Abstract
Background
We examined the effect of kidney disease (KD) on mortality in nondiabetic and diabetic Pima Indians aged ≥45 years old.
Methods
Deaths and person-years of follow-up were stratified in a time-dependent fashion into categories of (1) no proteinuria and normal serum creatinine (SCr); (2) proteinuria and normal SCr; (3) high SCr [SCr ≥133 μmol/L (1.5 mg/dL) in men, ≥124 μmol/L (1.4 mg/dL) in women] but not on renal replacement therapy (RRT); or (4) RRT.
Results
Among 1993 subjects, 55.8% had type 2 diabetes at baseline. Overall death rates increased with declining kidney function in both the nondiabetic and diabetic subjects (P < 0.0001). Death rates were similar in nondiabetic and diabetic subjects with comparable levels of kidney function, although the number of deaths among nondiabetic subjects with advanced KD was small. Infections and malignancy were the leading causes of death in nondiabetic subjects with KD. Among diabetic subjects, overall mortality increased with diabetes duration (P = 0.0001) and was highest in those on RRT (P < 0.0001). High SCr was associated with higher death rates from cardiovascular disease (CVD), diabetic nephropathy (DN), infections, and malignancy.
Conclusion
Death rates increased comparably with worsening kidney function in both nondiabetic and diabetic subjects and were similar in nondiabetic and diabetic subjects without KD. KD was associated with excess mortality from DN, CVD, infections, and malignancy in diabetic subjects, and from infections in those without diabetes.
Keywords: Pima Indians, kidney disease, type 2 diabetes, mortality
Chronic kidney disease, defined as kidney damage or decreased kidney function for 3 or more months, affects 11% of the United States (US) population [1], and it is associated with increased mortality from cardiovascular disease (CVD) and end-stage renal disease. Chronic kidney disease is twice as frequent in persons with diabetes, and those with diabetes also have the highest burden of comorbidities and the lowest survival [2]. Native Americans, African Americans, and Hispanics with diabetes are at particularly high risk of developing kidney failure [3], a factor that may influence mortality patterns in these populations.
In this study we examined the impact of kidney disease (KD), defined by proteinuria, elevated serum creatinine concentration, or onset of renal replacement therapy (RRT), on overall and cause-specific mortality in nondiabetic and diabetic Pima Indians from the Gila River Indian Community in Arizona. This population has a high incidence of type 2 diabetes [4], and the incidence of kidney failure attributable to type 2 diabetes is 14 times that of the US diabetic population aged 45 to 64 years [5].
METHODS
Patients and study design
The Pima Indians and the closely related Tohono O’odham (Papago) Indians who live in the Gila River Indian Community in the desert of central Arizona participate in a comprehensive longitudinal study of diabetes and its complications. Since 1965, each member of this community who is ≥5 years of age is invited to have a research examination approximately every 2 years, regardless of health. These biennial examinations include measurements of venous plasma glucose, obtained 2 hours after a 75 g oral glucose load, and assessment of the complications of diabetes. Protein and creatinine concentrations are measured in urine specimens collected at the end of the 2-hour glucose tolerance test. Among those with at least a trace of protein in the urine by dip-stick, urine protein concentration is determined quantitatively by the Shevky-Stafford acid/alcohol precipitation method [6]. The urine protein forms precipitates upon the addition of Tsuchiya’s reagent. The resulting precipitate is centrifuged (Forma Scientific, Inc., Marietta, GA, USA) at a speed of 1900 to 2000 rpm (relative centrifugal force of 990) for 10 minutes and then measured. Serum and urine creatinine concentrations are measured by a modification of the Jaffé reaction [7]. Proteinuria was defined by a protein-to-creatinine ratio ≥0.5 g protein/g creatinine, reflecting an approximate protein excretion rate of at least 0.5 g/day. Diabetes was diagnosed by World Health Organization criteria [8] and the date of diagnosis was determined from these research examinations or from review of clinical records if diabetes was diagnosed in the course of routine medical care. The onset of RRT was defined as the date of beginning chronic dialysis and was ascertained independently of the research examinations. The study population included subjects who were at least half-Pima or Tohono O’odham heritage, resided in the community between January 1, 1965 and December 31, 2001 and had 1 or more research examinations after 45 years of age. Each subject’s vital status as of December 31, 2001 was determined. For all deaths, the accuracy and completeness of the underlying cause was determined by review of clinical records, autopsy reports, and death certificates.
