Abstract
A series of antibacterial and antifungal amino acid-derived compounds and their cobalt(II), copper(II), nickel(II), and zinc(II) metal complexes have been synthesized and characterized by their elemental analyses, molar conductances, magnetic moments, and IR, and electronic spectral measurements. Ligands (L1)−(L5) were derived by condensation of β-diketones with glycine, phenylalanine, valine, and histidine and act as bidentate towards metal ions (cobalt, copper, nickel, and zinc) via the azomethine-N and deprotonated-O of the respective amino acid. The stoichiometric reaction between the metal(II) ion and synthesized ligands in molar ratio of M : L (1 : 1) resulted in the formation of the metal complexes of type [M(L)(H2O)4]Cl (where M = Co(II), Cu(II), and Zn(II)) and of M : L (1 : 2) of type [M(L)2(H2O)2] (where M = Co(II), Cu(II), Ni(II), and Zn(II)). The magnetic moment data suggested for the complexes to have an octahedral geometry around the central metal atom. The electronic spectral data also supported the same octahedral geometry of the complexes. Elemental analyses and NMR spectral data of the ligands and their metal(II) complexes agree with their proposed structures. The synthesized ligands, along with their metal(II) complexes, were screened for their in vitro antibacterial activity against four Gram-negative (Escherichia coli, Shigella flexeneri, Pseudomonas aeruginosa, and Salmonella typhi) and two Gram-positive (Bacillus subtilis and Staphylococcus aureus) bacterial strains and for in vitro antifungal activity against Trichophyton longifusus, Candida albicans, Aspergillus flavus, Microsporum canis, Fusarium solani, and Candida glaberata. The results of these studies show the metal(II) complexes to be more antibacterial/antifungal against one or more species as compared to the uncomplexed ligands. The brine shrimp bioassay was also carried out to study their in vitro cytotoxic properties. Five compounds, (3), (7), (10), (11), and (22), displayed potent cytotoxic activity as LD50 = 8.974 × 10−4, 7.022 × 10−4, 8.839 × 10−4, 7.133 × 10−4, and 9.725 × 10−4 M/mL, respectively, against Artemia salina.
INTRODUCTION
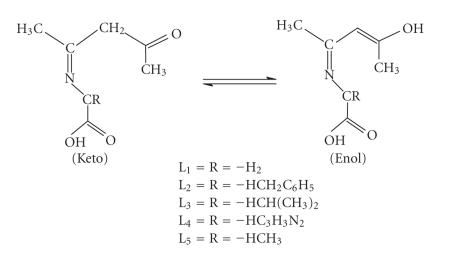
We have already drawn attention [1–5] to the strong relationship between metals or their complexes, and antibacterial [6–12], antitumour [13–15], and anticancer [16, 17] activities. A number of in vivo studies have indicated [18–20] that biologically active compounds become more bacteriostatic and carcinostatic upon chelation. Such interaction of transition-metal ions with amino acids and peptides is of immense biological importance [21–23]. It has been reported [24–28] that metal complexes of amino acid Schiff bases with transition metals possess anticarcinogenic activity. Various tumors tend to have poor blood supplies, and therefore amino acids have been effectively used to direct nitrogen mustards into the cancer cells. For example, phenylalanine mustard is used in controlling malignant myeloma [29] and Burkett's lymphoma [30], and similarly sarcolysine [31] is used to treat wide range of tumors. Indeed, certain tumors and cancer cells are unable to produce all the amino acids synthesized by the normal cells. Therefore, these cells require an external supply of such essential amino acids to pass on to the cancer cells by the blood stream. In the recent past, a number of studies have highlighted the use of acetylacetone in various significant applications [32–37]. In the present studies, ligands (L1)–(L5) (Figure 1) were obtained by the condensation reaction between amino acids (glycine, phenylalanine, alanine, valine, or histidine) and acetylacetone with this hope that it may provide us valuable theoretical information for exploring metal-based bacteriostatic and/or carcinostatic pharmaceuticals with high efficacy and low toxicity. In this effort, we have also introduced an azomethine (−C=N) linkage with the concern that it may permit a notable variety in the remarkable chemistry and behavior of such compounds. The synthesized amino acid-derived compounds (L1)–(L5) have been exposed to act as bidentate towards divalent metal atoms solely through the azomethine-N and carboxylato groups forming a stable 5-membered chelate ring system. The metal(II) complexes, (1)–(40) of the types [M(L)(H2O)4] and [M(L)2(H2O)2]Cl (where M = Co(II), Cu(II), Ni(II), and Zn(II) and L = amino acid-derived ligands (L1)–(L5)) were formed by a stoichiometric ratio of M : L as (1 : 2) and (1 : 1), respectively. These two different stoichiometric ratios of the ligand incorporated with the metal ion were used in order to study the effect of the presence of one or two ligands, respectively, on the biological activity. All these compounds have been characterized by their IR, NMR, molar conductance, magnetic moment, and elemental analyses. The IR of the ligands and their corresponding metal(II) complexes are in agreement with the proposed structures. The magnetic moment and electronic spectral data suggest for all the complexes to have an octahedral geometry. Elemental analyses and NMR spectral data of the ligands and their metal(II) complexes also agree with the structures as anticipated. All these ligands along with their metal(II) complexes were screened for their in vitro antibacterial activity against four Gram-negative (E coli, S flexenari, P aeruginosa, and S typhi) and two Gram-positive (B subtilis and S aureus) bacterial strains and for in vitro antifungal activity against T longifusus, C albicans, A flavus, M canis, F solani, and C glaberata. These compounds have shown varied antibacterial and antifungal activities against one or more bacterial/fungal strains and this activity enhanced on coordination/chelation. The reported compounds are not only good candidates as antibacterial and antifungal agents, but also are a promising addition of new class of compounds as the metal-based drugs.
Figure 1.
Proposed structure of the ligands (L1)–(L5).
EXPERIMENTAL
Material and methods
Solvents used were analytical grades; all metal(II) were used as chloride salts. IR spectra were recorded on the Philips Analytical PU 9800 FTIR spectrophotometer. NMR spectra were recorded on Perkin-Elmer 283B spectrometer. UV-visible spectra were obtained in DMF on a Hitachi U-2000 double-beam spectrophotometer. C, H, and N analyses, conductance and magnetic measurements were carried out on solid compounds using the respective instruments. Melting points were recorded on a Gallenkamp apparatus and are not corrected. The complexes were analyzed for their metal contents by EDTA titration [38]. Antibacterial and antifungal screening was done at HEJ Research Institute of Chemistry, International Center for Chemical Sciences, University of Karachi, Pakistan.
Preparation of Schiff-bases (L1)–(L5)
Acetylacetone (20 mmol) in ethanol (10 mL) was added to a stirred solution of the amino acid (20 mmol) in water (30 mL). The mixture was refluxed for 4–6 hours during which the color of the solution turned to yellow-orange. The completion of reaction was monitored through TLC. After completion of the reaction, it was cooled to afford a solid product. The solid residue was filtered, washed with ethanol, then with ether, and dried. Crystallization from a mixture of ethanol-propanol (60 : 40) afforded the desired ligands. The same method was applied for the preparation of all other ligands by using the corresponding amino acids and/or acetylacetone, working in the same conditions with their respective molar ratio.
{[(3-Hydroxy-1-methylbutyl)-2-en-1-ylidene] amino}acetic acid (L1)
Yield 52%; mp 294°C; IR (KBr, cm−1): 3444 (OH), 3015 (C=C), 1700 (COOH), 1635 (azomethine, HC=N); 1H NMR (DMSO-d6, δ, ppm): 1.85 (s, 6H, CH3), 2.83 (t, 2H, CH2), 5.18 (t, 1H, CH), 6.94 (s, 1H, azomethine), 10.27 (s, 1H, OH), 11.29 (s, 1H, COOH). Anal. Calcd. for C7H11NO3 (157.0): C, 53.50; H, 7.01; N, 8.92. Found: C, 53.32; H, 7.41; N, 8.86%. 1H NMR of Zn(II) complex (DMSO-d6, δ, ppm): 2.08 (s, 6H, CH3), 2.98 (t, 2H, CH2), 5.37 (t, 1H, CH), 7.48 (s, 1H, azomethine), 10.58 (s, 1H, OH), 11.36 (s, 4H, OH2).
