Abstract
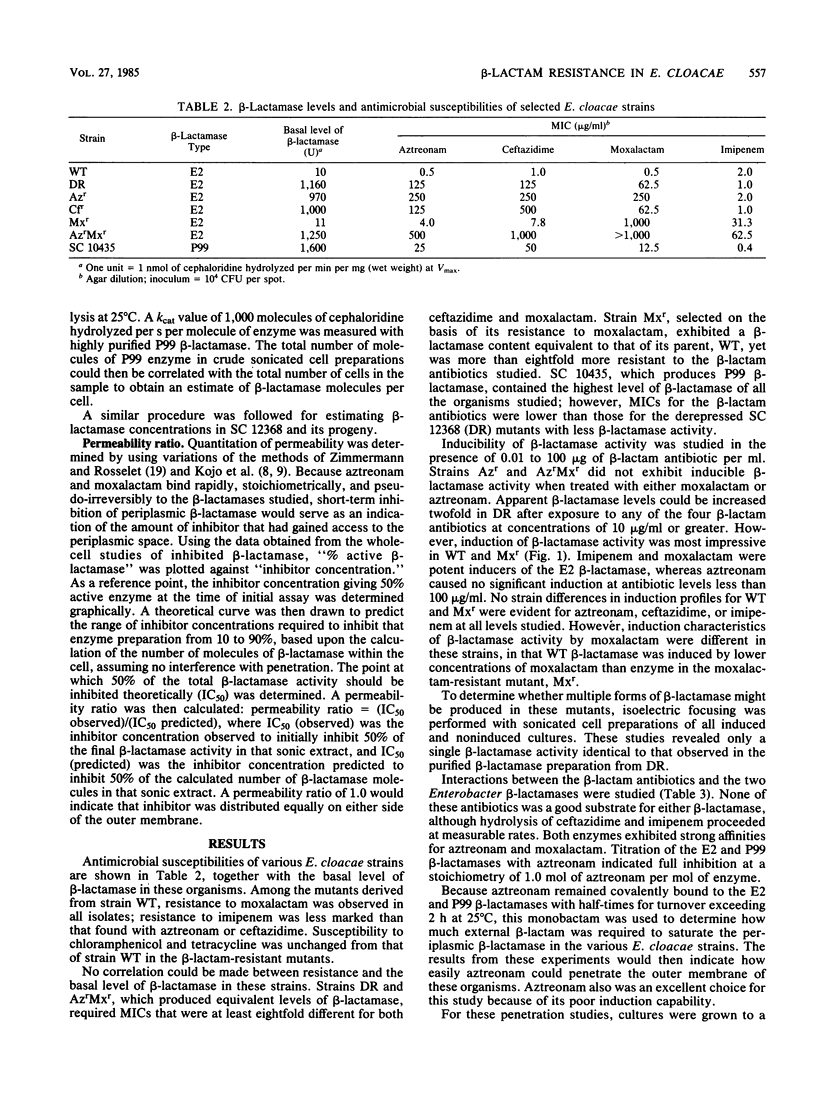
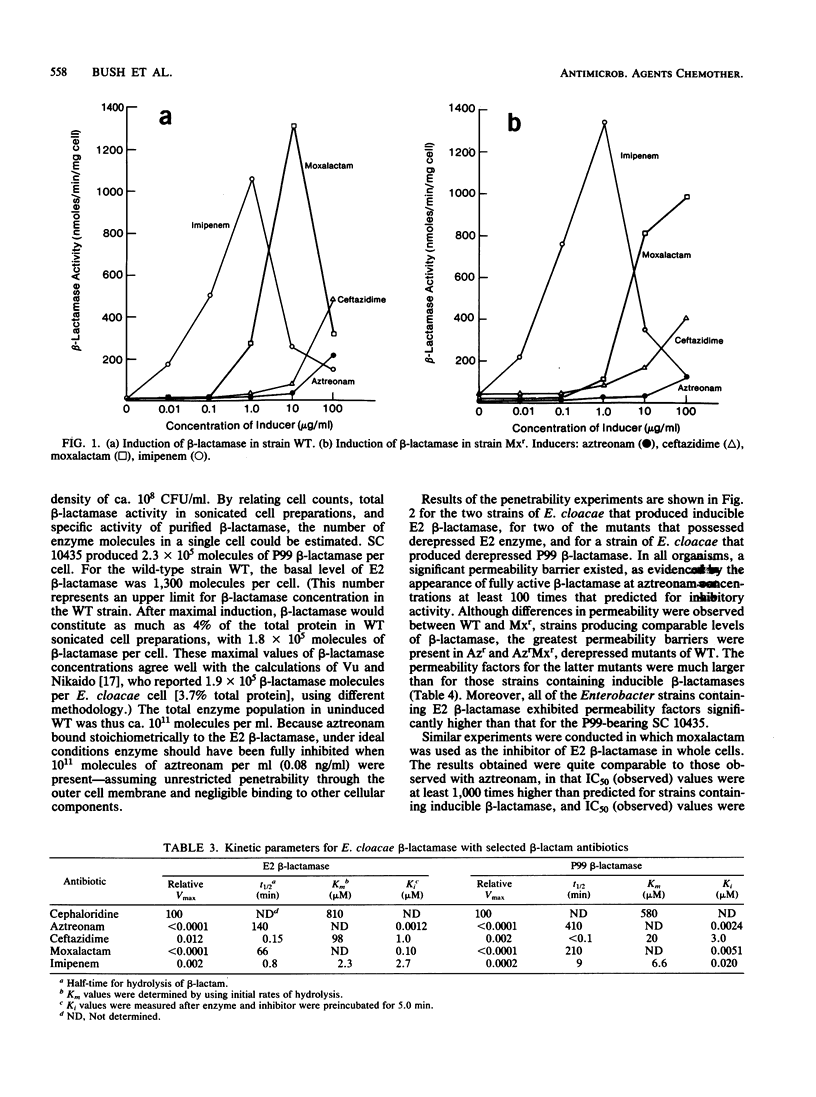
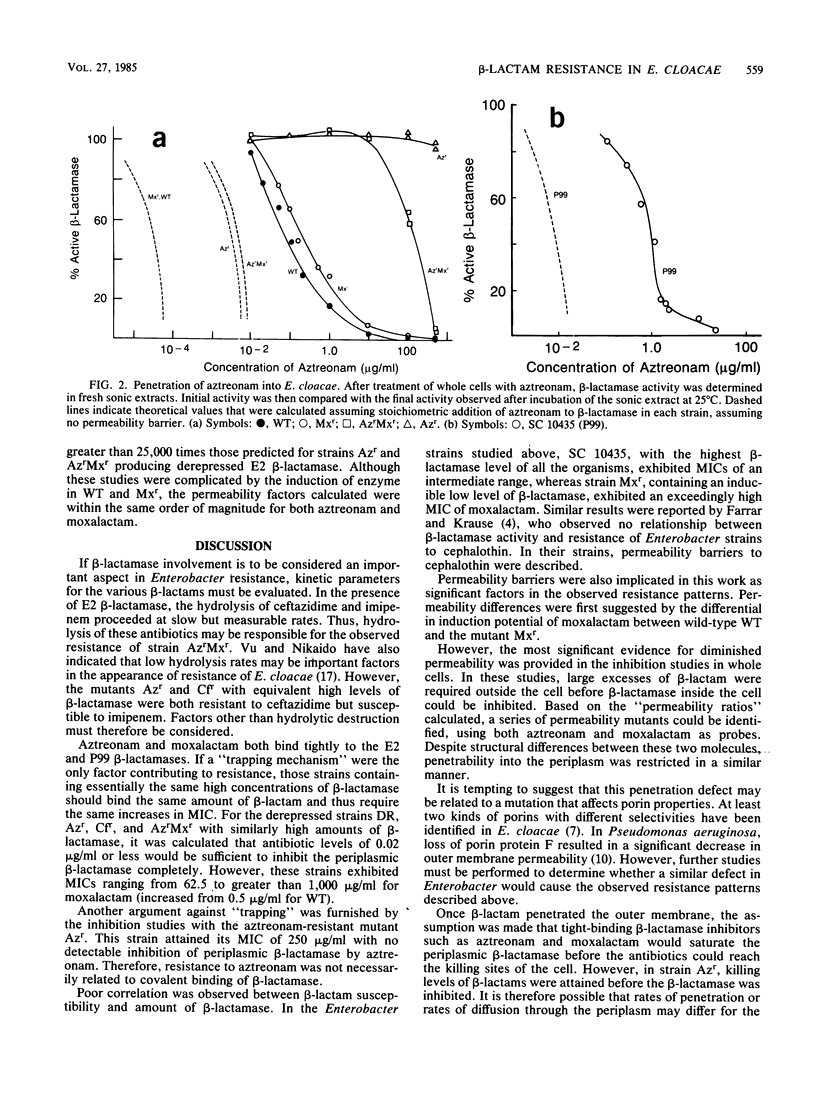
Strains of Enterobacter cloacae were selected on the basis of resistance to aztreonam, ceftazidime, moxalactam, or imipenem. All strains produced the same E2 beta-lactamase, with an isoelectric point greater than 9.5 and with high hydrolytic activity in the presence of cephaloridine. Resistance to beta-lactams could not be correlated with the amount of beta-lactamase present in the various strains. beta-Lactamase activity was induced strongly by moxalactam and imipenem in the wild-type and moxalactam-resistant strains, with beta-lactamase representing as much as 4% of the total cellular protein after induction (2 X 10(5) molecules per cell). Ceftazidime and aztreonam were poor inducers. None of the antibiotics studied was readily hydrolyzed by the E2 beta-lactamase; aztreonam and moxalactam inhibited the enzyme with apparent Ki values of 1.2 and 100 nM, respectively. Aztreonam, which bound covalently to the E2 beta-lactamase with a half-life of 2.3 h at 25 degrees C, was used to measure penetrability of beta-lactam into the periplasmic space of the resistant E. cloacae strains. In all of the E2-producing organisms studied, a significant permeability barrier existed. A maximum concentration of 0.02 microgram of aztreonam per ml should have saturated the periplasmic beta-lactamase in the highest enzyme producers studied. However, fully active beta-lactamase was observed in the periplasm of cells exposed to aztreonam at concentrations at least 1,000-fold higher than that theoretically necessary to inhibit the total enzyme within the cell. Thus, the major cause for resistance to beta-lactam antibiotics in these E. cloacae strains was lack of penetration across the outer membrane.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruderlein H., Daniel R., Perras D., DiPaola A., Fideliá A., Belleau B. An investigation of the destruction of the beta-lactam ring of penems by the albumin drug-binding site. Can J Biochem. 1981 Oct;59(10):857–866. doi: 10.1139/o81-118. [DOI] [PubMed] [Google Scholar]