Terminology and codes of the International Classification of Disease, Ninth Revision (ICD-9) were used for recording causes of death. Deaths were considered natural if they were due to disease (ICD-9 codes 001.0–799.9) and external if they were due to injury or poisoning (ICD-9 codes 800.0–999.9). The underlying cause of death was coded as diabetic nephropathy (ICD-9 code 250.4) in subjects who had kidney failure attributable to diabetes if they (1) did not receive RRT, (2) received RRT inconsistently or discontinued RRT, or (3) had dialysis-related complications as an immediate or contributory cause of death (e.g., malnutrition, shock, heart failure, septicemia, pericarditis, hypokalemia).
Statistical analysis
Death rates were calculated as the number of subjects who died per 1000 person-years of follow-up. The period of risk began at the first research examination after attaining the age of 45 years and extended until death or December 31, 2001, whichever came earlier. In subjects who did not have diabetes at the beginning of follow-up and developed diabetes during the course of the study, their person-time at risk was computed separately for each status. Follow-up within the nondiabetic and diabetic person-time strata was stratified in a time-dependent fashion according to decades of age and the following kidney function categories: normal SCr and no proteinuria; proteinuria (protein-to-creatinine ratio ≥0.5 g protein/g creatinine) with normal SCr; high SCr [serum creatinine ≥133 μmol/L (1.5 mg/dL) in men, ≥124 μmol/L (1.4 mg/dL) in women], with or without proteinuria, but not receiving RRT; receiving RRT.
Age- and sex-adjusted death rates and death rate ratios (DRR) were examined according to these categories and were standardized to the 1985 Pima Indian population aged 45 years and over. Tests for general association were computed by the Mantel-Haenszel test [9] and for linear association by the Mantel extension test [10].
RESULTS
During a median follow-up of 11.0 years (range 0.06–36.8), 332 deaths occurred among the 634 subjects who were nondiabetic throughout follow-up, and 709 deaths occurred among 1359 subjects who were diabetic at baseline (N = 1113) or developed diabetes during the study period (N = 246). Baseline characteristics of the study population at the first nondiabetic and diabetic examinations are presented in Table 1. At baseline, 848 nondiabetic subjects had normal kidney function and 32 had KD (proteinuria, high SCr, or RRT). At the end of nondiabetic follow-up, 841 (95.6%) of these subjects remained in the same category of kidney function, 31 (3.5%) worsened to more severe kidney disease and 8 (0.9%) improved their kidney function (Table 2). Among diabetic subjects, 1094 had normal kidney function and 265 had KD at baseline. Of these, 936 (68.9%) remained in the same kidney function category at the end of the study period, whereas 412 (30.4%) subjects progressed to more severe kidney disease and 11 (0.8%) improved.
Table 1.
Baseline clinical and demographic features of Pima Indians aged ≥45 years old
| Nondiabetic subjects (N = 880)a |
Diabetic subjects (N = 1359)a |
|||
|---|---|---|---|---|
| Mean ± SD | Centilesb | Mean ± SD | Centilesb | |
| Age years | 53.4 ± 10.0 | 46.3–57.8 | 53.2 ± 8.5 | 46.6–57.9 |
| Duration of diabetes years | – | – | 7.0 ± 7.2 | 0.4–11.7 |
| Mean arterial pressure mm Hg | 96.9 ± 14.1 | 86.7–105.3 | 100.3 ± 15.3 | 90.0–108.7 |
| BMI kg/m2 | ||||
| Men | 29.2 ± 6.1 | 25.0–32.0 | 30.7 ± 5.9 | 26.8–33.6 |
| Women | 33.5 ± 7.4 | 28.3–37.3 | 33.6 ± 7.2 | 28.4–37.0 |
| Serum cholesterol mg/dL | 182.2 ± 37.2 | 158.0–204.0 | 188.5 ± 46.2 | 159.0–212.0 |
| 2-hour plasma glucose mg/dL | 122.9 ± 31.5 | 100.0–145.0 | 335.9 ± 123.7 | 229.5–428.0 |
| Serum creatinine mg/dL | 0.8 ± 0.3 | 0.7–0.9 | 0.8 ± 0.5 | 0.6–0.9 |
Baseline data were missing for mean arterial pressure in 19 nondiabetic and 12 diabetic subjects, for 2-hour plasma glucose in 15 nondiabetic and 162 diabetic subjects, for BMI in 15 nondiabetic and 21 diabetic subjects, and for cholesterol in 2 diabetic subjects.
Two hundred forty-six of the nondiabetic subjects developed diabetes during the follow-up. The baseline characteristics from their first nondiabetic and first diabetic examination are included in the table.
The 25th and 75th centiles.
Table 2.
