Abstract
The activation of human platelets by thrombin is mediated primarily by protease-activated receptors (PARs). PAR1 and PAR4 are present on human platelets and are activated by the hexapeptides SFLLRN and GYPGQV, respectively. To further characterize the involvement of PAR1 and PAR4 in platelet activation, the ability of SFLLRN or GYPGQV to generate annexin V binding to newly exposed phospholipids on the platelet surface and generate procoagulant activity has been examined. Exposure of phosphatidylserine and phosphatidylethanolamine on platelets, as determined by an increase in annexin V binding, was strongly stimulated by SFLLRN, thrombin, and collagen, but only to a minor extent by GYPGQV. In a clotting assay initiated with factor VIIa, soluble tissue factor, and calcium, the clotting time in the absence of platelets was >5 min. In the presence of unstimulated platelets, the clotting time was 200 ± 20 sec. In the presence of platelets activated with SFLLRN or collagen, the clotting time decreased to 100 ± 10 sec. This shortening of the clotting time is equivalent to about a 5-fold increase in coagulant activity when stimulated platelets are compared with unstimulated platelets and activated platelets are used as a reference. These results indicate that thrombin initiates a very strong response in platelets through PAR1, leading to exposure of anionic phospholipids that support blood clotting. The response mediated by PAR4, however, was limited to platelet aggregation and similar to that triggered in platelets by weaker agonists such as ADP or epinephrine.
Keywords: clotting, phospholipid, annexin V, calcium flux
Thrombin mediates its cellular effects primarily through protease-activated receptors (PARs) (1). These receptors are activated by a unique mechanism in which the protease exposes a tethered ligand at the extracellular N terminus by specific minor proteolysis, resulting in intramolecular activation (2). Three of the four known PARs, PAR1, PAR3, and PAR4, are activated by thrombin (2–5). Current evidence suggests, however, that PAR1 and PAR4 are the major human platelet thrombin receptors (6). Specific agonist peptides have been designed for the thrombin-independent activation of PAR1 and PAR4 (2, 4, 5). No agonist peptide for PAR3 has been described (3).
Activation of platelet PAR1 by the PAR1-specific agonist peptide, SFLLRN, results in platelet aggregation and degranulation (7). Several discrepancies in platelet responses have been noted when platelets are stimulated by thrombin versus SFLLRN in vitro (8–12). These differences may be due in part to the presence of both PAR1 and PAR4 on human platelets (5, 6). Also, the thrombin and SFLLRN concentrations that were employed may not have been sufficient to elicit a maximal response. Additionally, the activation of PAR1 by SFLLRN versus thrombin may have different kinetic properties. Finally, many of the earlier platelet activation studies were carried out prior to the identification of PAR3 and PAR4.
Human and mouse PAR4 were recently cloned and subsequently shown to elicit platelet aggregation (4–5). The coexistence of two distinct platelet thrombin receptors raised several possibilities such as redundancy, synergy, and/or differential function. Accordingly, inositol trisphosphate (IP3) signaling by means of PAR1 was observed at low concentrations of thrombin (EC50 of 0.1 nM) (2), whereas IP3 signaling by means of PAR4 required much higher thrombin concentrations in transfected cell systems (2, 4, 5).
The physiologic activation of platelets by thrombin has been well characterized and includes platelet aggregation, granular secretion, and stimulation of platelet procoagulant activity (13, 14). This observed procoagulant activity was caused by exposure of phosphatidylserine (PS) and phosphatidylethanolamine (PE), which greatly accelerated the assembly of activated plasma coagulation factors on the platelet surface. Only strong platelet agonists such as thrombin, collagen, and calcium ionophore A23187 give rise to a significant increase of platelet PS and PE exposure (14, 15). In previous studies, selective activation of platelet PAR1 by SFLLRN failed to elicit a procoagulant response comparable to that by thrombin (8, 10). Thrombin activation of platelets is known to mediate IP3 signaling, resulting in an increased intracellular calcium flux (16). This increase in intracellular calcium concentration may result in the exposure of PS and PE through the calcium-dependent activation of “scramblase,” an intracellular membrane-associated protein known to translocate procoagulant anionic phospholipids to the outer surface (17).