- Bush K., Freudenberger J. S., Sykes R. B. Interaction of azthreonam and related monobactams with beta-lactamases from gram-negative bacteria. Antimicrob Agents Chemother. 1982 Sep;22(3):414–420. doi: 10.1128/aac.22.3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon M. The graphical determination of K m and K i . Biochem J. 1972 Aug;129(1):197–202. doi: 10.1042/bj1290197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Krause J. M. Relationship Between beta-Lactamase Activity and Resistance of Enterobacter to Cephalothin. Infect Immun. 1970 Nov;2(5):610–616. doi: 10.1128/iai.2.5.610-616.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gootz T. D., Sanders C. C., Goering R. V. Resistance to cefamandole: derepression of beta-lactamases by cefoxitin and mutation in Enterobacter cloacae. J Infect Dis. 1982 Jul;146(1):34–42. doi: 10.1093/infdis/146.1.34. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Yamaguchi A., Sawai T. Purification and characterization of two kinds of porins from the Enterobacter cloacae outer membrane. J Bacteriol. 1984 Jun;158(3):1179–1181. doi: 10.1128/jb.158.3.1179-1181.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kojo H., Shigi Y., Nishida M. A novel method for evaluating the outer membrane permeability to beta-lactamase-stable beta-lactam antibiotics. J Antibiot (Tokyo) 1980 Mar;33(3):310–316. doi: 10.7164/antibiotics.33.310. [DOI] [PubMed] [Google Scholar]
- Kojo H., Shigi Y., Nishida M. Enterobacter cloacae outer membrane permeability to ceftizoxime (FK 749) and five other new cephalosporin derivatives. J Antibiot (Tokyo) 1980 Mar;33(3):317–321. doi: 10.7164/antibiotics.33.317. [DOI] [PubMed] [Google Scholar]
- Nicas T. I., Hancock R. E. Pseudomonas aeruginosa outer membrane permeability: isolation of a porin protein F-deficient mutant. J Bacteriol. 1983 Jan;153(1):281–285. doi: 10.1128/jb.153.1.281-285.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross G. W., Boulton M. G. Purification of beta-lactamases on QAE-sephadex. Biochim Biophys Acta. 1973 Jun 6;309(2):430–439. doi: 10.1016/0005-2744(73)90041-7. [DOI] [PubMed] [Google Scholar]
