Abstract
Experimental animal models suggest that uric acid might have a pathogenic role in the early development of primary hypertension. We hypothesized that serum uric acid is correlated with blood pressure in children with new-onset, untreated, primary hypertension. We evaluated 125 consecutive children referred to the Baylor Pediatric Renal Program for evaluation of hypertension. None of the subjects had previously been evaluated or treated for hypertension. The children ranged in age from 6 to 18 years (mean, 13.4±3.3) and had normal renal function (creatinine clearance >80 mL · min−1 · 1.73 m−2). Sixty-three children had primary hypertension, 40 had secondary hypertension, and 22 had white-coat hypertension. Forty controls with normal blood pressure were recruited from the renal clinic. Uric acid levels were directly correlated with systolic (r=0.80, P=0.0002) and diastolic (r=0.66, P=0.0006) blood pressure in controls and in subjects with primary hypertension and were independent of renal function. Serum uric acid concentrations >5.5 mg/dL were found in 89% of subjects with primary hypertension, in 30% with secondary hypertension, in 0% with white-coat hypertension, and in 0% of controls. We conclude that serum uric acid is directly correlated with blood pressure in untreated children and that a serum uric acid value >5.5 mg/dL in an adolescent being evaluated for hypertension strongly suggests primary hypertension as opposed to white-coat or secondary hypertension. These results are consistent with the hypothesis that uric acid might have a role in the early pathogenesis of primary hypertension.
Keywords: uric acid; hypertension, essential; children; renal disease
Hypertension, the most common form of cardiovascular disease, is present in nearly 25% of adults and increases in prevalence with age. Hypertension results in increased morbidity and mortality by dramatically escalating the risk of myocardial infarction, congestive heart failure, stroke, peripheral vascular disease, and renal failure.1,2
Several clinical and laboratory observations are consistent with the hypothesis that uric acid might be important in the development of primary hypertension in humans. An association of gout with hypertension was first noted in 1879.3 Twenty-five percent to 40% of adult patients with untreated hypertension have hyperuricemia (>6.5 mg/dL), and many more have a high-normal serum uric acid value (5.0 to 6.5 mg/dL).4,5 The relation between uric acid and blood pressure (BP) is continuous and is observed in both African-Americans and whites.6 Furthermore, hyperuricemia predicts the development of, and is an independent risk factor for, hypertension.7,8 Finally, we demonstrated that hyperuricemic rats develop hypertension9 followed by preglomerular arteriolopathy.10 Early hypertension is dependent on the reninangiotensin system and nitric oxide pathways9; however, once preglomerular vascular disease develops, hypertension is driven by the kidney, and lowering uric acid levels is no longer protective.11 If substantiated in humans, then prevention of the renal microvascular lesions during childhood might reduce the incidence of long-term hypertension.
Although hypertension is less common in children and adolescents, evidence suggests that the roots of adult hyper-tension are present in childhood.12 Elevated BP in childhood is an excellent predictor of hypertension in adulthood.13,14 Numerous studies have demonstrated end-organ damage, including left ventricular hypertrophy15 and microalbuminuria,16 in many hypertensive children. Consequently, prevention of hypertension is the best means for avoiding long-term morbidity and mortality.
Pediatric hypertension often results from underlying renal, cardiovascular, or endocrine disease. Thirty percent to 60% of hypertensive children have an identifiable etiology.17,18 The most common causes are renal parenchymal diseases, whereas cardiovascular and endocrine etiologies are less frequent.19 Because pediatric hypertension is often secondary, children undergo meticulous laboratory and radiologic evaluation.19 Forty percent to 70% of hypertensive children do not have an identifiable etiology and are diagnosed with primary hypertension. The likelihood is greater among children diagnosed during adolescence or those with a significant family history for hypertension.20 Children with primary hypertension are frequently obese (50%) and almost universally progress to adult hypertension.20
In this report, we evaluated 125 consecutive children who were referred to the Baylor Pediatric Renal section with newly diagnosed hypertension. We hypothesized that the relation of serum uric acid to BP would be particularly strong in subjects with new-onset and untreated hypertension. A nearly linear relation was found between serum uric acid and BP. Furthermore, during the initial evaluation for hypertension in children with normal renal function, a serum uric acid level >5.5 mg/dL strongly suggests the diagnosis of primary hypertension and is inconsistent with a diagnosis of white-coat hypertension.