In this study the activation of human platelets with thrombin or PAR1- and PAR4-specific peptide agonists has been investigated. Selective PAR1 activation was as potent as thrombin in stimulating platelet aggregation, calcium flux, and anionic phospholipid exposure and development of a procoagulant surface. Selective PAR4 activation, however, resulted in a less potent platelet response giving rise to a lower calcium flux, limited anionic phospholipid exposure, and very little acceleration of the clotting cascade.
MATERIALS AND METHODS
Calcium ionophore A23187 and fura-2 were from Sigma. Calf tendon collagen was from Chrono-log (Havertown, PA). Recombinant factor VIIa and soluble tissue factor as well as the amidated hexapeptide SFLLRN were prepared at ZymoGenetics. Hexapeptides GYPGQV and GYPGKF were from Bethyl Laboratories (Montgomery, TX).
Preparation of Human α-Thrombin.
Human α-thrombin was prepared as follows: Prothrombin (40 mg in 10 ml of 25 mM Bis-Tris buffer, pH 6.5/40 mM NaCl) was activated with 0.5 mg of factor Xa in the presence of 4 mM CaCl2 for 2 hr at 37°C. The reaction was terminated by adding 10 mM EDTA. After the sample was dialyzed against 50 mM Bis-Tris, pH 6.5/40 mM NaCl, it was applied to a Waters Protein-Pak CM column (1 × 10 cm). Thrombin was eluted in the nonadsorbed fraction and appeared as a single band on nonreducing SDS/PAGE.
Preparation of FITC-Labeled Annexin V.
Human annexin V, C316S, was prepared by a procedure similar to that for annexin V-N6 (18). Annexin V was labeled with FITC according to the manufacturer’s instruction (Molecular Probes). Annexin V (13.4 mg/12 ml) was dialyzed overnight against 50 mM sodium borate buffer, pH 9.0. Then 150 μl of 25 mM FITC in dimethylformamide was added and allowed to stand at room temperature for 2 hr. The reaction was quenched by adding 2 ml of 0.5 M glycine followed by dialysis overnight against 0.1 M sodium phosphate buffer, pH 6.0/50 mM NaCl. The sample was then applied to a Waters Protein-Pak DEAE column (1 × 8 cm) equilibrated with 0.1 M sodium phosphate buffer, pH 6.0. After the column had been washed, proteins were eluted by a linear NaCl gradient to 0.5 M. Three major protein peaks emerged from the column. The protein in the first peak was fluorescent and migrated as a single band on SDS/PAGE slightly slower than unlabeled annexin V. The molar ratio of fluorescein to protein was determined to be 0.9 mol/mol. The protein concentration was determined by the BCA (bicinchoninic acid) protein assay (Pierce) using bovine albumin as a standard, and the incorporation of fluorescein was estimated, assuming a molar absorptivity at 494 nm of 65,700. The phospholipid binding activity of FITC-annexin V was determined by a kaolin-dependent clotting assay using normal human plasma and rabbit brain cephalin (19). FITC-annexin V showed essentially the same inhibitory activity on the clotting time as did native annexin V.
Platelet Preparation.
Platelets were prepared from freshly drawn human blood essentially as described by Timmons and Hawiger (20). Blood was collected from healthy fasting donors by antecubital venipuncture into citrate. Platelet-rich plasma was prepared by centrifugation of the anticoagulated whole blood at 1,000 × g for 4 min at room temperature. Prostaglandin E1 (PGE1) was added to the platelet-rich plasma to a final concentration of 1 μM, and the preparation was incubated for 5 min. Platelets were washed twice in an isotonic platelet buffer (10 mM Hepes, pH 7.35/55 mM dextrose/137 mM NaCl/2.7 mM KCl/1 mg/ml bovine albumin/1 μM PGE1) and recovered by centrifugation. Washed platelets were finally suspended in annexin V binding buffer (10 mM Hepes, pH 7.35/5 mM dextrose/150 mM NaCl/2.7 mM KCl/2.5 mM CaCl2/1 mg/ml bovine albumin) at a density of 250,000 platelets per μl.