{[2-(3-Hydroxy-1-methylbutyl)-2-en-1-ylidene]amino}-3-phenylpropanoic acid (L2)
Yield 56%; mp 242°C; IR (KBr, cm−1): 3444 (OH), 3049 (C=C), 1703 (COOH), 1635 (azomethine, C=N); 1H NMR (DMSO-d6, δ, ppm): 1H NMR (DMSO-d6, δ, ppm): 1.75 (s, 6H, CH3), 2.53 (t, 2H, CH2), 3.18 (t, 1H, CH2), 3.73 (t, 2H, CH2), 6.67 (s, 1H, azomethine), 7.16–7.79 (m, 5H, Ph), 10.27 (s, 1H, OH), 11.29 (s, 1H, COOH). Anal. Calcd. for C14H19NO2 (233.0): C, 68.02; H, 6.88; N, 5.67. Found: C, 68.33; H, 7.15; N, 5.83%. 1H NMR of Zn(II) complex (DMSO-d6, δ, ppm): 1.97 (s, 6H, CH3), 2.86 (t, 2H, CH2), 3.41 (t, 1H, CH2), 3.96 (t, 2H, CH2), 7.51 (s, 1H, azomethine), 7.36–7.93 (m, 5H, Ph), 10.58 (s, 1H, OH), 11.36 (s, 4H, OH2).
{[2-(3-Hydroxy-1-methylbutyl)-2-en-1-ylidene]amino}-3-methylbutanoic acid (L3)
Yield 54%; mp 210°C; IR (KBr, cm−1): 3444 (OH), 3049 (C=C), 1708 (COOH), 1635 (azomethine, C=N); 1H NMR (DMSO-d6, δ, ppm): 1.88 (s, 12H, CH3), 3.16 (t, 1H, CH), 3.73 (t, 1H, CH), 5.52 (t, 1H, CH), 10.27 (s, 1H, OH), 11.29 (s, 1H, COOH). Anal. Calcd. for C10H17NO3 (199.0): C, 60.30; H, 8.54; N, 7.04. Found: C, 60.64; H, 8.37; N, 7.46%. 1H NMR of Zn(II) complex (DMSO-d6, δ, ppm): 2.03 (s, 12H, CH3), 3.37 (t, 1H, CH), 3.96 (t, 1H, CH), 5.87 (t, 1H, CH), 10.56 (s, 1H, OH), 11.36 (s, 4H, OH2).
{[2-(3-Hydroxy-1-methylbutyl)-2-en-1-ylidene]amino}-3-(imidazol-4-yl) propanoic acid (L4)
Yield 51%; mp 194°C; IR (KBr, cm−1): 3444 (OH), 3045 (C=C), 1705 (COOH), 1635 (azomethine, C=N); 1H NMR (DMSO-d6, δ, ppm): 1H NMR (DMSO-d6, δ, ppm): 1.75 (s, 6H, CH3), 3.36 (t, 1H, CH), 3.78 (s, 1H, CH), 7.96 (s, 1H, imidazol), 8.26 (d, 1H, imidazol), 10.27 (s, 1H, OH), 10.84 (s, 1H, NH), 11.29 (s, 1H, COOH). Anal. Calcd. for C10H13N3O3 (223.0): C, 55.23; H, 7.11; N, 17.53. Found: C, 55.53; H, 7.38; N, 17.26%; 1H NMR of Zn(II) complex (DMSO-d6, δ, ppm): 2.07 (s, 6H, CH3), 3.58 (t, 1H, CH), 3.94 (s, 1H, CH), 8.25 (s, 1H, imidazol), 8.47 (dd, 1H, imidazol), 10.58 (s, 1H, OH), 11.13 (s, 1H, NH), 11.36 (s, 4H, OH2).
{[2-(3-Hydroxy-1-methylbutyl)-2-en-1-ylidene]amino}propanoic acid (L5)
Yield 53%; mp 160°C; IR (KBr, cm−1): 3444 (OH), 3018 (C=C), 1700 (COOH), 1635 (azomethine, C=N); 1H NMR (DMSO-d6, δ, ppm): 1.85 (s, 9H, CH3), 5.18 (t, 1H, CH), 5.34 (t, 1H, CH), 10.27 (s, 1H, OH), 11.29 (s, 1H, COOH). Anal. Calcd. for C8H13NO3 (171.0): C, 47.76; H, 7.46; N, 20.90. Found: C, 47.57; H, 7.28; N, 20.77%. 1H NMR of Zn(II) complex (DMSO-d6, δ, ppm): 2.12 (s, 9H, CH3), 5.41 (t, 1H, CH), 5.63 (t, 1H, CH), 10.58 (s, 1H, OH), 11.36 (s, 4H, OH2).
Preparation of metal(II) complexes
For the preparation of metal(II) complexes, a solution (30 mL) of the corresponding ligand in hot methanol was added to a stirred solution of metal(II) chloride in ethanol (25 mL) having a required molar ratio of M : L (1 : 1 and 1 : 2). The mixture was refluxed for 3 hours and then cooled to room temperature which solidified on cooling. The solid thus obtained was filtered, washed with methanol/ethanol and ether, and finally dried in air to afford the desired product. Crystallization from aqueous/ethanol (40 : 60) gave the expected metal complex.
BIOLOGICAL ACTIVITY
Antibacterial bioassay (in vitro)
All the synthesized ligands (L1)–(L5) and their corresponding metal(II) complexes (1)–(20) were screened in vitro for their antibacterial activity against four Gram-negative (E coli, S flexenari, P aeruginosa, and S typhi) and two Gram-positive (B subtilis and S aureus) bacterial strains using agar-well diffusion method [39]. Two to eight hours old bacterial inoculums containing approximately 104–106 colony forming units (CFU)/mL were used in these assays. The wells were dug in the media with the help of a sterile metallic borer with centers at least 24 mm. Recommended concentration (100 μl) of the test sample (1 mg/mL in DMSO) was introduced in the respective wells. Other wells supplemented with DMSO and reference antibacterial drug, imipenum served as negative and positive controls, respectively. The plates were incubated immediately at 37°C for 20 hours. Activity was determined by measuring the diameter of zones showing complete inhibition (mm). Growth inhibition was compared [40] with the standard drug. In order to clarify any participating role of DMSO in the biological screening, separate studies were carried out with the solutions alone of DMSO and they showed no activity against any bacterial strains.
Antifungal activity (in vitro)
Antifungal activities of all compounds were studied against six fungal cultures, T longifusus, C albicans, A flavus, M canis, F solani, and C glaberata. Sabouraud dextrose agar (Oxoid, Hampshire, England) was seeded with 105 (cfu) mL−1 fungal spore suspensions and was transferred to petri plates. Discs soaked in 20 mL (10 μg/mL in DMSO) of all compounds were placed at different positions on the agar surface. The plates were incubated at 32°C for seven days. The results were recorded as zones of inhibition in mm and were compared with standard drugs Miconazole and Amphotericin B.
Minimum inhibitory concentration (MIC)
Compounds containing antibacterial activity over 80% were selected for minimum inhibitory concentration (MIC) studies (Table 5). The minimum inhibitory concentration was determined using the disc diffusion technique [39] by preparing discs containing 10, 25, 50, and 100 μg/mL of the compounds and applying the protocol.
Table 5.
Results of minimum inhibitory concentration (M/mL) of the selected compounds (4), (12), (20), (24), and (40) against selected bacteria.
| Number | 4 | 12 | 20 | 24 | 40 |
|
| |||||
| Gram-negative | |||||
| E coli | — | — | 5.690 × 10−8 | — | — |
| P aeruginosa | 1.215 × 10−7 | — | — | — | — |
| S typhi | — | 5.046 × 10−8 | — | — | — |
|
| |||||
| Gram-positive | |||||
| S aureus | — | — | — | — | 2.933 × 10−8 |
| B subtilis | — | — | — | 7.648 × 10−8 | — |
Cytotoxicity (in vitro)
Brine shrimp (Artemia salina leach) eggs were hatched in a shallow rectangular plastic dish (22×32 cm), filled with artificial seawater, which was prepared [24] with commercial salt mixture and double distilled water. An unequal partition was made in the plastic dish with the help of a perforated device. Approximately 50 mg of eggs were sprinkled into the large compartment, which was darkened while the matter compartment was opened to ordinary light. After two days, nauplii were collected by a pipette from the lighted side. A sample of the test compound was prepared by dissolving 20 mg of each compound in 2 mL of DMF. From this stock solutions, 500, 50, and 5 μg/mL were transferred to 9 vials (three for each dilution were used for each test sample and LD50 is the mean of three values) and one vial was kept as control having 2 mL of DMF only. The solvent was allowed to evaporate overnight. After two days, when shrimp larvae were ready, 1 mL of seawater and 10 shrimps were added to each vial (30 shrimps/dilution) and the volume was adjusted with seawater to 5 mL per vial. After 24 hours, the numbers of survivors were counted. Data were analyzed by Finney computer program to determine the LD50 values [41].