The number of nondiabetic and diabetic subjects according to baseline kidney function and progression or regression of kidney function at the last examination
| Kidney function at first nondiabetic examination | Normal | Proteinuria | High SCr | RRT | Total at last examination | |
|---|---|---|---|---|---|---|
| Normal | 823 | 7 | 0 | 0 | 830 | |
| Kidney function at last nondiabetic examination | Proteinuria | 18 | 15 | 1 | 0 | 34 |
| High SCr | 4 | 0 | 3 | 0 | 7 | |
| RRT | 3 | 0 | 6 | 0 | 9 | |
| Total | 848 | 22 | 10 | 0 | 880 | |
| Kidney function at first diabetic examination | Normal | Proteinuria | High SCr | RRT | Total at last examination | |
| Kidney function at end of follow-up | Normal | 796 | 10 | 0 | 0 | 806 |
| Proteinuria | 148 | 109 | 1 | 0 | 258 | |
| High SCr | 28 | 21 | 28 | 0 | 77 | |
| RRT | 122 | 68 | 25 | 3 | 218 | |
| Total | 1094 | 208 | 54 | 3 | 1359 |
Subjects with stable kidney function neither worsened nor improved their kidney function (bold numbers). In each column, the numbers below the bold number represent subjects with worsening kidney function, whereas those above the bold number represent subjects who had improved kidney function.
The age- and sex-adjusted death rates from natural causes in nondiabetic subjects ranged from 23.8/1000 person-years (95% CI 20.8–26.8) in those without kidney disease to 84.5/1000 person-years (95% CI 1.6–167.4) in subjects with high SCr (P < 0.0001) (Table 3). The range among diabetic subjects with comparable degrees of kidney disease was similar, rising from 25.3/1000 person-years (95%CI 22.3–28.2) in subjects with normal kidney function, to 105.1/1000 person-years (95% CI 75.7–134.5) in those with high SCr (Table 3). With progression to RRT, the death rate increased further to 208.7/1000 person-years (95%CI 171.1–246.3) in those with diabetes (P < 0.0001). With only 9 nondiabetic subjects on RRT during 28.4 person-years of follow-up, insufficient data were available to compute an age- and sex-adjusted death rate in this category. Although the number of deaths in nondiabetic subjects with KD was small, the excess mortality was due primarily to higher death rates from infections. On the other hand, diabetic subjects with proteinuria had higher age- and sex-adjusted death rates from DN, infections, and CVD. Those with high SCr also had increased mortality due to malignant neoplasms. In both nondiabetic and diabetic subjects, age- and sex-adjusted death rates for other underlying causes did not differ significantly across the kidney function groups.
Table 3.
Number of deaths, death rates, and death rate ratios (DRR) with 95% CI for leading underlying causes in nondiabetic and diabetic Pima Indians, according to kidney function category
| Normal SCr
|
Proteinuria
|
High serum creatinine
|
RRT
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Underlying cause of death (ICD-9 codes) | Deaths (N) | Death ratea | Deaths (N) | Death ratea | DRRb (95% CI) | Deaths (N) | Death ratea | DRRb (95% CI) | Deaths (N) | Death ratea | DRRb (95% CI) |
| Nondiabetic subjects | |||||||||||
| Cardiovascular disease (410.0–414.9, 431.0–437.9) | 38 | 3.3 | 2 | 4.0 | 1.2 (0.3–5.0) | 0 | 0 | 0 | 2 | – | – |
| Infectionsd | 40 | 3.5 | 6 | 10.2 | 2.9 (1.2–6.8) | 3 | 20.6 | 5.8 (1.7–19.9) | 0 | – | – |