Platelet Aggregation.
Platelet aggregation was followed turbidometrically at 37°C in a platelet aggregometer (PACKS-4, Helena Laboratories, Beaumont, TX). Fibrinogen was added to washed platelets resuspended in annexin V binding buffer to a concentration of 100 μg/ml as described by Brass et al. (21). Aggregation under stirring conditions was initiated by the addition of agonist peptides SFLLRN (0.1 mM), GYPGQV (1 mM), or GYPGKF (0.5 mM) and turbidity was monitored for 10 min.
Calcium Flux.
Transient increase in cytoplasmic calcium was measured by fura-2 fluorescence based on the method of Heemskeerk et al. (11). Briefly, washed platelets were loaded with fura-2 by incubation in isotonic platelet buffer with 2 mM fura-2 for 45 min at room temperature in the dark. The platelets were washed in platelet buffer and resuspended to a density of 300,000 platelets per μl. Agonists were added, and transient fura-2 fluorescence, monitored by excitation at 340 nm and emission at 510 nm, was recorded on a Perkin–Elmer LS-5 fluorescence spectrophotometer.
Annexin V Binding.
Binding of FITC-labeled annexin V to platelets was monitored by using a method modified from that of Thiagarajan and Tait (15). Briefly, washed platelets in annexin V binding buffer were activated by agonists at room temperature for 30 min without stirring in the presence of FITC-labeled annexin V (200 nM). Unbound annexin V was removed by repeated centrifugation (1,000 × g, 2 min). Bound FITC-labeled annexin V was released from platelets in two sequential washes with platelet buffer containing 50 mM EDTA, and the amount of released FITC-annexin V was quantified by fluorimetry (excitation 494 nm, emission 525 nm).
Procoagulant Activity.
The ability of platelets activated by various agonists to support coagulation was measured in a phospholipid-dependent coagulation assay. Platelets activated by agonists were mixed with an equal volume of pooled human plasma and 4 nM factor VIIa and 4 nM soluble tissue factor. Clotting was initiated by the addition of calcium to a final concentration of 10 mM. In the time curve analysis, the concentrations of factor VIIa and soluble tissue factor were increased to 10 nM.
RESULTS AND DISCUSSION
Platelet Aggregation.
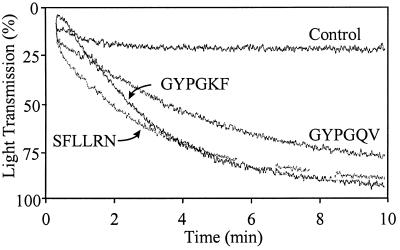
The hexapeptides SFLLRN and GYPGQV, corresponding to the human PAR1 and PAR4 tethered ligands, readily induce platelet aggregation at concentrations of 100 μM and 1000 μM, respectively. These results are similar to those recently published by Kahn et al. (6). The hexapeptide GYPGKF, corresponding to the mouse PAR4 tethered ligand, also induced aggregation of human platelets at 500 μM under similar conditions (Fig. 1). These data indicate that both PAR1 and PAR4 activation results in a sufficient intracellular calcium flux leading to the surface exposure of the functional glycoprotein IIb/IIIa complex, which is the platelet fibrinogen receptor. In these experiments, a 10-fold higher peptide concentration of GYPGQV versus SFLLRN was required to induce a similar response in the platelet aggregation assay. These differences may be due in part to the 3-fold higher abundance of PAR1 than PAR4 on human platelets (6). Also, signaling differences between the two receptors may exist because of the divergent sequences of PAR1 and PAR4 intracellular domains, although they are both known to signal through the IP3 pathway (2, 5). Interestingly, the agonist peptide GYPGKF, modeled after the mouse PAR4 tethered ligand sequence, was more effective in inducing human platelet aggregation than was the corresponding human PAR4 tethered ligand sequence GYPGQV (Fig. 1). This difference may reflect subtle variations in the affinity of short synthetic peptides for the native uncleaved PAR4 receptor on human platelets. Further studies are necessary to confirm these differences. These data show that specific and selective activation of either PAR1 or PAR4 from human platelets results in the calcium-dependent exposure of functional GPIIb/IIIa complexes leading to platelet aggregation.