RESULT AND DISCUSSION
Physicochemical properties of obtained compounds
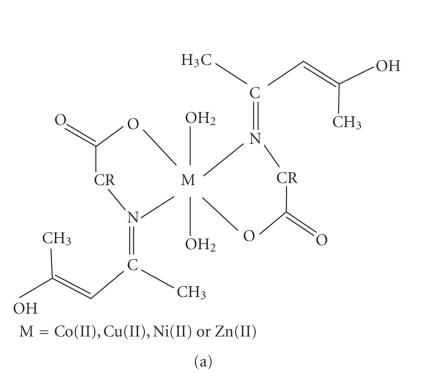
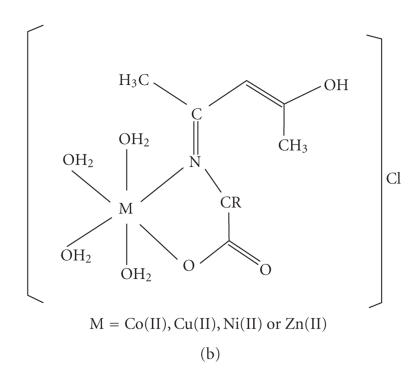
The ligands (L1)–(L5) were prepared by refluxing an appropriate amount of respective amino acid with the corresponding acetylacetone in ethanol. The structures of the synthesized ligands were established with the help of their IR, NMR, and microanalytical data. All metal(II) complexes (1)–(40) of these ligands were prepared by using the respective metal salts as chloride with the corresponding ligands in two different molar ratios of metal : ligand as 1 : 2 and 1 : 1. All these complexes are intensively colored air and moisture stable amorphous solids which decompose without melting. They are insoluble in common organic solvents and only soluble in water, DMF, and DMSO. Molar conductance values of the soluble complexes in DMF (10−3 M solution at 25°C) indicated that complexes having molar ratio of metal : ligand as 1 : 2 have lower values (26–35 Ohm−1 cm−2 mol−1) indicating that they are all nonelectrolytic in nature. However, the complexes having molar ratio of metal : ligand as 1 : 1 showed higher values (122–128 Ohm−1 cm−2 mol−1) indicating them as electrolytic [42]. The elemental analyses data (Table 1) agree well with the proposed formulae for the ligands and also confirmed the [M(L)2(OH2)2] (Figure 2(a)) and [M(L)(OH2)4]Cl (Figure 2(b)) composition of the metal(II) chelates. Efforts to grow good crystals of the ligands and their metal chelates for X-ray diffraction studies were unsuccessful due to their poor solubility in common organic solvents.
Table 1.
Physical and analytical data of the metal(II) complexes (1)–(40).
| Number | Metal chelate | MP (°C) | Yield (%) | Calc (found) % | ||
| C | H | N | ||||
|
| ||||||
| (1) | [Co(L1)2(H2O)2] [406.9] | 336–338 | 71 | 41.28 (41.61) | 5.90 (5.42) | 6.88 (6.13) |
| C14H24CoN2O8 | ||||||
| (2) | [Cu(L1)2(H2O)2] [411.5] | 328–330 | 73 | 40.82 (40.44) | 5.83 (5.52) | 6.80 (6.45) |
| C14H24CuN2O8 | ||||||
| (3) | [Ni(L1)2(H2O)2] [406.7] | 330–332 | 70 | 41.31 (41.65) | 5.90 (5.98) | 6.88 (6.57) |
| C14H24NiN2O8 | ||||||
| (4) | [Zn(L1)2(H2O)2] [411.4] | 331–332 | 70 | 40.84 (40.63) | 5.83 (5.62) | 6.81 (6.96) |
| C14H24ZnN2O8 | ||||||
| (5) | [Co(L2)2(H2O)2] [586.9] | 378–380 | 72 | 57.25 (57.53) | 6.13 (6.55) | 4.77 (4.63) |
| C28H36CoN2O8 | ||||||
| (6) | [Cu(L2)2(H2O)2] [563.5] | 335–337 | 72 | 56.80 (56.66) | 6.09 (6.37) | 4.73 (4.58) |
| C28H36CuN2O8 | ||||||
| (7) | [Ni(L2)2(H2O)2] [586.7] | 338–340 | 73 | 57.27 (57.14) | 6.14 (6.47) | 4.77 (4.84) |
| C28H36NiN2O8 | ||||||
| (8) | [Zn(L2)2(H2O)2] [591.4] | 332–334 | 72 | 56.82 (56.98) | 6.09 (5.84) | 4.73 (4.65) |
| C28H36ZnN2O8 | ||||||
| (9) | [Co(L3)2(H2O)2] [490.9] | 339–341 | 74 | 48.89 (48.73) | 7.33 (7.62) | 5.70 (5.53) |
| C20H36CoN2O8 | ||||||
| (10) | [Cu(L3)2(H2O)2] [495.5] | 344–346 | 73 | 48.43 (48.87) | 7.26 (7.18) | 5.65 (5.85) |
| C20H36CuN2O8 | ||||||
| (11) | [Ni(L3)2(H2O)2] [490.7] | 340–342 | 73 | 48.91 (48.76) | 7.34 (7.58) | 5.71 (5.43) |
| C20H36NiN2O8 | ||||||
| (12) | [Zn(L3)2(H2O)2] [495.4] | 337–339 | 72 | 48.45 (48.63) | 7.27 (7.47) | 5.65 (5.96) |
| C20H36ZnN2O8 | ||||||
| (13) | [Co(L4)2(H2O)2] [566.9] | 238–240 | 72 | 46.57 (46.66) | 5.64 (5.53) | 14.82 (14.72) |
| C22H32CoN6O8 | ||||||
| (14) | [Cu(L4)2(H2O)2] [571.5] | 230–232 | 70 | 46.19 (46.54) | 5.60 (5.43) | 14.70 (14.57) |
| C22H32CuN6O8 | ||||||
| (15) | [Ni(L4)2(H2O)2] [566.7] | 227–229 | 71 | 46.59 (46.62) | 5.65 (5.57) | 14.82 (14.66) |
| C22H32NiN6O8 | ||||||
| (16) | [Zn(L4)2(H2O)2] [571.4] | 225–227 | 72 | 46.20 (46.06) | 5.60 (5.81) | 14.70 (14.98) |
| C22H32ZnN6O8 | ||||||
| (17) | [Co(L5)2(H2O)2] [434.9] | 240–242 | 73 | 44.15 (44.48) | 6.44 (6.16) | 6.44 (6.82) |
| C16H28CoN2O8 | ||||||
| (18) | [Cu(L5)2(H2O)2] [439.5] | 244–246 | 72 | 43.68 (43.36) | 6.37 (6.56) | 6.37 (6.73) |
| C16H28CuN2O8 | ||||||
| (19) | [Ni(L5)2(H2O)2] [434.7] | 245–247 | 70 | 44.16 (44.44) | 6.44 (6.38) | 6.44 (6.16) |
| C16H28NiN2O8 | ||||||
| (20) | [Zn(L5)2(H2O)2] [439.4] | 236–238 | 69 | 43.70 (43.34) | 6.37 (6.15) | 6.37 (6.62) |
| C16H28ZnN2O8 | ||||||
| (21) | [Co(L1)(H2O)4]Cl [322.4] | 206–208 | 70 | 26.05 (26.37) | 5.58 (5.41) | 4.34 (4.13) |
| C7H18CoNO7Cl | ||||||
| (22) | [Cu(L1)(H2O)4]Cl [327.0] | 216–218 | 71 | 25.68 (25.44) | 5.50 (5.82) | 4.28 (4.45) |
| C7H18CuNO7Cl | ||||||
| (23) | [Ni(L1)(H2O)4]Cl [322.2] | 212–214 | 72 | 26.07 (26.38) | 5.59 (5.88) | 4.35 (4.54) |
| C7H18NiNO7Cl | ||||||
| (24) | [Zn(L1)(H2O)4]Cl [326.9] | 202–204 | 70 | 25.70 (25.53) | 5.51 (5.62) | 4.28 (4.11) |
| C7H18ZnNO7Cl | ||||||
| (25) | [Co(L2)(H2O)4]Cl [412.4] | 218–220 | 73 | 40.73 (40.93) | 5.82 (5.55) | 3.39 (3.18) |
| C14H24CoNO7Cl | ||||||
| (26) | [Cu(L2)(H2O)4]Cl [417] | 227–229 | 72 | 40.28 (40.46) | 5.75 (5.64) | 3.36 (3.67) |
| C14H24CuNO7Cl | ||||||
| (27) | [Ni(L2)(H2O)4]Cl [412.2] | 220–222 | 73 | 40.76 (40.43) | 5.82 (5.64) | 3.40 (3.13) |
| C14H24NiNO7Cl | ||||||
| (28) | [Zn(L2)(H2O)4]Cl [416.9] | 214–216 | 72 | 40.30 (40.48) | 5.76 (5.40) | 3.36 (3.58) |
| C14H24ZnNO7Cl | ||||||
| (29) | [Co(L3)(H2O)4]Cl [364.4] | 230–232 | 70 | 32.93 (32.67) | 6.59 (6.35) | 3.84 (3.53) |
| C10H24CoNO7Cl | ||||||
| (30) | [Cu(L3)(H2O)4]Cl [369.0] | 238–240 | 71 | 32.52 (32.84) | 6.50 (6.18) | 3.79 (3.88) |
| C10H24CuNO7Cl | ||||||
| (31) | [Ni(L3)(H2O)4]Cl [364.2] | 240–242 | 72 | 32.95 (33.28) | 6.59 (6.34) | 3.84 (3.63) |
| C10H24NiNO7Cl | ||||||
| (32) | [Zn(L3)(H2O)4]Cl [368.9] | 235–237 | 73 | 32.53 (32.43) | 6.51 (6.87) | 3.80 (3.96) |
| C10H24ZnNO7Cl | ||||||
| (33) | [Co(L4)(H2O)4]Cl [402.4] | 233–235 | 73 | 32.80 (32.66) | 5.47 (5.53) | 10.44 (10.72) |
| C11H22CoN3O7Cl | ||||||
| (34) | [Cu(L4)(H2O)4]Cl [407.0] | 235–237 | 74 | 32.43 (32.64) | 5.40 (5.27) | 10.32 (10.57) |
| C11H22CuN3O7Cl | ||||||
| (35) | [Ni(L4)(H2O)4]Cl [402.2] | 220–222 | 73 | 32.82 (32.58) | 5.47 (5.65) | 10.44 (10.68) |
| C11H22NiN3O7Cl | ||||||
| (36) | [Zn(L4)(H2O)4]Cl [406.9] | 238–240 | 72 | 32.44 (32.06) | 5.41 (5.83) | 10.32 (10.78) |
| C11H22ZnN3O7Cl | ||||||
| (37) | [Co(L5)(H2O)4]Cl [336.4] | 244–246 | 73 | 28.53 (28.68) | 5.94 (5.64) | 4.16 (4.52) |
| C8H20CoNO7Cl | ||||||