Methods
Patient Population
One hundred twenty-five children, aged 6 to 18 years, referred consecutively to the Pediatric Renal Clinic for hypertension, were screened for serum uric acid levels. Normotensive controls (n=40) were referred for hematuria, proteinuria, or enuresis. All subjects and controls had normal renal function (Schwartz formula, creatinine clearance >80 mL · min−1 · 1.73 m−2). The study was approved by the Baylor Institutional Review Board.
Diagnosis of Hypertension
Hypertension was defined in accordance with the Second National Task Force on High Blood Pressure in Children and Adolescents as systolic or diastolic BP >95th percentile for age, height, and gender on 3 consecutive visits.17 Each data point was the mean of 3 relaxed, seated, right arm readings with a blood pressure monitor (Dinamap) and cuff of an appropriate size.17 Subjects with confirmed hypertension and no identified cause of secondary hypertension were diagnosed with primary hypertension.
Diagnosis of White-Coat Hypertension
Children with systolic and diastolic BP indices (ratio of BP to 95th percentile <1.2) underwent 24-hour ambulatory BP monitoring. Children with higher BP indices had a very low probability of white-coat hypertension21 and were at sufficient risk that it would have been unethical to withhold treatment for study purposes. These children remained in the study because to exclude all severely hypertensive children could have severely biased the results, and only clinic BP measurements were used for analysis. Ambulatory BP monitoring was performed with a commercially available device (SpaceLabs 90217) for 24 hours with measurements every 20 minutes. Activity diaries defined sleep periods. Monitor data were considered adequate when >75% of measurements were successful. We used a more conservative definition of white-coat hypertension than most authors21-25 by requiring that ambulatory BP monitoring data reveal mean daytime and 24-hour systolic and diastolic BPs be <95th percentile relative to normative data26 and that 24-hour systolic and diastolic BP loads be <25%, despite in-clinic hypertension.
Evaluation of Hypertension
Laboratory analyses, performed at Texas Children's Hospital Clinical Laboratory, included blood tests for the evaluation of serum electrolytes, blood urea nitrogen, creatinine, calcium, uric acid, renin, and thyroid function tests. Glomerular filtration rate (GFR) was calculated by the Schwartz formula. Complete urinalyses were performed by the nephrology faculty. Hypertensive patients underwent a renal ultrasound with Doppler and a dimercaptosuccinic acid (DMSA) renal perfusion scan. Subjects with abdominal bruits, suggestive history (William's syndrome, neurofibromatosis, or family history of fibromuscular dysplasia), suggestive ultrasound findings (renal asymmetry or Doppler abnormalities), or severe hyper-tension and elevated plasma renin levels underwent renal angiography (9 children). Urinary catecholamines, to screen for pheochromocytoma, were collected in children with severe hyper-tension (30 mm Hg >95th percentile); complaints of diaphoresis, flushing, and/or palpitations; or a family history of catecholamine-secreting tumors.
Statistical Analysis
Significance testing of the difference between means was done by a paired 2-tailed Student t test, and correlations were assessed by Pearson coefficients. ANOVA was used to test for differences owing to covariates and multiple-regression modeling to distinguish the contribution of body mass index (BMI) to the effect of uric acid on BP. All statistical analyses were performed with commercially available software (Statistica 6.0, StatSoft).
Results
Patient Population
Among the 125 children evaluated for hypertension, 63 had essential hypertension, 40 had secondary hypertension, and 22 had white-coat hypertension. Fifty percent of the population with essential hypertension is consistent with the reported prevalence in adolescent populations.20 The majority of children with secondary hypertension had renal parenchymal scars leading to renin-mediated hypertension (32 children). Other etiologies included renal artery stenosis in 3 (2 of whom also had William's syndrome), pheochromocytoma (2 patients), coarctation of the aorta (2 patients), and 1 with the syndrome of apparent mineralocorticoid excess.