Figure 1.
Effects of PAR1 and PAR4 agonists on the aggregation of washed human platelets in the presence of calcium and fibrinogen. Concentrations employed: 100 μM SFLLRN, 1000 μM GYPGQV, 500 μM GYPGKF, and 100 μg/ml fibrinogen. Aggregation was studied under stirred conditions.
Annexin V Binding.
Exposure of anionic phospholipid on platelet surfaces resulting from PAR1 and PAR4 activation was measured by the binding of FITC-labeled annexin V. Annexin V is a ubiquitous cytosolic protein, which binds with very high affinity to PS and PE moieties (15). The platelet agonists, thrombin, collagen, the calcium ionophore A23187, and the PAR1 agonist peptide SFLLRN all gave rise to 2-fold or higher increases in annexin V binding (Table 1). The potencies of PAR4-specific agonist peptides GYPGQV and GYPGKF were significantly less, although they both gave rise to a 50% higher annexin V binding relative to unstimulated platelets (baseline). The calcium ionophore was the most potent agonist, giving rise to a 20-fold increase in annexin V binding over baseline. This result was consistent with prior observations (15). The relative strengths of the agonists examined in this study were as follows: GYPGQV (1000 μM), GYPGKF (500 μM) < thrombin (50 nM), SFLLRN (100 μM) < collagen (20 μg/ml) < calcium ionophore (5 μM). The effect of adding both collagen and thrombin was synergistic, as was the effect of adding both collagen and SFLLRN. This synergistic effect was expected, because thrombin and collagen stimulate a calcium flux through distinct platelet receptors and signaling pathways (22, 23). The increase in annexin V binding by adding SFLLRN and GYPGQV together was not significantly different from the increase caused by adding SFLLRN alone. These results show that while PAR4 activation leads to platelet aggregation, it does not significantly contribute to the surface exposure of PS and PE.
Table 1.
Annexin V binding of platelets after stimulation with various platelet agonists
| Agonist | Annexin V binding, fold increase |
|---|---|
| None | 1.00 ± 0.38 |
| Thrombin | 2.16 ± 0.40 |
| Collagen | 3.75 ± 0.40 |
| SFLLRN | 1.98 ± 0.14 |
| GYPGQV | 1.44 ± 0.31 |
| GYPGKF | 1.57 ± 0.57 |
| Thrombin + collagen | 9.35 ± 0.69 |
| SFLLRN + collagen | 9.44 ± 1.13 |
| SFLLRN + GYPGQV | 2.51 ± 0.54 |
| SFLLRN + GYPGKF | 2.59 ± 0.20 |
The fold increase in annexin V binding was calculated by dividing the fluorescence resulting from release of FITC-labeled annexin V from activated platelets by the corresponding fluorescence from unactivated platelets.
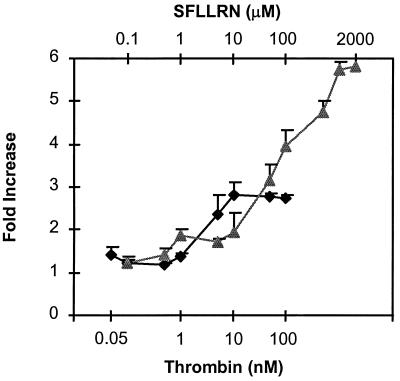
In preliminary experiments, SFLLRN at high concentrations was shown to be more potent than thrombin in stimulating platelet annexin V binding. Accordingly, dose–response curves were generated for thrombin and SFLLRN to determine the concentrations at which maximal annexin V binding was reached (Fig. 2). Surprisingly, maximal annexin V binding resulting from activation with SFLLRN was 2-fold higher than that with thrombin. This difference may be due to differences in the kinetics between activating with a peptide ligand and a proteolytic enzyme.