| (38) | [Cu(L5)(H2O)4]Cl [341.0] | 248–250 | 72 | 28.15 (28.36) | 5.86 (5.56) | 4.11 (4.43) |
| C8H20CuNO7Cl | ||||||
| (39) | [Ni(L5)(H2O)4]Cl [336.2] | 244–246 | 73 | 28.56 (28.74) | 5.95 (5.78) | 4.16 (4.56) |
| C8H20NiNO7Cl | ||||||
| (40) | [Zn(L5)(H2O)4]Cl [340.9] | 247–249 | 72 | 28.16 (28.48) | 5.87 (5.65) | 4.11 (4.42) |
| C8H20ZnNO7Cl | ||||||
Figure 2.
Proposed structures of the metal(II) complexes (1)–(40).
IR spectra
Diketones and related compounds such as acetylacetone in the present studies are capable of exhibiting keto-enol tautomerism and react with metal cations to form metal complexes. The selected IR spectra of the ligands and its metal(II) complexes along with their tentative assignments are reported in “experimental” and in Table 2, respectively. The IR spectra of all the ligands show [43] the absence of bands at 3245 and 1745 cm−1 due to ν(HN2) group of amino acids and ν(C=O) of acetylacetone. Instead, a new prominent band at 1635 cm−1 due to azomethine ν(C=N) linkage appeared in all the ligands indicating [44] that condensation between ketone moiety of acetylacetone and that of amino group of amino acid has taken place resulting into the formation of the desired ligands (L1)–(L5). Also, the presence of bands at 3015–3025 and 3444–3450 cm−1 due to ν(C=C) and ν(OH) in the ligands clearly gave an evidence [43] of establishing keto-enol tautomeric system in which these ligands behave as enol. Moreover, on comparison of the IR spectra of the ligands with their metal(II) complexes showed [45] a major shift to lower wave numbers by 15–20 cm−1 in azomethine ν(C=N) at 1610–1620 cm−1 suggesting involvement of the azomethine-N with the metal(II) ion. Also, disappearance of the stretching frequency at 1700–1708 cm−1 assigned to ν(COOH) and appearance of new ν as and ν s modes of the (−CO2) group at 1590 and 1385 cm−1, respectively, the Δν value (205 cm−1) is consistent with carboxylate coordination with the metal atoms. These overall data suggest that the azomethine-N and carboxylate-O groups are involved in coordination with the metal(II) ion in complexes (1)–(40). In the low-frequency region, spectra of the metal(II) complexes (Table 1) exhibited [46] new bands which are not present in the spectra of the ligands. These bands are located at 525 and 470 cm−1, which are attributed to ν(M−O) and ν(M−N). The coordinated water in all the metal(II) complexes presents different peaks at 990 cm−1 (rocking) and 760 cm−1 (wagging), whereas none of these vibrations appear in the spectra of uncoordinated ligands.
Table 2.
Physical and spectral data of the metal(II) complexes (1)–(40).
| Number | Color | BM (μ eff) | IR ( cm−1) | λ max ( cm−1) |
|
| ||||
| (1) | Dark brown | 4.4 | 3444 (OH), 3020 (OH2), | 17543, 21739, 29290 |
| 1610 (C=N), 1385 (C−O), | ||||
| 525 (M−O), 470 (M−N) | ||||
| (2) | Light blue | 1.7 | 3450 (OH), 3025 (OH2), | 15151, 30235 |
| 1620 (C=N), 1335 (C−O), | ||||
| 440 (M−N), 520 (M−O) | ||||
| (3) | Dull green | 3.1 | 3445 (OH), 3015 (OH2), | 12897, 16528, 24390, 30215 |
| 1615 (C=N), 1335 (C−O), | ||||
| 430 (M−N), 535 (M−O) | ||||
| (4) | Off-white | Dia | 3448 (OH), 3025 (OH2), | 28445 |
| 1610 (C=N), 1335 (C−O), | ||||
| 435 (M−N), 545 (M−O) | ||||
| (5) | Dark brown | 4.2 | 3444 (OH), 3025 (OH2), | 18018, 22222, 29565 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (6) | Dark blue | 1.7 | 3444 (OH), 3015 (OH2), | 15873, 30380 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (7) | Dark green | 3.1 | 3448 (OH), 3020 (OH2), | 13333, 16667, 25000, 30365 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (8) | Cream | Dia | 3445 (OH), 3020 (OH2), | 28680 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (9) | Brown | 4.5 | 3448 (OH), 3025 (OH2), | 17750, 21535, 29310 |
| 1610 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (10) | Bluish green | 1.8 | 3450 (OH), 3015 (OH2), | 15470, 30355 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (11) | Dark green | 3.3 | 3444 (OH), 3015 (OH2), | 12975, 16585, 24685, 30310 |
| 1610 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (12) | Pale yellow | Dia | 3450 (OH), 3020 (OH2), | 28525 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (13) | Tea pink | 4.3 | 3445 (OH), 3015 (OH2), | 17850, 21950, 29410 |
| 1610 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (14) | Green | 1.9 | 3448 (OH), 3025 (OH2), | 15510, 30290 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (15) | Sea green | 3.2 | 3445 (OH), 3025 (OH2), | 13230, 16660, 24880, 30360 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (16) | Off-white | Dia | 3444 (OH), 3020 (OH2), | 30360 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (17) | Dark brown | 4.5 | 3450 (OH), 3015 (OH2), | 17985, 22125, 29490 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (18) | Blue | 1.8 | 3450 (OH), 3020 (OH2), | 15750, 30360 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (19) | Dark green | 3.4 | 3444 (OH), 3020 (OH2), | 13215, 16575, 24910, 30355 |
| 1610 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (20) | Cream | Dia | 3445 (OH), 3020 (OH2), | 28610 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (21) | Dark blue | 4.2 | 3450 (OH), 3025 (OH2), | 18010, 21745, 29290 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (22) | Green | 1.7 | 3450 (OH), 3015 (OH2), | 15545, 30235 |
| 1610 (C=N), 1335 (C−O), | ||||
| 440 (M−N), 520 (M−O) | ||||
| (23) | Dirty green | 3.1 | 3450 (OH), 3015 (OH2), | 12897, 16580, 24490, 30215 |
| 1615 (C=N), 1335 (C−O), | ||||
| 430 (M−N), 535 (M−O) | ||||
| (24) | Off-white | Dia | 3450 (OH), 3025 (OH2), | 28445 |
| 1620 (C=N), 1335 (C−O), | ||||
| 435 (M−N), 545 (M−O) | ||||
| (25) | Dark blue | 4.4 | 3448 (OH), 3020 (OH2), | 17500, 22124, 29565 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (26) | Dirty green | 1.7 | 3450 (OH), 3025 (OH2), | 15795, 30380 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (27) | Sea green | 3.1 | 3448 (OH), 3015 (OH2), | 13233, 16590, 25000, 30365 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (28) | Pale yellow | Dia | 3450 (OH), 3020 (OH2), | 28680 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (29) | Royal blue | 4.5 | 3450 (OH), 3025 (OH2), | 17750, 21995, 29310 |