The clinical and laboratory characteristics of the children are shown in the Table. The data presented are mean±SD. A moderate male predominance was found in all groups. Based on nominal interpretation of the 2-tailed t test, there were no significant differences in gender distribution, age, or height between the controls and the subject diagnostic groups. The children with primary and white-coat hypertension were heavier than controls, and the greater weight was reflected in their greater BMIs and body surface area. Because normal BMI varies significantly in 6- to 18-year-olds, BMI percentile for age was used for comparison rather than the BMI itself. No significant differences in age, height, or GFR were found between controls and the subjects.
Clinical Characteristics of the Study Population
| Clinical Characteristicq | Controls (n=40) | Primary Hypertension (n=63) | Secondary Hypertension (n=40) | White Coat Hypertension (n=22) |
|---|---|---|---|---|
| Gender, % male | 56 | 67 (0.13) | 62 (0.25) | 56 (0.54) |
| Age, y | 13.7±3.6 | 15.0±2.8 (0.06) | 12.7±4.1 (0.57) | 12.5±3.5 (0.42) |
| SBP, mm Hg* | 108±11.4 | 146±10.7 (0.0002) | 139±15.9 (0.0008) | 129±9.2† (0.005) |
| DBP, mm Hg* | 62.4±6.4 | 82.2±11.2 (0.001) | 82.7±12.1 (0.001) | 77.1±8.6† (0.004) |
| Height, cm | 158±12.4 | 166±15 (0.07) | 150±18 (0.27) | 151±17 (0.36) |
| Weight, kg | 57.6±26 | 86±27 (0.002) | 53.4±29 (0.21) | 61.6±24 (0.10) |
| BMI percentile | 56±26 | 87±20 (0.0003) | 66±37 (0.14) | 84±23 (0.001) |
| GFR, mL/min/1.73m2 | 136±30 | 130±25 (0.22) | 132±42 (0.29) | 133±31 (0.19) |
| Uric acid, mg/dL | 3.6±0.8 | 6.7±1.3 (0.000004) | 4.3±1.4 (0.008) | 3.6±0.7 (0.80) |
| Males | 3.8±0.8 | 6.8±1.1 (0.00009) | 4.5±1.3 (0.02) | 3.4±0.6 (0.24) |
| Females | 3.4±0.8 | 6.4±1.5 (0.0002) | 3.9±1.5 (0.08) | 3.7±0.7 (0.19) |
P values, compared to controls, are in parentheses.
All blood pressures shown are the mean of casual measurements.
Blood pressures measured by ambulatory blood pressure monitoring were substantially lower: 113±6.5 and 64±4.7 for mean daytime systolic and diastolic, respectively.
Serum Uric Acid Levels
Figure 1 shows the serum uric acid levels for children in each group of the cohort. The mean serum uric acid level (±SD) in controls was 3.6±0.8 mg/dL. The mean serum uric acid value was the same in children with white-coat hypertension (3.6±0.7 mg/dL, P=0.80) but was significantly elevated in children with primary (6.7±1.3 mg/dL, P=0.000004) and secondary (4.3±1.4 mg/dL, P=0.008) hypertension.
Figure 1.
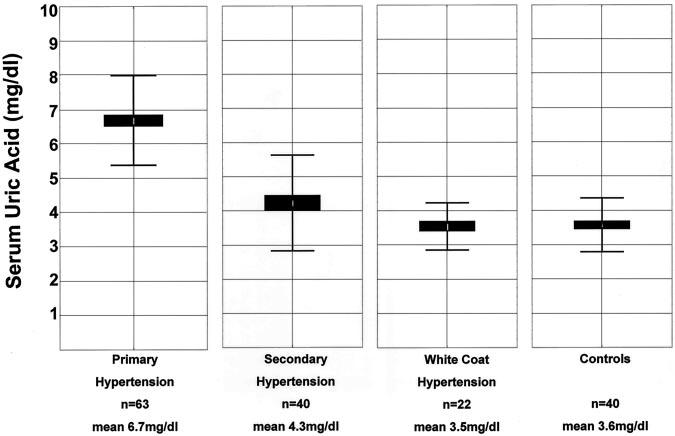
Box-and-whisker plot of serum uric acid levels in children with hypertension and normal BP. The mean and SD for serum uric acid levels for children with primary hypertension, secondary hypertension, white-coat hypertension, and normal BP controls are shown. When compared with controls, uric acid levels were significantly higher in children with essential (P=0.000004) and secondary (P=0.008) hypertension.