Figure 2.
Annexin V binding to platelets as a function of thrombin and SFLLRN concentrations. The concentrations of thrombin (♦) and SFLLRN (▴) were in the range of 0.05–100 nM and 0.1–2000 μM, respectively. Fold increase in binding was based on fluorescence measurements as described in the legend for Table 1.
Overall, annexin V binding initiated by PAR1 activation by SFLLRN readily accounts for the ability of thrombin to stimulate platelets, leading to the exposure of negatively charged phospholipids. In addition, the stimulation of platelets with PAR4 activating peptides, GYPGQV or GYPGKF, does not significantly increase annexin V binding to platelets. These data indicated that PAR4 does not play a major role in the generation of platelet procoagulant phospholipid. On the basis of these results, it was concluded that the anionic phospholipid exposure resulting from the thrombin activation of platelets can be accounted for by the activation of PAR1 alone.
Calcium Flux.
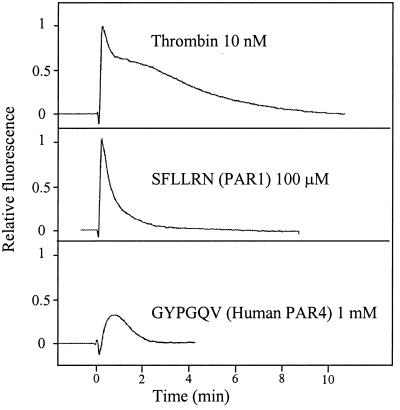
To determine the relative contributions of PAR1 and PAR4 stimulation to intracellular calcium flux, the increase in fluorescence generated by stimulating fura-2-loaded platelets with thrombin was compared with signals generated by the PAR1 and PAR4 agonist peptides. Ten nanomolar thrombin and 100 μM SFLLRN gave rise to similar heights of the calcium spike, whereas 1 mM GYPGQV induced a calcium spike only about half as high (Fig. 3). These data, together with the annexin V binding, indicate that PAR4 activation leads to a limited calcium flux that is sufficient for glycoprotein IIb/IIIa exposure and platelet aggregation. This intracellular calcium concentration of about 0.3 μM is sufficient for platelet aggregation (13) but is inadequate to cause significant exposure of PE and PS on the platelet surface generating procoagulant activity. To effectively stimulate platelet procoagulant activity, the cytosolic calcium concentration has to be higher than the level required for aggregation (24, 25) and is probably in the range of 0.8 μM (13). PAR1 activation, which leads to a high calcium influx into the cytoplasm, induces both platelet aggregation and significant surface exposure of PE and PS. Because the platelet calcium fluxes induced by thrombin and SFLLRN were similar, it was concluded that PAR1 was the major functional thrombin receptor with respect to platelet activation.
Figure 3.
The calcium fluxes in human platelets resulting from thrombin, SFLLRN, and GYPGQV. The calcium flux was monitored in fura-2-loaded platelets at concentrations of 10 nM, 100 μM, and 1 mM for thrombin, SFLLRN, and GYPGQV, respectively.
Clotting.