| Malignant neoplasms (140.0–208.9) | 44 | 4.2 | 4 | 7.9 | 1.9 (0.7–5.3) | 1 | 4.8 | 1.1 (0.2–8.2) | 0 | – | – |
| Alcoholic liver disease (571.0–571.3) | 24 | 2.7 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | – | – |
| Other natural causesc | 104 | 10.0 | 5 | 20.7 | 2.1 (0.7–5.9) | 3 | 59.1 | 5.9 (1.5–22.6) | 1 | – | – |
| All natural causes | 250 | 23.8 | 17 | 42.8 | 1.8 (1.0–3.3) | 7 | 84.5 | 3.6 (1.3–9.6) | 4 | – | – |
| All external causes | 50 | 5.2 | 2 | 11.4 | 2.2 (0.5–9.4) | 0 | 0 | 0 | 2 | – | – |
| All causes | 300 | 29.0 | 19 | 54.2 | 1.9 (1.1–3.3) | 7 | 84.5 | 2.9 (1.1–7.8) | 6 | – | – |
| Diabetic subjects | |||||||||||
| Cardiovascular disease (410.0–414.9, 431.0–437.9) | 86 | 7.0 | 38 | 12.7 | 1.8 (1.2–2.7) | 11 | 22.2 | 3.2 (1.6–6.4) | 51 | 63.5 | 9.1 (6.2–13.3) |
| Diabetic nephropathy (250.4) | 5 | 0.4 | 12 | 3.2 | 8.5 (2.8–25.5) | 29 | 46.8 | 126.4 (47.2–338.5) | 55 | 72.7 | 196.5 (75.9–508.6) |
| Infectionsd | 45 | 3.3 | 24 | 7.1 | 2.1 (1.3–3.7) | 4 | 10.2 | 3.1 (1.1–8.6) | 23 | 34.8 | 10.5 (6.0–18.3) |
| Malignant neoplasms (140.0–208.9) | 55 | 4.3 | 12 | 3.4 | 0.8 (0.4–1.6) | 8 | 11.7 | 2.7 (1.2–6.0) | 2 | 3.2 | 0.8 (0.2–3.3) |
| Alcoholic liver disease (571.0–571.3) | 36 | 3.3 | 6 | 2.1 | 0.6 (0.3–1.5) | 1 | 2.8 | 0.9 (0.1–6.3) | 3 | 3.1 | 0.9 (0.3–3.0) |
| Other natural causesc | 82 | 7.0 | 28 | 9.0 | 1.3 (0.8–2.1) | 6 | 11.4 | 1.6 (0.7–3.9) | 24 | 31.4 | 3.7 (2.3–6.0) |
| All natural causes | 309 | 25.3 | 120 | 37.4 | 1.5 (1.2–1.9) | 59 | 105.1 | 4.2 (3.1–5.6) | 158 | 208.7 | 8.3 (6.7–10.2) |
| All external causes | 44 | 4.0 | 9 | 3.8 | 1.0 (0.5–2.0) | 2 | 2.4 | 0.6 (0.1–2.6) | 8 | 9.9 | 2.5 (1.1–5.8) |
| All causes | 353 | 29.2 | 129 | 41.3 | 1.4 (1.1–1.8) | 61 | 107.5 | 3.7 (2.7–5.0) | 166 | 218.6 | 7.5 (6.1–9.2) |
Death rate is reported per 1000 person-years, age and sex adjusted.
DRR, death rate ratio relative to normal SCr. Adjusted rates and rate ratios are missing for categories with small number of person-years follow-up.
Includes deaths from cardiovascular diseases other than IHD or stroke, from other diabetic causes, and from other natural causes.
ICD-9 codes 001.0–139.8; 320.0–326.9; 460.0–466.1; 480.0–487.8; 540.0–543.9; 572.0; 599.0–590.9; 599.0; 680.0–686.9; 729.4.
The major underlying infectious cause of death among subjects, regardless of the presence or absence of kidney disease, was pneumonia, accounting for 62% of deaths from infectious diseases in those without diabetes and 51% of deaths from infectious diseases in those with diabetes. Deaths from malignant neoplasms in diabetic subjects with kidney disease were too few (12 in the proteinuria group, 8 in the high SCr group, 2 in RRT group) to reliably determine trends by types of neoplasms. Nevertheless, in those receiving RRT, the 2 deaths from malignancy were attributed to neoplasms of the kidney.
Figure 1 shows a strong effect of kidney function on natural and CVD mortality in all categories of diabetes duration, but a lesser effect of duration for any category of kidney function. The higher overall mortality in those with longer duration of diabetes is due primarily to the greater proportion of person-years of follow-up falling in the categories of worse kidney function, as shown in Figure 1C.
Fig. 1. Trends in age- and sex-adjusted death rates from natural causes (A) and cardiovascular disease (CVD) (B).