| 1610 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (30) | Green | 1.8 | 3448 (OH), 3015 (OH2), | 15490, 30355 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (31) | Dull green | 3.3 | 3448 (OH), 3020 (OH2), | 12995, 16655, 24685, 30310 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (32) | Yellow | Dia | 3450 (OH), 3025 (OH2), | 28525 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (33) | Purple blue | 4.3 | 3450 (OH), 3025 (OH2), | 17855, 21925, 29410 |
| 1610 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (34) | Bluish green | 1.9 | 3448 (OH), 3015 (OH2), | 15515, 30290 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (35) | Dirty green | 3.2 | 3450 (OH), 3020 (OH2), | 13130, 16565, 24880, 30360 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (36) | Pale yellow | Dia | 3450 (OH), 3025 (OH2), | 30360 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (37) | Dark brown | 4.5 | 3448 (OH), 3015 (OH2), | 17985, 22125, 29490 |
| 1615 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (38) | Green | 1.8 | 3450 (OH), 3020 (OH2), | 15750, 30360 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (39) | Light green | 3.4 | 3448 (OH), 3020 (OH2), | 13215, 16570, 24910, 30355 |
| 1610 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
| (40) | Cream | Dia | 3450 (OH), 3015 (OH2), | 28610 |
| 1620 (C=N), 1335 (C−O), | ||||
| 425 (M−O), 390 (M−N) | ||||
NMR spectra
The 1H NMR spectral data are reported along with the possible assignments in “experimental.” All the protons were found as to be in their expected region [47]. The conclusions drawn from these studies lend further support to the mode of bonding discussed in their IR spectra. In the spectra of diamagnetic Zn(II) complexes, coordination of the ligands via azomethine-N and carboxylate-O was established by downfield shifting of these signals in the Zn(II) complexes due to the increased conjugation and coordination [48]. The number of protons calculated from the integration curves and those obtained from the values of the expected CHN analyses agree with each other. It was observed that DMSO did not have any coordinating effect neither on the spectra of the ligands nor on its metal complexes.
Electronic spectra
The Co(II) complexes exhibited well-resolved bands at 17543–18018 cm−1 and a strong high-energy band at 21739–22222 cm−1 (Table 2) and are assigned [49] to the transitions 4T1g(F)→4T2g(F), 4T1g(F)→4T1g(P) for a high-spin octahedral geometry [50]. A high-intensity band at 28565–29215 cm−1 was assigned to the metal to ligand charge transfer. The magnetic susceptibility measurements (4.7–4.9 BM) for the solid Co(II) complexes are also indicative of three unpaired electrons per Co(II) ion suggesting [51] consistency with their octahedral environment. The electronic spectra of the Cu(II) complexes (Table 2) showed two low-energy weak bands at 15151–15873 cm−1 and a strong high-energy band at 30255–30420 cm−1. The low-energy band in this position typically is expected for an octahedral configuration and may be assigned to 10 Dq corresponding to the transition 2Eg→2T2g [49]. The strong high-energy band, in turn, is assigned to metal → ligand charge transfer. Also, the magnetic moment values (1.9–2.2 BM) for the copper(II) are indicative of antiferromagnetic spin-spin interaction through molecular association. Hence, the copper(II) complexes appear to be in the octahedral geometry with d2 x–d2 y ground state [51]. The electronic spectra of the Ni(II) complexes showed d-d bands in the regions 24390–25000, 16528–16667, and 12987–13333 cm−1. These are assigned to the spin-allowed transitions 3A2g(F)→3T2g(F), 3A2g(F)→3T1g(F), and 3A2g(F)→3T1g(P), respectively, consistent with their well-defined octahedral configuration. The band at 29815–30335 cm−1 was assigned to metal → ligand charge transfer. The magnetic measurements (3.0–3.3 BM) showed two unpaired electrons per Ni(II) ion suggesting [52] also an octahedral geometry for the Ni(II) complexes. The electronic spectra of the Zn(II) complexes exhibited only a high-intensity band at 28 350–29 145 cm−1 and are assigned [49] to a ligand-metal charge transfer.
Biological activity
The antibacterial activity results presented in Table 3 show that the newly synthesized compounds (L1)–(L5) and their metal(II) complexes (1)–(40) possess biological activity. These new derivatives obtained by condensation of the amino group of amino acid with salicylaldehyde were screened for their antibacterial activity against E coli, B subtillis, S flexenari, S aureus, P aeruginosa, and S typhi and for antifungal activity (Table 4) against T longifusus, C albicans, A flavus, M canis, F solani, and C glaberata. These results exhibited markedly an enhancement in activity on coordination with the metal ions against one or more testing bacterial strains. This enhancement in the activity is rationalized on the basis of the structures of, (L1)–(L5) by possessing an additional azomethine (C=N) linkage which imports in elucidating the mechanism of transamination and resamination reactions in biological system [53, 54]. It has also been suggested [55–65] that the ligands with nitrogen and oxygen donor systems might inhibit enzyme production, since the enzymes which require these groups for their activity appear to be especially more susceptible to deactivation by the metal ions upon chelation. Chelation reduces the polarity [55–65] of the metal ion mainly because of the partial sharing of its positive charge with the donor groups and possibly the π-electron delocalization within the whole chelate ring system thus formed during coordination. This process of chelation thus increases the lipophilic nature of the central metal atom, which in turn favors its permeation through the lipoid layer of the membrane. This in turn is responsible for increasing the hydrophobic character and liposolubility of the molecule in crossing cell membrane of the microorganism, and hence enhances the biological utilization ratio and activity of the testing drug/compound.
Table 3.
Results of antibacterial bioassay (concentration used 1 mg/mL of DMSO). (a) E coli, (b) S flexenari, (c) P aeruginosa, (d) S typhi, (e) S aureus, (f) B subtilis 10 <: weak; > 10: moderate; > 16: significant.