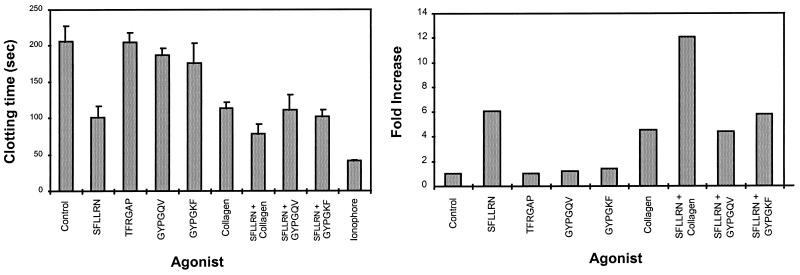
An increase in intracellular calcium in platelets resulting from stimulation by a calcium ionophore or by receptor-mediated signaling may activate scramblase (26), which causes a rapid randomization and exposure of anionic phospholipids PE and PS on the platelet surface (14, 25). It has been shown that the procoagulant properties of a phospholipid surface depend entirely on the composition of the phospholipid, particularly the PS content. Clot-promoting activity was proportional to the content of PS and was maximal at ≈20% (27). To determine the effect of increased surface exposure of PE and PS induced by PAR1 and PAR4 activation on coagulation, clotting time in a phospholipid-dependent clotting assay was recorded. Without an added membrane surface, this system has a clotting time of >5 min. Washed platelets, in the absence of an agonist, reduced the clotting time to ≈200 sec. This reduction suggests that some degree of anionic phospholipid exposure has been induced during the platelet isolation procedure. Platelets activated with the calcium ionophore A23187 reduced the clotting time to 40 sec (Fig. 4). This result is consistent with the observation that the calcium ionophore, the most potent platelet agonist known, caused a rapid rise in intracellular calcium, resulting in rapid and maximal exposure of PS and PE on platelet surfaces. SFLLRN and collagen, which were equally potent in stimulating platelets to support clot formation, reduced the clotting time to ≈100 sec. Stimulation of platelets with a combination of SFLLRN and collagen further reduced the clotting time to 80 sec. This effect is consistent with the fact that SFLLRN and collagen bind to distinct receptors, and initiate signaling through different pathways (23, 28). Stimulation of platelets with a PAR3 tethered ligand peptide (TFRGAP) did not change the clotting time relative to control unstimulated platelets. This is consistent with the observation that human PAR3 was not activated by this peptide (3). Interestingly, platelets stimulated with the human and mouse PAR4-specific agonist peptides GYPGQV and GYPGKF have no significant effect on the clotting time (Fig. 4). Similarly, these PAR4-specific agonist peptides, when added in combination with SFLLRN, failed to further reduce the clotting time compared with SFLLRN alone (Fig. 4), indicating that PAR4 activation did not lead to or contribute to the development of a procoagulant surface. These data suggest that activation of PAR4 mediates a response in platelets similar to that of a weak agonist.
Figure 4.
(Left) Clot formation in the presence of platelets activated with various agonists. Concentrations of agonists: SFLLRN, 100 μM; TFRGAP, 500 μM, GYPGQV, 1000 μM; GYPGKF, 500 μM; collagen, 20 mg/ml; Ca ionophore A23187, 5 μM. TFRGAP, which did not lower the clotting time, was used as a negative hexapeptide control. The data shown are the results from independent experiments, using platelets adjusted to 300,000 per μl. (Right) The fold increase in clot acceleration was calculated by using an activated platelet standard curve which plots clotting time as a function of platelet dilution in a double logarithmic plot.
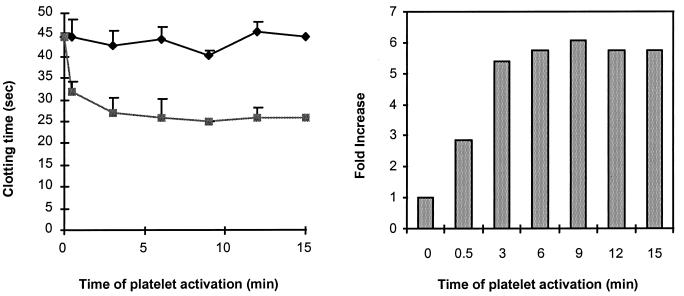
To further elaborate on the significance of the platelet procoagulant response resulting from PAR1 activation, the clotting time was measured as a function of the duration that platelets had been activated. To carry out these studies, the concentration of SFLLRN was increased to 200 mM, the platelets were increased to 500,000 per μl, and the factor VIIa/tissue factor concentration was increased to 10 nM. Because of the experimental procedure, the earliest time point after adding agonist to the platelet suspension was approximately 30 sec. The results in Fig. 5 show that the development of procoagulant properties was immediate. Approximately 80% of the decrease in clotting time was reached after the platelets were activated for 30 sec, while a maximal response was observed after 3–6 min of activation. Accordingly, the rapid development of procoagulant properties resulting from PAR1 activation is physiologically relevant for vascular damage, where rapid clot formation is crucial.
