of aging [8]. However, previous work examining the effect of exercise on the AHP in motor neurons indicates that the AHP magnitude is increased for a subset of motor neurons following increased exercise [2]. Regardless, the fact that the reduction in the AHP associated with environmental enrichment was observed only in aged animals indicates that the responsiveness to the enrichment procedure is different across the two age groups.
Contrary to the current results, a decrease in the amplitude of the AHP is observed in both aged [25,30] and young animals [4,33] that exhibit learning on a hippocampal dependent tasks. Moreover, aged animals that do not exhibit learning do not exhibit a reduction in the AHP. This raises the interesting possibility that the cellular pathways associated with environmental enrichment may not be the same as those associated with more conventional learning paradigms. Alternatively, differences may be due to age-related impairments in the adaptive response to the environmental or behavioral setting. The relationship between intense stress and corticosterone on neural function and behavior are highly similar to those observed during aging. Furthermore, the ability of stress to modify the AHP may depend on the level of stress and subsequent level of corticosterone released [16,18]. Elevated corticosteroid levels associated with intense stress enhance the AHP, modify synaptic plasticity, and impair learning on a spatial discrimination task [11]. In contrast, exposure to novelty is a relatively mild stress [17] and recent studies indicate that a mild stress can reduce the AHP [32]. Thus, the reduction in the AHP, specific for aged animals may be related to differences in the stress response under the two situations. In this regard, the level of stress associate with exposure to a novel environment may be similar to that observed for learning on a specific task and much milder relative to stress experienced by animals that fail to learn. Thus, at least for aged animals, the reduction in the AHP may be due to mild stress associated with exposure to novelty.
If the reduction in the AHP is linked to mild stress associated with exposure to novelty, the question arises as to why young animals exhibit a reduction in the AHP during learning, but not after 8-10 weeks of environmental enrichment. In considering changes in the stress response during aging, it is clear that the response following exposure to a novel environment and the ability to habituate the stress response following repeated exposure to a mild stressor is modified by aging [15,27]. Indeed, previous work indicates that the reduction of the AHP associated with learning is rather short lived in young animals [4,33]. Thus, the absence of an effect on the AHP in young animals may be due to habituation to novelty over the 8-10 weeks of differential experience.
Previous research indicates that there are several ways to regulate the amplitude of the AHP and pharmacological treatments designed to reduce the AHP can improve learning in aged animals [6,29]. While the mechanism for the reduction in the AHP was not examined in the present study, the results suggest that mild behavioral stress associated with environmental enrichment may improve learning in aged animals through increased cell excitability. Indeed, environmental enrichment has been shown to have beneficial effects on the rate of learning in aged animals [7,12,28]. Similarly, exposure to a mild swim stress for four weeks improved acquisition of spatial information in aged animals when tested following a one hour intertrial interval [23]. However, this same study indicated that the treatment did not ameliorate an age-related deficit in memory retention/consolidation that manifest with longer (24 hr) retention intervals. Thus, the parameters of enrichment including the level of behavioral stress are likely to influence treatment effects on learning and on the biological markers of aging. Together the results are consistent with the idea that environmental enrichment can ameliorate senescent physiology by reducing the AHP, and that the reduction in the AHP may facilitate learning. Future studies should address the role of exercise and stress in mediating age-related differences in the reduction of the AHP following enrichment and determine whether amelioration of this marker of aging is associated with better memory.
4. Experimental procedures
4.1 Animals
Procedures involving animal subjects have been reviewed and approved by the Institutional Animal Care and Use Committee and were in accordance with guidelines established by the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals. Male Fischer 344 rats, young (3-6 months) and aged (20-22 months) were obtained from National Institute on Aging aged rat colony and were randomly assigned to either environmentally enriched conditions (EC, aged/young adult, n = 11/10) or individual cage (IC, aged/young adult, n = 6/12). All animals were maintained on a 12:12 hr light schedule, and provided ad lib access to food and water.