| Bacteria | |||||||
|
|
|||||||
| Gram-negative | Gram-positive | ||||||
|
| |||||||
| (a) | (b) | (c) | (d) | (e) | (f) | ||
|
| |||||||
| Compound (zone of inhibition) | L1 | 12 | 07 | 13 | 11 | 16 | 15 |
| L2 | 14 | 07 | 14 | 14 | 15 | 16 | |
| L3 | 14 | 08 | 12 | 15 | 16 | 17 | |
| L4 | 13 | 05 | 14 | 14 | 17 | 14 | |
| L5 | 12 | 07 | 15 | 15 | 17 | 15 | |
| 1 | 16 | 10 | 16 | 16 | 18 | 17 | |
| 2 | 15 | 11 | 15 | 17 | 18 | 18 | |
| 3 | 15 | 10 | 17 | 18 | 18 | 18 | |
| 4 | 16 | 12 | 22 | 18 | 19 | 19 | |
| 5 | 15 | 10 | 17 | 18 | 19 | 18 | |
| 6 | 15 | 10 | 16 | 17 | 19 | 17 | |
| 7 | 16 | 11 | 17 | 18 | 20 | 18 | |
| 8 | 16 | 11 | 18 | 19 | 21 | 19 | |
| 9 | 17 | 10 | 17 | 17 | 18 | 18 | |
| 10 | 16 | 10 | 18 | 16 | 19 | 19 | |
| 11 | 17 | 11 | 16 | 17 | 19 | 18 | |
| 12 | 19 | 12 | 17 | 24 | 20 | 19 | |
| 13 | 16 | 10 | 16 | 19 | 19 | 18 | |
| 14 | 16 | 11 | 17 | 17 | 17 | 18 | |
| 15 | 17 | 10 | 18 | 18 | 18 | 17 | |
| 16 | 18 | 11 | 17 | 20 | 20 | 20 | |
| 17 | 14 | 09 | 17 | 17 | 18 | 18 | |
| 18 | 17 | 10 | 18 | 18 | 19 | 19 | |
| 19 | 19 | 09 | 16 | 18 | 19 | 19 | |
| 20 | 25 | 10 | 19 | 18 | 20 | 21 | |
| 21 | 12 | 07 | 13 | 12 | 15 | 17 | |
| 22 | 11 | 06 | 14 | 13 | 16 | 18 | |
| 23 | 12 | 06 | 12 | 12 | 17 | 16 | |
| 24 | 15 | 09 | 16 | 14 | 18 | 24 | |
| 25 | 12 | 08 | 14 | 13 | 16 | 16 | |
| 26 | 12 | 07 | 15 | 12 | 15 | 17 | |
| 27 | 14 | 08 | 14 | 12 | 17 | 19 | |
| 28 | 15 | 09 | 16 | 14 | 18 | 19 | |
| 29 | 11 | 08 | 12 | 12 | 14 | 15 | |
| 30 | 12 | 07 | 12 | 11 | 16 | 16 | |
| 31 | 13 | 07 | 14 | 13 | 15 | 16 | |
| 32 | 14 | 10 | 15 | 15 | 17 | 18 | |
| 33 | 13 | 08 | 14 | 14 | 16 | 17 | |
| 34 | 14 | 09 | 13 | 15 | 15 | 16 | |
| 35 | 12 | 07 | 14 | 15 | 16 | 17 | |
| 36 | 14 | 11 | 16 | 17 | 17 | 18 | |
| 37 | 11 | 09 | 15 | 14 | 15 | 18 | |
| 38 | 12 | 08 | 15 | 15 | 16 | 16 | |
| 39 | 13 | 09 | 14 | 16 | 17 | 17 | |
| 40 | 15 | 10 | 16 | 17 | 26 | 19 | |
| *SD | 30 | 27 | 26 | 27 | 30 | 28 | |
*SD: standard drug (Imipenem).
Table 4.
Results of antifungal bioassay (concentration used 200 μg/mL). (a) T longifucus, (b) C albicans, (c) A flavus, (d) M canis, (e) F solani, (f) C glaberata.
| Organism | |||||||
|
|
|||||||
| (a) | (b) | (c) | (d) | (e) | (f) | ||
|
| |||||||
| Compound (zone of inhibition) | L1 | 16 | 00 | 15 | 10 | 00 | 18 |
| L2 | 00 | 07 | 00 | 00 | 15 | 00 | |
| L3 | 17 | 00 | 00 | 00 | 00 | 00 | |
| L4 | 20 | 00 | 00 | 15 | 00 | 20 | |
| L5 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 1 | 17 | 00 | 18 | 15 | 00 | 20 | |
| 2 | 18 | 00 | 20 | 14 | 00 | 18 | |
| 3 | 20 | 00 | 19 | 12 | 00 | 19 | |
| 4 | 22 | 00 | 20 | 21 | 00 | 22 | |
| 5 | 00 | 10 | 00 | 00 | 17 | 00 | |
| 6 | 10 | 17 | 00 | 00 | 18 | 17 | |
| 7 | 00 | 15 | 00 | 00 | 18 | 00 | |
| 8 | 00 | 18 | 00 | 00 | 20 | 00 | |
| 9 | 19 | 00 | 00 | 00 | 00 | 00 | |
| 10 | 20 | 00 | 17 | 00 | 00 | 00 | |
| 11 | 22 | 00 | 00 | 00 | 00 | 00 | |
| 12 | 24 | 00 | 00 | 00 | 00 | 00 | |
| 13 | 22 | 00 | 00 | 00 | 00 | 00 | |
| 14 | 24 | 20 | 00 | 25 | 20 | 20 | |
| 15 | 23 | 00 | 00 | 00 | 00 | 00 | |
| 16 | 25 | 00 | 18 | 30 | 00 | 00 | |
| 17 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 18 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 19 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 20 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 21 | 00 | 00 | 00 | 19 | 00 | 00 | |
| 22 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 23 | 00 | 18 | 00 | 00 | 00 | 00 | |
| 24 | 20 | 00 | 00 | 00 | 24 | 18 | |
| 25 | 00 | 17 | 17 | 17 | 17 | 00 | |
| 26 | 00 | 00 | 15 | 00 | 00 | 17 | |
| 27 | 00 | 00 | 00 | 00 | 15 | 00 | |
| 28 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 29 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 30 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 31 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 32 | 00 | 20 | 00 | 19 | 00 | 00 | |
| 33 | 00 | 20 | 20 | 20 | 20 | 20 | |
| 34 | 00 | 00 | 00 | 00 | 00 | 20 | |
| 35 | 00 | 00 | 19 | 00 | 00 | 00 | |
| 36 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 37 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 38 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 39 | 00 | 00 | 00 | 00 | 00 | 00 | |
| 40 | 00 | 00 | 19 | 00 | 00 | 20 | |
| *SD | A | B | C | D | E | F | |
*SD = standard drugs MIC μg/mL; A = Miconazole (70 μg/mL: 1.6822 × 10−7 M), B = Miconazole (110.8 μg/mL: 2.6626 × 10−7 M), C = Amphotericin B (20 μg/mL: 2.1642×10−8 M), D=Miconazole (98.4 μg/mL: 2.3647 × 10−7 M), E = Miconazole (73.25 μg/mL: 1.7603 × 10−7 M), F = Miconazole (110.8 μg/mL: 2.66266 × 10−7 M).
Cytotoxic bioassay
All the synthesized compounds were screened for their cytotoxicity (brine shrimp bioassay) using the protocol of Meyer et al [66]. From the data recorded in Table 6, it is evident that only five compounds (3), (7), (10), (11), and (22) displayed potent cytotoxic activity as LD50 = 8.974 × 10−4, 7.022 × 10−4, 8.839×10−4, 7.133×10−4, and 9.725×10−4 M/mL, respectively, against Artemia salina while all other compounds were almost inactive for this assay.
Table 6.
Brine shrimp bioassay data of the ligands (L1)–(L5) and their metal(II) complexes (1)–(40).
| Compound | LD50 (M/mL) |
|
| |
| L1 | 6.369 × 10−3 |
| L2 | 4.292 × 10−3 |
| L3 | 5.025 × 10−3 |
| L4 | 4.484 × 10−3 |
| L5 | 5.848 × 10−3 |
| 1 | 2.458 × 10−3 |
| 2 | 2.430 × 10−3 |
| 3 | 8.975 × 10−4 |
| 4 | 2.431 × 10−3 |
| 5 | 1.704 × 10−3 |
| 6 | 1.691 × 10−3 |
| 7 | 7.022 × 10−4 |
| 8 | 1.691 × 10−3 |
| 9 | 2.037 × 10−3 |
| 10 | 8.839 × 10−4 |
| 11 | 7.133 × 10−4 |
| 12 | 2.018 × 10−3 |
| 13 | 1.764 × 10−3 |
| 14 | 1.750 × 10−3 |
| 15 | 1.765 × 10−3 |
| 16 | 1.750 × 10−3 |
| 17 | 2.299 × 10−3 |
| 18 | 2.275 × 10−3 |
| 19 | 2.300 × 10−3 |
| 20 | 2.276 × 10−3 |
| 21 | 3.102 × 10−3 |
| 22 | 9.725 × 10−4 |
| 23 | 3.104 × 10−3 |
| 24 | 3.059 × 10−3 |
| 25 | 2.425 × 10−3 |
| 26 | 2.398 × 10−3 |
| 27 | 2.426 × 10−3 |
| 28 | 2.399 × 10−3 |
| 29 | 2.744 × 10−3 |
| 30 | 2.710 × 10−3 |
| 31 | 1.112 × 10−3 |
| 32 | 2.711 × 10−3 |
| 33 | 2.485 × 10−3 |
| 34 | 2.457 × 10−3 |
| 35 | 2.486 × 10−3 |
| 36 | 2.458 × 10−3 |
| 37 | 2.973 × 10−3 |
| 38 | 1.246 × 10−3 |
| 39 | 2.974 × 10−3 |
| 40 | 2.933 × 10−3 |
CONCLUSION
The synthesized amino acid-derived compounds showed antibacterial/antifungal properties. In comparison, the cobalt (II), copper(II), nickel(II), and zinc(II) metal complexes of these compounds showed more activity against one or more bacterial/fungal strains, thus introducing a novel class of metal-based bactericidal and fungicidal agents.
ACKNOWLEDGMENT
We are grateful to HEJ Research Institute of Chemistry, University of Karachi, Pakistan, for providing us with help in taking NMR spectra and also antibacterial and antifungal assays.