Figure 5.
(Left) Time curve for the activation of platelets by SFLLRN. A final concentration of 200 μM SFLLRN was used to activate platelets, and aliquots were examined for clot acceleration in a clotting assay initiated by the addition of 10 nM VIIa and soluble tissue factor and 10 mM calcium chloride. ⧫, No agonist; ■, activation with SFLLRN. (Right) The fold increase in clot acceleration was calculated by using an activated platelet standard curve, which plots clotting time as a function of platelet dilution in a double-logarithmic plot.
These data show that thrombin mediates a strong platelet agonist effect through the PAR1 receptor, resulting in both platelet aggregation and the rapid stimulation of platelet procoagulant activity as measured by annexin V binding and clotting assays. The selective activation of PAR4 results in weak platelet stimulation, functionally characterized by platelet aggregation without a high enough exposure of anionic phospholipid to significantly accelerate clotting. Accordingly, PAR4 activation is comparable to platelet stimulation with weak agonists such as ADP or epinephrine (13).
Acknowledgments
We are grateful to Mark Corwin for the preparation of the annexin V and Jonathan Tait and Jeff Harris for technical help. This work was supported in part by Grant HL16919 of the National Institutes of Health and a fellowship to H.A. from the Danish Research Academy.
ABBREVIATIONS
- PAR
protease-activated receptor
- IP3
inositol trisphosphate
- PS
phosphatidylserine
- PE
phosphatidylethanolamine
References
- 1.Coughlin S R. Arterioscler Thromb Vasc Biol. 1998;18:514–518. doi: 10.1161/01.atv.18.4.514. [DOI] [PubMed] [Google Scholar]
- 2.Vu T K, Hung D T, Wheaton V I, Coughlin S R. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 3.Ishihara H, Connolly A J, Zeng D, Kahn M L, Zheng Y W, Timmons C, Tram T, Coughlin S R. Nature (London) 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 4.Kahn M L, Zheng Y W, Huang W, Bigornia V, Zeng D, Moff S, Farese R V, Jr, Tam C, Coughlin S R. Nature (London) 1998;394:690–694. doi: 10.1038/29325. [DOI] [PubMed] [Google Scholar]
- 5.Xu W F, Andersen H, Whitmore T E, Presnell S R, Yee D P, Ching A, Gilbert T, Davie E W, Foster D C. Proc Natl Acad Sci USA. 1998;95:6642–6646. doi: 10.1073/pnas.95.12.6642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kahn M L, Nakanishi-Matsui M, Shapiro M J, Ishihara H, Coughlin S R. J Clin Invest. 1999;103:879–887. doi: 10.1172/JCI6042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hung D T, Vu T H, Nelken N A, Coughlin S R. J Cell Biol. 1992;116:827–832. doi: 10.1083/jcb.116.3.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kinlough Rathbone R L, Perry D W, Guccione M A, Rand M L, Packham M A. Thromb Haemostasis. 1993;70:1019–1023. [PubMed] [Google Scholar]