4.2. Environmental Enrichment
The IC rats were independently housed in wire cages (48L×25W×22H cm) and only handled for regular maintenance. The EC rats were group housed (2 per cage) and were allowed to explore a novel environment for 1-2 hours/day. The novel environments consisted of a large box, recording cage, or empty maze pool. Each environment contained several three dimensional objects (e.g. toys, boxes, large plastic pipes, coffee mug, water bottle). The environment and availability of objects was rotated randomly. Following 8 weeks of differential experience, rats (1 per day) were killed for collection of electrophysiology. Differential experience was maintained for remaining rats until all animals had been euthanized (2 weeks). Thus, differential experience was maintained for 8-10 weeks and animals in the enriched condition were exposed to a novel environment at least 24 hr prior to harvesting the hippocampus.
4.3. Hippocampal slice preparation
Rats were overdosed with CO2 and hippocampi were dissected. Hippocampal slices (∼400 μm) were cut parallel to the alvear fibers using a Vibratome (Technical Products International Inc., St. Louis, Missouri). Slices were then transferred to a standard interface-recording chamber that was continuously perfused (1 ml/min) with oxygenated artificial cerebrospinal fluid containing (in mM): NaCl 124, KCl 2, KH2PO4 1.25, MgSO4 2, CaCl2 2, NaHCO3 26, and glucose 10. Slices were maintained at 30-32°C and humidified air (95% O2, 5% CO2) was blown over the slices.
4.4. Intracellular recording
Microelectrodes were pulled from thin-wall 1.0 mm microfiber-filled borosilicate capillaries using a Flaming/Brown horizontal micropipette puller (Sutter Instruments, San Rafael, California). The resistance of microelectrodes when filled with 3 M potassium acetate ranged from 50 to 100 MΩ. Microelectrodes were visually positioned in the CA1 pyramidal cell layer using a dissecting microscope (SZH10, Optical Elements Corporation, Washington D.C). Extracellular sharp electrode recordings were obtained from CA1 hippocampal pyramidal neurons. The signals were amplified using an Axoclamp 2B amplifier (Axon Instruments, Foster City, CA), and recordings were sampled at 1 kHz, and stored on computer disk for off-line analysis (Data Wave Technologies, Longmont, CO). An acceptance criterion was established for cell health such that only neurons with resting membrane potential less than −60 mV measured in the absence of injected holding current, an input resistance >20 MΩ, and an action potential amplitude of 70 mV were included in the analysis. Voltage deflections resulting from hyperpolarizing current pulses (100 ms, 0.2 nA) were used to determine input resistance. For examination of the AHP, the membrane potential was maintained at ∼ 64 mV with a constant current injection (0.48 ± 0.02 nA, mean ±sem) to minimize the effects of voltage-dependent alterations in membrane conductance. Depolarizing current pulses (100 msec, 0.1-1.2 nA) were delivered every 20 sec through the intracellular electrode to elicit a sodium spike bursts of 4 action potentials. The AHP was measured as the difference between the holding potential during the 100 msec period immediately before the onset of the depolarizing current and the membrane potential 500 msec after the offset of the depolarizing current.