References
- 1.Chohan ZH, Praveen M, Ghaffar A. Structural and biological behaviour of Co(II), Cu(II) and Ni(II) metal complexes of some amino acid derived Schiff-bases. Metal-Based Drugs. 1997;4(5):267–272. doi: 10.1155/MBD.1997.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chohan ZH, Scozzafava A, Supuran CT. Unsymmetrical 1,1′-disubstituted ferrocenes: synthesis of Co(II), Cu(II), Ni(II) and Zn(II) chelates of ferrocenyl -1-thiadiazolo-1′-tetrazole, -1-thiadiazolo-1′-triazole and -1-tetrazolo-1′-triazole with antimicrobial properties. Journal of Enzyme Inhibition and Medicinal Chemistry. 2002;17(4):261–266. doi: 10.1080/1475636021000006261. [DOI] [PubMed] [Google Scholar]
- 3.Chohan ZH, Kausar S. Synthesis, structural and biological studies of nickel(II), copper(II) and zinc(II) chelates with tridentate Schiff bases having NNO and NNS donor systems. Chemical and Pharmaceutical Bulletin. 1993;41(5):951–953. doi: 10.1248/cpb.41.951. [DOI] [PubMed] [Google Scholar]
- 4.Ul-Hassan M, Chohan ZH, Scozzafava A, Supuran CT. Carbonic anhydrase inhibitors: Schiff's bases of aromatic and heterocyclic sulfonamides and their metal complexes. Journal of Enzyme Inhibition and Medicinal Chemistry. 2004;19(3):263–267. doi: 10.1080/14756360410001689595. [DOI] [PubMed] [Google Scholar]
- 5.Ul-Hassan M, Chohan ZH, Supuran CT. Antibacterial Zn(II) compounds of Schiff bases derived from some benzothiazoles. Main Group Metal Chemistry. 2002;25(5):291–296. [Google Scholar]
- 6.Chohan ZH, Scozzafava A, Supuran CT. Zinc complexes of benzothiazole-derived Schiff bases with antibacterial activity. Journal of Enzyme Inhibition and Medicinal Chemistry. 2003;18(3):259–263. doi: 10.1080/1475636031000071817. [DOI] [PubMed] [Google Scholar]
- 7.Seven MJ, Johnson LA. Metal Binding in Medicine. 4th ed. Philadelphia, Pa: Lippincott; 1960. [Google Scholar]
- 8.Srivastava RS. Studies on some antifungal transition metal chelates of 2-(2-hydroxybenzylideneamino) benzimidazole. Indian Journal of Chemistry. 1990;29A:1024. [Google Scholar]
- 9.Patel VK, Vasanwala AM, Jejurkar CN. Synthesis of mixed Schiff base complexes of copper(II) and nickel(II) and their spectral, magnetic and antifungal studies. Indian Journal of Chemistry. 1989;28A:719. [Google Scholar]
- 10.Maggio F, Pellerito A, Pellerito L, Grimaudo S, Mansueto C, Vitturi R. Organometallic complexes with biological molecules II. Synthesis, solid-state characterization and in vivo cytotoxicity of diorganotin(IV)chloro and triorganotin(IV)chloro derivatives of penicillin G. Applied Organometallic Chemistry. 1994;8(1):71–85. [Google Scholar]
- 11.Vitturi R, Mansueto C, Gianguzza A, Maggio F, Pellerito A, Pellerito L. Organometallic complexes with biological molecules. III: in vivo cytotoxicity of diorganotin(IV)chloro and triorganotin(IV)chloro derivatives of penicillin g on chromosomes of aphanius fasciatus (pisces, cyprinodontiformes) Applied Organometallic Chemistry. 1994;8(6):509–515. [Google Scholar]
- 12.Pellerito L, Maggio F, Consigilo A, Pellerito A, Stocco GC, Gremaudo S. Organometallic complexes with biological molecules. IV: Di- and tri-organotin(IV)amoxicillin derivatives: solid-state and solution-phase spectroscopic investigations. Applied Organometallic Chemistry. 1995;9(3):227–239. [Google Scholar]
- 13.Vitturi R, Zava B, Colomba MS, Pellerito A, Maggio F, Pellerito L. Organometallic complexes with biological molecules. V: in vivo cytotoxicity of diorganotin(IV)-amoxicillin derivatives in mitotic chromosomes of rutilus rubilio (pisces, Cyprinidae) Applied Organometallic Chemistry. 1995;9(7):561–566. [Google Scholar]
- 14.Rosenberg B, VanCamp L. The successful regression of large solid sarcoma 180 tumors by platinum compounds. Cancer Research. 1970;30(6):1799–1802. [PubMed] [Google Scholar]
- 15.Cleare MJ, Hoeschele JD. Studies on the antitumor activity of group VIII transition metal complexes. I. Platinum (II) complexes. Bioinorganic Chemistry. 1973;2(3):187–210. [Google Scholar]
- 16.Narayanan VA, Nasr M, Paull KD. In: Tin Based Antitumour Drugs. Vol H 37. Berlin, Germany: Springer; 1990. NATO ASI Series. [Google Scholar]
- 17.Crowe AJ. In: Metal Based Antitumour Drugs. Vol. 1. London, UK: Freud; 1988. The antitumour activity of tin compounds; pp. 103–149. [Google Scholar]
- 18.Saxena AK. Organotin compounds: toxicology and biomedicinal applications. Applied Organometallic Chemistry. 1987;1(1):39–56. [Google Scholar]
- 19.Furst A. The Chemistry of Chelation in Cancer. 3rd ed. Springfield, Ill: Thomas; 1963. [Google Scholar]
- 20.Williams DR. Thermodynamic considerations in co-ordination. Part X. A potentiometric and calorimetric investigation of copper(II) histidine complexes in solution. Journal of the Chemical Society. Dalton Transactions. 1972;(7):790–797. [Google Scholar]
- 21.Jones AD, Williams DR. Thermodynamic considerations in co-ordination. Part IX. Heat capacity investigations into complex formation between some lanthanide(III) ions and histidine. Journal of the Chemical Society A. 1971:3159–3162. [Google Scholar]
- 22.Pratt JM. Inorganic Chemistry of Vitamin B12. London, UK: Academic Press; 1972. [Google Scholar]
- 23.Shapiro SK, Schlenk F. Transmethylation and Methionine Biosynthesis. Chicago, Ill: University of Chicago Press; 1965. [Google Scholar]
- 24.Sakyan I, Logoglu E, Arslan S, Sari N, Akiyan N. Antimicrobial activities of N-(2-hydroxy-1-naphthalidene)-amino acid (glycine, alanine, phenylalanine, histidine, tryptophane) Schiff bases and their manganese(III) complexes. Biometals. 2004;17(2):115–120. doi: 10.1023/b:biom.0000018380.34793.df. [DOI] [PubMed] [Google Scholar]
- 25.Sari N, Arslan S, Logoglu E, Sakiyan I. Antibacterial activities of some Amino acid Schiff bases. G.U.J.Sci. 2003;16(2):283–288. [Google Scholar]
- 26.Williams RJP. Role of transition metal ions in biological processes. Royal Institute of Chemistry Reviews. 1968;1(1):13–38. [Google Scholar]
- 27.Sigel H, McCormick DB. Discriminating behavior of metal ions and ligands with regard to their biological significance. Accounts of Chemical Research. 1970;3(6):201–208. [Google Scholar]
- 28.Sinn E, Harris CM. Schiff base metal complexes as ligands. Coordination Chemistry Reviews. 1969;4(4):391–422. [Google Scholar]
- 29.Stock JA. In: The Biology of Cancer. Chapter 9. London, UK: D. Van Nostrand; 1966. The chemotherapy of cancer; p. 176. [Google Scholar]
- 30.Clifford P, Singh S, Stjernswärd J, Klein G. Long-term survival of patients with Burkitt's lymphoma: an assessment of treatment and other factors which may relate to survival. Cancer Research. 1967;27(12):2578–2615. [PubMed] [Google Scholar]
- 31.Stock JA. Chemotherapy of cancer. Chemistry in Britain. 1970;6(1):11–16. [PubMed] [Google Scholar]
- 32.Maurya RC, Mishra DD, Pillai S. Studies on some mixed-ligand chelates of cobalt(II) involving acetoacetylarylamides and biologically active heterocyclic oxygen donors. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 1997;27(10):1453–1466. [Google Scholar]
- 33.Maurya RC, Verma R, Trivedi PK, Singh H. Synthesis, magnetic and spectral studies of some mixed-ligand chelates of Bis(2-hydroxyacetophenonato)copper(II) with 2-or 3-pyrazoline-5-one derivatives. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 1998;28(2):311–329. [Google Scholar]