A serum uric acid level >5.5 mg/dL was useful in identifying children with primary hypertension. Fifty-six of 63 children (89%) with primary hypertension had a serum uric acid value >5.5 mg/dL, whereas this value was present in only 12 of 40 children (30%) with secondary hypertension, 0 of 22 children with white-coat hypertension, and 0 of 40 controls. In this population of children with normal renal function who were undergoing initial evaluation for hyper-tension, a serum uric acid value >5.5 mg/dL had a positive predictive value of 82% for primary hypertension. The sensitivity and specificity of a serum uric acid value >5.5 mg/dL for primary hypertension were 87% and 86%, respectively. In this population, a serum uric acid value >5.5 mg/dL strongly supports the diagnosis of primary hypertension and essentially excludes the diagnosis of white-coat hypertension.
Correlation Between Serum Uric Acid and Systolic and Diastolic BP
Because the children with primary hypertension had no identifiable etiology for their elevated BP and they tended to have higher serum uric acid levels, consistent with the hypothesis of a causal link, we evaluated the correlation between BP and serum uric acid in children with primary hypertension compared with control children with normal BP. The correlations for uric acid and systolic BP (r=0.8053, P=0.00002) and uric acid and diastolic BP (r=0.6606, P=0.0006) are shown in Figure 2. The correlation of serum uric acid with BP is continuous and linear, with a change in uric acid of 1.0 mg/dL corresponding to an increase of 10 mm Hg in systolic BP. A correlation is also present in secondary hypertension between uric acid and SBP (r=0.4357 (P=0.002) and between uric acid and DBP (r=0.3076 (P=0.005) but is weak relative to that in primary hypertension (graphical data not shown).
Figure 2.

A, Serum uric acid is plotted against systolic BP for children with normal BP (controls) and primary hypertension. B, Serum uric acid is plotted against diastolic BP for children with normal BP and primary hypertension. Data do not include individuals with secondary hypertension. Solid and dotted lines in both panels represent the best fit and 95% confidence intervals, respectively, and demonstrate the linear relation between uric acid concentration and BP. Pearson correlation coefficients are r=0.8053 (P=0.000004) for systolic BP and r=0.6606 (P=0.0014) for diastolic BP.
GFR Is Not the Determinant of BP or Serum Uric Acid
Although renal insufficiency can lead to both hypertension and hyperuricemia, decreased renal function does not account for the differences between children with normal and elevated BP in this study. The mean corrected GFR was the same for both groups (Table), and no correlation existed between GFR and serum uric acid (r=0.0407; Figure 3).
Figure 3.

No correlation between uric acid and GFR. Serum uric acid is plotted against GFR for children with normal BP and primary hypertension (same population as in Figure 2). The correlation coefficient is r=0.0407.
Obesity, Hyperuricemia, and Hypertension
Obesity has been associated with both hypertension and hyperuricemia; however, obesity could not explain the presence of either condition in these patients. When the mean BMI percentile (for age and gender) was assessed for each of the diagnostic groups, the link between hyperuricemia and obesity was inconsistent (Table). The children with primary hypertension and white-coat hypertension were quite obese, but only those with true hypertension had elevated serum uric acid levels. In contrast, children with secondary hypertension had modestly elevated uric acid levels, but their BMI was the same as that of the controls. Furthermore, in multiple regression analysis, the contribution of BMI percentile to systolic and diastolic BP was small. Controlling for the effect of BMI percentile as a covariate left a residual correlation between serum uric acid and SBP of r=0.7309 (P=0.00004) and between serum uric acid and DBP of r=0.6095 (P=0.003).