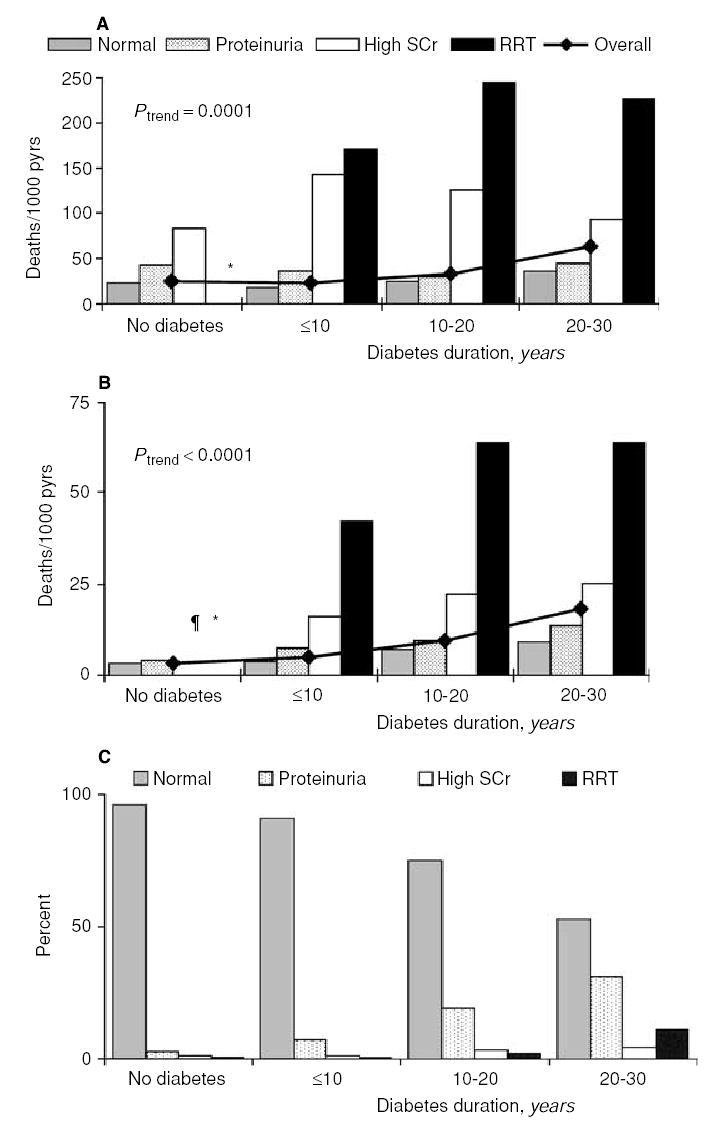
The bars represent mortality rates stratified by diabetes and its duration in the 4 kidney function categories. The line shows overall death rates by diabetes and its duration. Overall mortality rates were positively associated with duration of diabetes and were similar in the nondiabetic subjects and those with diabetes of <10 years’ duration. Panel (C) is a frequency distribution of person-years stratified by diabetes and its duration in the 4 kidney function categories. The fraction of person-years of follow-up among persons with normal kidney function ranged from 96% in nondiabetic subjects to 53% in those with 20 to 30 years of diabetes. Conversely, the fraction of person-years among persons on renal replacement therapy (RRT) ranged from 0.4% in nondiabetic subjects to 12% in those with 20 to 30 years of diabetes. ¶ Rate is null; *insufficient data.
DISCUSSION
Overall mortality in Pima Indians was greater with greater severity of kidney disease in both nondiabetic and diabetic subjects. The death rates in nondiabetic and diabetic subjects without kidney disease were virtually identical and increased to a similar degree with worsening kidney function in both groups, suggesting that kidney disease rather than diabetes per se is the major determinant of increased mortality among the diabetic population.
Cardiovascular disease was the leading cause of death in diabetic Pima Indians without kidney disease and in those with early kidney disease characterized by proteinuria. With progression of KD, CVD mortality increased further, but became the second leading cause of death behind DN. The greatest increase in CVD deaths occurred in subjects receiving RRT, in whom the death rate was 9 times as high as in those with normal kidney function. Indeed, the introduction and widespread use of RRT, while prolonging life and reducing deaths that would otherwise be attributable to DN, is largely responsible for the rise in deaths from cardiovascular disease in diabetic Pima Indians [11]. These findings extend our previous observation [12] that overall mortality in diabetic Pima Indians with clinical proteinuria was 3.5 times as high as in those without proteinuria, with the excess largely attributable to uremia and to cardiovascular disease.
Although SCr is not an ideal measurement of kidney function, the association between SCr concentration and mortality has been reported in several other studies. The choice of cut points, although arbitrary, was made to ensure that we identified an advanced stage of kidney disease. Alternative cut points did not change the overall conclusions of the study (data not shown).