- 9.Kramer R M, Roberts E F, Hyslop P A, Utterback B G, Hui K Y, Jakubowski J A. J Biol Chem. 1995;270:14816–14823. doi: 10.1074/jbc.270.24.14816. [DOI] [PubMed] [Google Scholar]
- 10.Goodwin C A, Wheeler Jones C P, Kakkar V V, Deadman J J, Authi K S, Scully M F. Biochem Biophys Res Commun. 1994;202:321–327. doi: 10.1006/bbrc.1994.1930. [DOI] [PubMed] [Google Scholar]
- 11.Heemskerk J W, Feijge M A, Henneman L, Rosing J, Hemker H C. Eur J Biochem. 1997;249:547–555. doi: 10.1111/j.1432-1033.1997.00547.x. [DOI] [PubMed] [Google Scholar]
- 12.Vickers J D, Packham M A, Kinlough Rathbone R L. Am J Hematol. 1997;54:288–295. doi: 10.1002/(sici)1096-8652(199704)54:4<288::aid-ajh5>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 13.Holmsen H. In: Hemostasis and Thrombosis: Basic Principles and Clinical Practice. Colman R W, Hirsh J, Marder V J, Salzman E W, editors. Philadelphia: Lippincott; 1994. pp. 524–544. [Google Scholar]
- 14.Bevers E M, Comfurius P, Zwaal R F. Blood Rev. 1991;5:146–154. doi: 10.1016/0268-960x(91)90031-7. [DOI] [PubMed] [Google Scholar]
- 15.Thiagarajan P, Tait J F. J Biol Chem. 1990;265:17420–17423. [PubMed] [Google Scholar]
- 16.Rittenhouse-Simmons S. J Clin Invest. 1979;63:580–587. doi: 10.1172/JCI109339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassé F, Stout J G, Sims P J, Wiedmer T. J Biol Chem. 1996;271:17205–17210. doi: 10.1074/jbc.271.29.17205. [DOI] [PubMed] [Google Scholar]
- 18.Tanaka K, Einaga K, Tsuchiyama H, Tait J F, Fujikawa K. Biochemistry. 1996;35:922–929. doi: 10.1021/bi951528x. [DOI] [PubMed] [Google Scholar]
- 19.Funakoshi T, Heimark R L, Hendrickson L E, McMullen B A, Fujikawa K. Biochemistry. 1987;26:5572–5578. doi: 10.1021/bi00391a053. [DOI] [PubMed] [Google Scholar]
- 20.Timmons S, Hawiger J. Methods Enzymol. 1989;169:11–21. doi: 10.1016/0076-6879(89)69046-5. [DOI] [PubMed] [Google Scholar]
- 21.Brass L F, Vassallo R R, Jr, Belmonte E, Ahuja M, Cichowski K, Hoxie J A. J Biol Chem. 1992;267:13795–13798. [PubMed] [Google Scholar]
- 22.Blake R A, Schieven G L, Watson S P. FEBS Lett. 1994;353:212–216. doi: 10.1016/0014-5793(94)01037-4. [DOI] [PubMed] [Google Scholar]
- 23.Brass L F. Coron Artery Dis. 1997;8:49–58. doi: 10.1097/00019501-199701000-00008. [DOI] [PubMed] [Google Scholar]
- 24.Stout J G, Zhou Q, Wiedmer T, Sims P J. Biochemistry. 1998;37:14860–14866. doi: 10.1021/bi9812930. [DOI] [PubMed] [Google Scholar]
- 25.Zwaal R F A, Schroit A J. Blood. 1997;89:1121–1132. [PubMed] [Google Scholar]
- 26.Zhou Q, Zhao J, Stout J G, Luhm R A, Wiedmer T, Sims P J. J Biol Chem. 1997;272:18240–18244. doi: 10.1074/jbc.272.29.18240. [DOI] [PubMed] [Google Scholar]
- 27.Nelsestuen G L, Broderius M. Biochemistry. 1977;16:4172–4177. doi: 10.1021/bi00638a006. [DOI] [PubMed] [Google Scholar]
- 28.Inoue K, Ozaki Y, Satoh K, Wu Y, Yatomi Y, Shin Y, Morita T. Biochem Biophys Res Commun. 1999;256:114–120. doi: 10.1006/bbrc.1999.0295. [DOI] [PubMed] [Google Scholar]