Acknowledgements
This work was supported by National Institutes of Health Grant AG14979 and the Evelyn F. McKnight Brain Research Foundation. Special thanks to Asha Rani and Prasanna Durairaj for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aggleton JP, Brown MW. Contrasting hippocampal and perirhinal cortex function using immediate early gene imaging. Q J Exp Psychol B. 2005;58:218–33. doi: 10.1080/02724990444000131. [DOI] [PubMed] [Google Scholar]
- 2.Beaumont E, Gardiner P. Effects of daily spontaneous running on the electrophysiological properties of hindlimb motoneurones in rats. J Physiol. 2002;540:129–38. doi: 10.1113/jphysiol.2001.013084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Countryman RA, Kaban NL, Colombo PJ. Hippocampal c-fos is necessary for long-term memory of a socially transmitted food preference. Neurobiol Learn Mem. 2005;84:175–83. doi: 10.1016/j.nlm.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Disterhoft JF, Thompson LT, Moyer JR, Jr., Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–20. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- 5.Disterhoft JF, Thompson LT, Moyer JR, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59:413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- 6.Disterhoft JF, Wu WW, Ohno M. Biophysical alterations of hippocampal pyramidal neurons in learning, ageing and Alzheimer's disease. Ageing Res Rev. 2004;3:383–406. doi: 10.1016/j.arr.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Fernandez CI, Collazo J, Bauza Y, Castellanos MR, Lopez O. Environmental enrichment-behavior-oxidative stress interactions in the aged rat: issues for a therapeutic approach in human aging. Ann N Y Acad Sci. 2004;1019:53–7. doi: 10.1196/annals.1297.012. [DOI] [PubMed] [Google Scholar]
- 8.Foster TC. Biological markers of age-related memory deficits : treatment of senescent physiology. CNS Drugs. 2006;20:153–66. doi: 10.2165/00023210-200620020-00006. [DOI] [PubMed] [Google Scholar]
- 9.Foster TC, Dumas TC. Mechanism for increased hippocampal synaptic strength following differential experience. J Neurophysiol. 2001;85:1377–83. doi: 10.1152/jn.2001.85.4.1377. [DOI] [PubMed] [Google Scholar]
- 10.Foster TC, Gagne J, Massicotte G. Mechanism of altered synaptic strength due to experience: relation to long-term potentiation. Brain Res. 1996;736:243–50. doi: 10.1016/0006-8993(96)00707-x. [DOI] [PubMed] [Google Scholar]
- 11.Foster TC, Norris CM. Age-associated changes in Ca(2+)-dependent processes: relation to hippocampal synaptic plasticity. Hippocampus. 1997;7:602–12. doi: 10.1002/(SICI)1098-1063(1997)7:6<602::AID-HIPO3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Frick KM, Fernandez SM. Enrichment enhances spatial memory and increases synaptophysin levels in aged female mice. Neurobiol Aging. 2003;24:615–26. doi: 10.1016/s0197-4580(02)00138-0. [DOI] [PubMed] [Google Scholar]
- 13.Frick KM, Stearns NA, Pan JY, Berger-Sweeney J. Effects of environmental enrichment on spatial memory and neurochemistry in middle-aged mice. Learn Mem. 2003;10:187–98. doi: 10.1101/lm.50703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagne J, Gelinas S, Martinoli MG, Foster TC, Ohayon M, Thompson RF, Baudry M, Massicotte G. AMPA receptor properties in adult rat hippocampus following environmental enrichment. Brain Res. 1998;799:16–25. doi: 10.1016/s0006-8993(98)00451-x. [DOI] [PubMed] [Google Scholar]
- 15.Herman JP, Larson BR, Speert DB, Seasholtz AF. Hypothalamo-pituitary-adrenocortical dysregulation in aging F344/Brown-Norway F1 hybrid rats. Neurobiol Aging. 2001;22:323–32. doi: 10.1016/s0197-4580(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 16.Joels M, de Kloet ER. Effect of corticosteroid hormones on electrical activity in rat hippocampus. J Steroid Biochem Mol Biol. 1991;40:83–6. doi: 10.1016/0960-0760(91)90170-a. [DOI] [PubMed] [Google Scholar]