The NHANES I study [13] found that diabetic persons with moderate renal insufficiency [SCr of 122 to 177 μmol/L (1.4 to 2.0 mg/dL) in men and 104 to 146 μmol/L (1.2 to 1.7 mg/dL) in women] had higher total and CVD mortality than those without diabetes or renal insufficiency. There was, however, no significant association between serum creatinine and total or cardiovascular mortality in this population after adjustment for cardiovascular risk factors such as sex, age, hypercholesterolemia, hypertension, diabetes, and smoking. Hence, the authors suggested that the association between renal insufficiency and cardiovascular disease reported in other studies [14–17], some of which excluded subjects with diabetes, was due to the co-occurrence of other established cardiovascular risk factors. The authors proposed that elevated serum creatinine was simply a marker for the presence of these risk factors. Similarly, in the Framingham community-based study [18], mild renal insufficiency [SCr of 136 to 265 μmol/L (1.5 to 3.0 mg/dL) in men and 120 to 265 μmol/L (1.4 to 3.0 mg/dL) in women] was associated with higher total mortality in men, but there was no association of KD with age-adjusted cardiovascular mortality in either sex. On the other hand, the risk of death in the US Medicare population aged 65 years and older [19] with chronic KD but no diabetes was 11 times as high as the risk of progression to RRT, and 6 times as high as the risk of RRT for those with both diabetes and chronic KD. Likewise, in the ARIC study [20], the higher CVD incidence in middle-aged nondiabetic and diabetic patients with KD was strongly associated with risk factors that were more prevalent as GFR declined. The NHANES II Mortality Study [21] found a graded relationship between proteinuria and the risk of total or CVD mortality in diabetic as well as nondiabetic subjects. In NHANES II, CVD mortality was highest among those with glomerular filtration rate (GFR) <70 mL/min. These findings suggest that higher mortality associated with KD reflects increased rates of CVD.
The independent, inverse association between estimated GFR, computed from a formula described in the Modification of Diet in Renal Disease study (MDRD formula) [22], and mortality rates from CVD was confirmed by recent studies in 2 different populations. In a large, ethnically diverse outpatient sample of the adult population in the San Francisco Bay Area [23], independent associations were found between lower estimated GFR and risk of cardiovascular events and death from CVD. Similarly, among patients with a previous acute myocardial infarction enrolled in the VALIANT study [24], lower GFR was associated with higher rates of death or recurrent cardiovascular events. Nonetheless, the MDRD formula has not been validated in diabetic kidney disease or in ethnic groups other than Caucasian and African Americans and may be less accurate in persons with normal kidney function [25]. The MDRD formula was developed from measures of GFR in people with GFR <60 mL/min/1.73m2 so its use is not recommended by the National Kidney Foundation in those with GFR ≥60 mL/min/1.73m2 [26]. Therefore, we chose to define stages of kidney function by SCr concentration and level of urinary protein excretion and not by the estimated GFR. When we analyzed the data using the estimated GFR instead of SCr concentration to define kidney disease, however, the overall conclusions of the study did not change.
By contrast to other populations, Pima Indians have a low incidence of fatal CVD, particularly among those without diabetes [27]. The absence of an association between KD and CVD mortality in nondiabetic Pima Indians may be due in part to the small number of nondiabetic subjects with KD. Among diabetic Pima Indians, CVD mortality is strongly related to diabetes duration, and diabetic Pima Indians with KD are more likely to die from CVD after the onset of kidney failure, when cardiovascular risk factors, including dyslipidemia and hypertension, become more prevalent or worsen [28].
The increased frequency of infections in subjects with KD is related to comorbidity and to depressed humoral and cellular immunity that progresses during the development of uremia [29]. Infectious diseases accounted for 18% of all natural mortality in the nondiabetic subjects, and 18% of these deaths occurred in those with kidney disease. Pneumonia was the leading infectious cause of death, accounting for 33% of deaths in nondiabetic subjects with proteinuria and 67% in those with high SCr. In the diabetic subjects, 15% of all natural mortality was due to infectious diseases and 53% of these deaths occurred in subjects with kidney disease. Pneumonia was the leading infectious cause of death in the predialysis patients, followed by kidney infections and skin infections. In the general US population with chronic kidney disease, mortality due to infectious diseases is 3.2 times as high as in those without KD [2]. Among patients with end-stage renal disease, infectious diseases are the second leading cause of death following CVD. Sepsis accounts nationally for 75% of these infectious deaths, followed by pulmonary infections and viral infections [2]. In Pima Indians on RRT, infectious disease is the third leading cause of death following DN and CVD. Pneumonia was the leading infectious cause of death (34.8%), followed by coccidioidomycosis (17.4%) and urinary tract infections (13%).
Although some reports suggest that diabetes increases the risk of death from several types of cancer, a relationship between KD and deaths from malignant neoplasms has not been described [30, 31]. Nevertheless, a 10-fold higher risk of renal cell carcinoma has been reported [32] in hemodialysis patients than in the nondialysis population. Notably, renal cell carcinoma was the only cause of death from malignancies among diabetic Pima Indians on RRT in this study.