- 34.Maurya RC, Mishra DD, Mukherjee S, Trivedi PK. Novel mixed-ligand derivatives of bis(acetylacetonato)-bis(methylacetoacetato) and bis(ethylacetoacetate)-nickel(II) with some potentially mono-, bi- and tri-dentate 2/3-pyrazoline-5-ones. Transition Metal Chemistry. 1991;16(5):524–527. [Google Scholar]
- 35.Maurya RC, Mishra DD, Choubey V, Khan IB. Studies on some novel mixed-ligand derivatives of bis(acetylacetonato)-, bis(methylacetoacetato)- and bis(ethylacetoacetato) cobalt(II) with 8-hydroxyquinoline sulfonamides and pyrazolone. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 1990;20:1103. [Google Scholar]
- 36.Joshi KC, Pathak VK. Metal chelates of fluorinated 1,3-diketones and related compounds. Coordination Chemistry Reviews. 1977;22(1-2):37–122. [Google Scholar]
- 37.Maurya RC, Mishra DD, Trivedi PK. Synthesis, magnetic and spectral studies of some mixed-ligand chelates of mercury(II) with acetoacetanilide/o-hydroxyacetophenone and 2/3-pyrazoline-5-one derivatives. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 1994;24:17. [Google Scholar]
- 38.Vogel AI. A Textbook of Quantitative Inorganic Analysis. 4th ed. London, UK: ELBS and Longman; 1978. [Google Scholar]
- 39.Atta-ur-Rahman, Choudhary MI, Thomsen WJ. Bioassay Techniques for Drug Development. Amsterdam, The Netherlands: Harwood Academic; 2001. [Google Scholar]
- 40.McLauglin JL, Chang CJ, Smith DL. “Bench top” bioassay for the discovery of bioactive natural products: an update. In: Atta-ur-Rahman, editor. Studies in Natural Products Chemistry. Vol. 9. Amsterdam, The Netherlands: Elsevier Science; 1991. pp. 383–409. [Google Scholar]
- 41.Finney DJ. Probit Analysis. 3rd ed. Cambridge, UK: Cambridge University Press; 1971. [Google Scholar]
- 42.Geary WJ. The use of conductivity measurements in organic solvents for the characterisation of coordination compounds. Coordination Chemistry Reviews. 1971;7(1):81–122. [Google Scholar]
- 43.Carlin RL. Transition Metal Chemistry. 2nd ed. New York, NY: Marcel Decker; 1965. [Google Scholar]
- 44.Bellamy LJ. The Infrared Spectra of Complex Molecules. New York, NY: John Wiley & Sons; 1971. [Google Scholar]
- 45.Ferrero JR. Low-Frequency Vibrations of Inorganic and Coordination Compound. New York, NY: John Wiley & Sons; 1971. [Google Scholar]
- 46.Nakamoto K. Infrared Spectra of Inorganic and Coordination Compounds. 2nd ed. New York, NY: Wiley Interscience; 1970. [Google Scholar]
- 47.Simmons WW. The Sadtler Handbook of Proton NMR Spectra. Philadelphia, Pa: Sadtler Research Laboratories; 1978. [Google Scholar]
- 48.Pasto DJ. Organic Structure Determination. London, UK: Prentice Hall; 1969. [Google Scholar]
- 49.Lever ABP, Lewis J, Nyholm RS. Square-planar bisethylenediamine-metal complexes. Journal of Chemical Society. 1963:2552. [Google Scholar]
- 50.Estes WE, Gavel DP, Hatfield WE, Hodgson DJ. Magnetic and structural characterization of dibromo- and dichlorobis(thiazole)copper(II) Inorganic Chemistry. 1978;17(6):1415–1421. [Google Scholar]
- 51.Ballhausen CJ. An Introduction to Ligand Field Theory. New York, NY: McGraw Hill; 1962. [Google Scholar]
- 52.Lever ABP. Inorganic Electronic Spectroscopy. Amsterdam, The Netherlands: Elsevier; 1984. [Google Scholar]
- 53.Lau KY, Mayr A, Cheung K-K. Synthesis of transition metal isocyanide complexes containing hydrogen bonding sites in peripheral locations. Inorganica Chimica Acta. 1999;285(2):223–232. [Google Scholar]
- 54.Dholakiya PP, Patel MN. Metal complexes: Preparation, magnetic, spectral, and biocidal studies of some mixed-ligand complexes with Schiff bases containing NO and NN donor atoms. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 2004;34(3):553–563. [Google Scholar]
- 55.Chohan ZH. Synthesis and biological properties of Cu(II) complexes with 1,1′-disubstituted ferrocenes. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 2004;34(5):833–846. [Google Scholar]
- 56.Chohan ZH, Supuran CT, Scozzafava A. Metalloantibiotics: synthesis and antibacterial activity of cobalt(II), copper(II), nickel(II) and zinc(II) complexes of kefzol. Journal of Enzyme Inhibition and Medicinal Chemistry. 2004;19(1):79–84. doi: 10.1080/14756360310001624939. [DOI] [PubMed] [Google Scholar]
- 57.Chohan ZH, Scozzafava A, Supuran CT. Synthesis of biologically active Co(II), Cu(II), Ni(II), and Zn(II) complexes of symmetrically 1,1′-disubstituted ferrocene-derived compounds. Synthesis and Reactivity in Inorganic and Metal-Organic Chemistry. 2003;33(2):241–257. [Google Scholar]
- 58.Chohan ZH. Antibacterial copper(II) complexes of 1,1′-symmetric ferrocene-derived Schiff-base ligands: studies of the effect of anions on their antibacterial properties. Applied Organometallic Chemistry. 2002;16(1):17–20. [Google Scholar]
- 59.Chohan ZH, Farooq MA, Scozzafava A, Supuran CT. Antibacterial schiff bases of oxalyl-hydrazine/diamide incorporating pyrrolyl and salicylyl moieties and of their zinc(II) complexes. Journal of Enzyme Inhibition and Medicinal Chemistry. 2002;17(1):1–7. doi: 10.1080/14756360290005598. [DOI] [PubMed] [Google Scholar]
- 60.Rehman SU, Chohan ZH, Gulnaz F, Supuran CT. In-vitro antibacterial, antifungal and cytotoxic activities of some coumarins and their metal complexes. Journal of Enzyme Inhibition and Medicinal Chemistry. 2005;20(4):333–340. doi: 10.1080/14756360500141911. [DOI] [PubMed] [Google Scholar]
- 61.Chohan ZH, Supuran CT. Organometallic compounds with biologically active molecules: in vitro antibacterial and antifungal activity of some 1,1′-(dicarbohydrazono) ferrocenes and their cobalt(II), copper(II), nickel(II) and zinc(II) complexes. Applied Organometallic Chemistry. 2005;19(12):1207–1214. [Google Scholar]
- 62.Chohan ZH, Supuran CT. In-vitro antibacterial and cytotoxic activity of cobalt (II), copper (II), nickel (II) and zinc (II) complexes of the antibiotic drug cephalothin (keflin) Journal of Enzyme Inhibition and Medicinal Chemistry. 2005;20(5):463–468. doi: 10.1080/10485250500219765. [DOI] [PubMed] [Google Scholar]
- 63.Chohan ZH, Supuran CT, Scozzafava A. Metal binding and antibacterial activity of ciprofloxacin complexes. Journal of Enzyme Inhibition and Medicinal Chemistry. 2005;20(3):303–307. doi: 10.1080/14756360310001624948. [DOI] [PubMed] [Google Scholar]
- 64.Chohan ZH. Antibacterial and antifungal ferrocene incorporated dithiothione and dithioketone compounds. Applied Organometallic Chemistry. 2006;20(2):112–116. [Google Scholar]
- 65.Chohan ZH, Arif M, Shafiq Z, Yaqub M, Supuran CT. In vitro antibacterial, antifungal & cytotoxic activity of some isonicotinoylhydrazide Schiff's bases and their cobalt (II), copper (II), nickel (II) and zinc (II) complexes. Journal of Enzyme Inhibition and Medicinal Chemistry. 2006;21(1):95–103. doi: 10.1080/14756360500456806. [DOI] [PubMed] [Google Scholar]
- 66.Meyer BN, Ferrigni NR, Putnam JE, Jacobsen LB, Nichols DE, McLaughlin JL. Brine shrimp: a convenient general bioassay for active plant constituents. Planta Medica. 1982;45(1):31–34. [PubMed] [Google Scholar]