- 17.Kabbaj M, Devine DP, Savage VR, Akil H. Neurobiological correlates of individual differences in novelty-seeking behavior in the rat: differential expression of stress-related molecules. J Neurosci. 2000;20:6983–8. doi: 10.1523/JNEUROSCI.20-18-06983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karst H, Joels M. The induction of corticosteroid actions on membrane properties of hippocampal CA1 neurons requires protein synthesis. Neurosci Lett. 1991;130:27–31. doi: 10.1016/0304-3940(91)90219-j. [DOI] [PubMed] [Google Scholar]
- 19.Kempermann G, Gast D, Gage FH. Neuroplasticity in old age: sustained fivefold induction of hippocampal neurogenesis by long-term environmental enrichment. Ann Neurol. 2002;52:135–43. doi: 10.1002/ana.10262. [DOI] [PubMed] [Google Scholar]
- 20.Kumar A, Foster TC. 17beta-Estradiol benzoate decreases the AHP amplitude in CA1 pyramidal neurons. J Neurophysiol. 2002;88:621–626. doi: 10.1152/jn.2002.88.2.621. [DOI] [PubMed] [Google Scholar]
- 21.Kumar A, Foster TC. Enhanced long-term potentiation during aging is masked by processes involving intracellular calcium stores. J Neurophysiol. 2004;91:2437–44. doi: 10.1152/jn.01148.2003. [DOI] [PubMed] [Google Scholar]
- 22.Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- 23.Mabry TR, McCarty R, Gold PE, Foster TC. Age and stress history effects on spatial performance in a swim task in Fischer-344 rats. Neurobiol Learn Mem. 1996;66:1–10. doi: 10.1006/nlme.1996.0038. [DOI] [PubMed] [Google Scholar]
- 24.Mohammed AH, Henriksson BG, Soderstrom S, Ebendal T, Olsson T, Seckl JR. Environmental influences on the central nervous system and their implications for the aging rat. Behav Brain Res. 1993;57:183–91. doi: 10.1016/0166-4328(93)90134-c. [DOI] [PubMed] [Google Scholar]
- 25.Moyer JR, Jr., Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20:5476–82. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyer JR, Jr., Thompson LT, Black JP, Disterhoft JF. Nimodipine increases excitability of rabbit CA1 pyramidal neurons in an age- and concentration-dependent manner. J Neurophysiol. 1992;68:2100–9. doi: 10.1152/jn.1992.68.6.2100. [DOI] [PubMed] [Google Scholar]
- 27.Sapolsky RM, Krey LC, McEwen BS. The adrenocortical axis in the aged rat: impaired sensitivity to both fast and delayed feedback inhibition. Neurobiol Aging. 1986;7:331–5. doi: 10.1016/0197-4580(86)90159-4. [DOI] [PubMed] [Google Scholar]
- 28.Soffie M, Hahn K, Terao E, Eclancher F. Behavioural and glial changes in old rats following environmental enrichment. Behav Brain Res. 1999;101:37–49. doi: 10.1016/s0166-4328(98)00139-9. [DOI] [PubMed] [Google Scholar]
- 29.Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV, Brewer LD, Landfield PW. Calcium dysregulation in neuronal aging and Alzheimer's disease: history and new directions. Cell Calcium. 1998;24:417–33. doi: 10.1016/s0143-4160(98)90064-1. [DOI] [PubMed] [Google Scholar]
- 30.Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25:2609–16. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Waters NS, Klintsova AY, Foster TC. Insensitivity of the hippocampus to environmental stimulation during postnatal development. J Neurosci. 1997;17:7967–73. doi: 10.1523/JNEUROSCI.17-20-07967.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiss C, Sametsky E, Sasse A, Spiess J, Disterhoft JF. Acute stress facilitates trace eyeblink conditioning in C57BL/6 male mice and increases the excitability of their CA1 pyramidal neurons. Learn Mem. 2005;12:138–43. doi: 10.1101/lm.89005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zelcer I, Cohen H, Richter-Levin G, Lebiosn T, Grossberger T, Barkai E. A cellular correlate of learning-induced metaplasticity in the hippocampus. Cereb Cortex. 2006;16:460–8. doi: 10.1093/cercor/bhi125. [DOI] [PubMed] [Google Scholar]