Because the confounding and intermediate effects of blood pressure, body mass index, serum cholesterol concentration, and plasma glucose concentration could not be distinguished in this analysis, we chose not to control for them. Models that did control for these variables changed only the point estimates and confidence intervals, but not the overall conclusions of the study (data not shown).
The level of kidney function in this study, with the exception of RRT, was determined at research examinations. The frequency of these examinations could therefore influence whether a subject was classified appropriately at the time of death. Among those who died, all available medical records were reviewed systematically and elevations of serum creatinine concentration ≥2.0 mg/dL were noted. These data were not used in this analysis, however, because they were available only for those who died. Accordingly, mortality rates in the pre-RRT groups could be underestimated to the extent that subjects who may have developed higher levels of KD were not identified as such. Nonetheless, only 9% of the subjects classified as having normal kidney function at all exams had a serum creatinine concentration ≥2 mg/dL in the medical record before death, suggesting that the extent of kidney function misclassification was small.
CONCLUSION
Death rates from natural causes in nondiabetic Pima Indians are comparable to those in diabetic Pima Indians when rates are stratified by the level of kidney disease. RRT occurred almost exclusively in subjects with diabetes, and mortality was highest in these subjects, with deaths from diabetic nephropathy, cardiovascular disease, and infections being primarily responsible for the excess mortality. The impact of cardiovascular disease on mortality was highest after the onset of RRT. Preventing the onset and progression of diabetic kidney disease would undoubtedly reduce the frequency of kidney failure, but might also reduce death rates from cardiovascular disease, infections, and malignancy in diabetic Pima Indians.
Acknowledgments
Dr. M.E. Pavkov was supported by a Mentor-Based Fellowship award from the American Diabetes Association. The authors are indebted to the members of the Gila River Indian Community for participating in this investigation.
References
- 1.Coresh J, Astor BC, Greene T, et al. Prevalence of chronic kidney disase and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis. 2003;41:1–12. doi: 10.1053/ajkd.2003.50007. [DOI] [PubMed] [Google Scholar]
- 2.US R enal Data System: USRDS 2003 Annual Data Report, Atlas of End-Stage Renal Disease in the United States, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2003
- 3.Cowie CC, Port FK, Wolfe RA, et al. Disparities in incidence of diabetic end-stage renal disease according to race and type of diabetes. N Engl J Med. 1989;321:1074–1079. doi: 10.1056/NEJM198910193211603. [DOI] [PubMed] [Google Scholar]
- 4.Knowler WC, Bennett PH, Hamman RF, Miller M. Diabetes incidence and prevalence in Pima Indians: A 19-fold greater incidence than in Rochester, Minnesota. Am J Epidemiol. 1978;108:497–505. doi: 10.1093/oxfordjournals.aje.a112648. [DOI] [PubMed] [Google Scholar]
- 5.Nelson RG, Newman JM, Knowler WC, et al. Incidence of end-stage renal disease in type 2 (non–insulin-dependent) diabetes mellitus in Pima Indians. Diabetologia. 1988;31:730–736. doi: 10.1007/BF00274774. [DOI] [PubMed] [Google Scholar]
- 6.Shevky M, Stafford M. A clinical method for the estimation of protein in urine and other body fluids. Arch Intern Med. 1923;32:222–225. [Google Scholar]
- 7.Chasson AL, Grady HJ, Stanley MA. Determination of creatinine by means of automatic chemical analysis. Tech Bull Regist Med Technol. 1960;30:207–212. [PubMed] [Google Scholar]
- 8.W orld Health Organization: Diabetes mellitus, Geneva, Switzerland, World Health Organization (Tech. Rep. Ser. no. 727), 1985
- 9.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:419–448. [PubMed] [Google Scholar]
- 10.Mantel N. Chi-square tests with one degree of freedom: Extension of the Mantel-Haenszel procedure. J Am Stat Assoc. 1963;59:690–700. [Google Scholar]
- 11.Pavkov ME, Sievers ML, Knowler WC, et al. An explanation for the increase in heart disease mortality rates in diabetic Pima Indians: Effect of renal replacement therapy. Diabetes Care. 2004;27:1132–1136. doi: 10.2337/diacare.27.5.1132. [DOI] [PubMed] [Google Scholar]