- Sanders C. C., Sanders W. E., Jr, Goering R. V. In vitro antagonism of beta-lactam antibiotics by cefoxitin. Antimicrob Agents Chemother. 1982 Jun;21(6):968–975. doi: 10.1128/aac.21.6.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeberg A. H., Tolxdorff-Neutzling R. M., Wiedemann B. Chromosomal beta-lactamases of Enterobacter cloacae are responsible for resistance to third-generation cephalosporins. Antimicrob Agents Chemother. 1983 Jun;23(6):918–925. doi: 10.1128/aac.23.6.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sykes R. B., Bonner D. P., Bush K., Georgopapadakou N. H. Azthreonam (SQ 26,776), a synthetic monobactam specifically active against aerobic gram-negative bacteria. Antimicrob Agents Chemother. 1982 Jan;21(1):85–92. doi: 10.1128/aac.21.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Then R. L., Angehrn P. Trapping of nonhydrolyzable cephalosporins by cephalosporinases in Enterobacter cloacae and Pseudomonas aeruginosa as a possible resistance mechanism. Antimicrob Agents Chemother. 1982 May;21(5):711–717. doi: 10.1128/aac.21.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu H., Nikaido H. Role of beta-lactam hydrolysis in the mechanism of resistance of a beta-lactamase-constitutive Enterobacter cloacae strain to expanded-spectrum beta-lactams. Antimicrob Agents Chemother. 1985 Mar;27(3):393–398. doi: 10.1128/aac.27.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterworth P. M., Emmerson A. M. Dissociated resistance among cephalosporins. Antimicrob Agents Chemother. 1979 Apr;15(4):497–503. doi: 10.1128/aac.15.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann W., Rosselet A. Function of the outer membrane of Escherichia coli as a permeability barrier to beta-lactam antibiotics. Antimicrob Agents Chemother. 1977 Sep;12(3):368–372. doi: 10.1128/aac.12.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]