- 12.Nelson RG, Pettitt DJ, Carraher MJ, et al. Effect of proteinuria on mortality in NIDDM. Diabetes. 1988;37:1499–1504. doi: 10.2337/diab.37.11.1499. [DOI] [PubMed] [Google Scholar]
- 13.Garg AX, Clark WF, Haynes RB, House AA. Moderate renal insufficiency and the risk of cardiovascular mortality: Results from the NHANES I. Kidney Int. 2002;61:1486–1494. doi: 10.1046/j.1523-1755.2002.00270.x. [DOI] [PubMed] [Google Scholar]
- 14.Flack JM, Neaton JD, Daniels B, Esunge P. Ethnicity and renal disease: Lessons from the Multiple Risk Factor Intervention Trial and the Treatment of Mild Hypertension Study. Am J Kidney Dis. 1993;21(Suppl 1):S31–S40. doi: 10.1016/s0272-6386(12)80859-6. [DOI] [PubMed] [Google Scholar]
- 15.Mann JF, Gerstein HC, Pogue J, et al. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: The HOPE randomized trial. Ann Intern Med. 2001;134:629–636. doi: 10.7326/0003-4819-134-8-200104170-00007. [DOI] [PubMed] [Google Scholar]
- 16.Hemmelgarn BR, Ghali WA, Quan H, et al. Poor long-term survival after coronary angiography in patients with renal insufficiency. Am J Kidney Dis. 2001;37:64–71. doi: 10.1053/ajkd.2001.20586. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Kronmal RA, Newman AB, et al. Risk factors for 5-year mortality in older adults. The Cardiovascular Health Study. JAMA. 1998;279:585–592. doi: 10.1001/jama.279.8.585. [DOI] [PubMed] [Google Scholar]
- 18.Culleton BF, Larson MG, Wilson PWF, et al. Cardiovascular disease and mortality in a community-based cohort with mild renal insufficiency. Kidney Int. 1999;56:2214–2219. doi: 10.1046/j.1523-1755.1999.00773.x. [DOI] [PubMed] [Google Scholar]
- 19.Foley NR, Murray AM, Shuling L, et al. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 20.Muntner P, Jiang H, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: Results from the Atherosclerosis Risk in Communities Study. J Am Soc Nephrol. 2004;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 21.Muntner P, He J, Hamm L, et al. Renal insufficiency and subsequent death resulting from cardiovascular disease in the United States. J Am Soc Nephrol. 2002;13:745–753. doi: 10.1681/ASN.V133745. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 23.Go AS, Chertow GM, Fan D, et al. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1306. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 24.Anavekar NS, McMurray JJV, Velazquez EJ, et al. Relation between renal dysfunction and cardiovascular outcomes after myocardial infarction. N Engl J Med. 2004;351:1285–1295. doi: 10.1056/NEJMoa041365. [DOI] [PubMed] [Google Scholar]
- 25.Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: Accuracy in good health and chronic kidney disease. Ann Intern Med. 2004;141:929–937. doi: 10.7326/0003-4819-141-12-200412210-00009. [DOI] [PubMed] [Google Scholar]
- 26.Clinical Practice Guidelines for Chronic Kidney Disease. Evaluation, Classification, and Stratification. Am J Kidney Dis. 2002;39:S46–S75. [PubMed] [Google Scholar]
- 27.Nelson RG, Sievers ML, Knowler WC, et al. Low incidence of fatal coronary heart disease in Pima Indians despite high prevalence of non–insulin-dependent diabetes. Circulation. 1990;81:987–995. doi: 10.1161/01.cir.81.3.987. [DOI] [PubMed] [Google Scholar]
- 28.Krane V, Wanner C. Cardiovascular disease and predisposing factors in chronic renal failure. J Clin Basic Cardiol. 2001;4:97–100. [Google Scholar]
- 29.Vanholder R, Ringoir S. Infectious morbidity and defects of phagocytic function in end-stage renal disease: A review. J Am Soc Nephrol. 1993;3:1541–1554. doi: 10.1681/ASN.V391541. [DOI] [PubMed] [Google Scholar]
- 30.Wannamethee SG, Shaper AG, Perry IJ. Serum creatinine concentration and risk of cardiovascular disease. A possible marker for increased risk of stroke. Stroke. 1997;28:557–563. doi: 10.1161/01.str.28.3.557. [DOI] [PubMed] [Google Scholar]
- 31.Miettinen H, Haffner SM, Lehto S, et al. Proteinuria predicts stroke and other atherosclerotic vascular disease events in nondiabetic and non–insulin-dependent diabetic subjects. Stroke. 1996;27:2033–2039. doi: 10.1161/01.str.27.11.2033. [DOI] [PubMed] [Google Scholar]
- 32.Ishikawa I. Renal cell carcinoma in chronic hemodialysis patients—A 1998 questionnaire study. J Jpn Soc Dial Ther. 2000;33:181–188. [Google Scholar]