Discussion
In this study, we examined the relation of serum uric acid to BP in newly diagnosed, untreated, childhood hypertension. The study was based on strong epidemiologic data that have linked serum uric acid to hypertension in humans4-8,27,28 and experimental animal data, which suggest that hyperuricemia causes hypertension.9-11,29 The experimental studies further demonstrated that hyperuricemia caused preglomerular vascular disease via a BP-independent pathway,10 and once vascular changes were established, the hypertension was driven by the kidney, and lowering uric acid levels was no longer protective.11 The observation from the Framingham study that the correlation of uric acid levels with BP was attenuated in the study population as they aged is consistent with these data.30,31 We therefore hypothesized that if serum uric acid were important in the genesis of primary hypertension, then the relation would be greatest in the young.
To test this hypothesis, we evaluated 125 children referred for hypertension. Twenty-two children were diagnosed with white-coat hypertension, 40 with secondary hypertension, and 63 with primary hypertension. The children with primary hypertension had, on average, moderately severe systolic hypertension (>15 mm Hg >95th percentile) but only mild diastolic hypertension. The children with secondary hyper-tension showed a trend toward slightly lower systolic BPs (139±15.9 vs 146±10.7 mm Hg), but the difference did not achieve statistical significance. The modest trend likely reflects the age and height difference between the subjects with primary and secondary hypertension. The finding that there are similar degrees of hypertension in the primary and secondary groups despite the very large difference in serum uric acid values suggests that uric acid is not merely a marker for the severity of hypertension.
To maximize the clinical relevance of the findings, we evaluated the relation between serum uric acid and the diagnostic criteria for hypertension that are most readily available to pediatricians, that of in-clinic BPs. When safe, we also obtained ambulatory BP monitoring data to assist in categorizing the children by type of hypertension; however, because the children were recruited at the time of initial evaluation, some children were sufficiently hypertensive to be at risk if treatment were delayed. Such children did not undergo diagnostic ambulatory monitoring. Sorof and colleagues21 estimated the probability of white-coat hypertension in the pediatric population as a function of BP index (BPI; the ratio of the child's casual BP to the 95th percentile for the child's age, height, and gender). As the BPI increased from 1.0 to 1.2, the probability of white-coat hypertension fell from 87% to 15% and was <5% for a BPI of 1.3. For the 8 children with primary hypertension who did not have ambulatory monitoring, their mean systolic BPI was 1.34 (range, 1.29 to 1.44) and for the 19 children with secondary hyper-tension who did not have ambulatory monitoring, their mean BPI was 1.26 (range, 1.20 to 1.51). The lack of ambulatory monitoring in these children is unlikely to have resulted in a significant rate of misdiagnosis. Furthermore, when correlation analysis was performed only on the children who underwent ambulatory monitoring, the correlation between uric acid and mean (ambulatory) daytime systolic BP was r=0.8773 for primary hypertension and r=0.5123 for secondary hypertension. Consequently, the clinically motivated decision to not perform ambulatory BP monitoring on certain patients had little or no effect on the observed relation between primary hypertension and serum uric acid.
Remarkably, the vast majority of children with primary hypertension (89%) had serum uric acid levels >5.5 mg/dL in contrast to 30% of children with secondary hypertension and none with white-coat hypertension or controls. The relation of serum uric acid with systolic and diastolic BP was continuous and much stronger (r=0.8053 for systolic BP) than that typically observed in adults.30,31 The increase in serum uric acid level could not be accounted for by differences in GFR, for which there was no correlation, and although the BMI percentile was higher in children with primary hypertension, multiple regression analysis revealed that BMI contributed relatively little to the observed effect of serum uric acid on BP.
Two large studies, the Moscow Children's Hypertension Study32 and NHANES III,33 have reported an association of serum uric acid with hypertension in children. The Hungarian Children's Health Study34 also reported that an elevated serum uric acid could predict the development of hypertension in children. Although these studies were quite large, none reported renal function, type of hypertension, severity of hypertension, or other medical diagnoses, making generalization impossible. In a small study of adolescents with primary hypertension, Gruskin35 found that hypertensive children had elevated serum uric acid (mean >6.5 mg/dL) and plasma renin activity. These data are consistent with the current study.
What is the mechanism for the increased serum uric acid level in children with primary hypertension? It is not decreased renal function, because GFR was similar in all groups. Likewise, although elevated uric acid levels are observed in subjects with obesity and hyperinsulinism,36 uric acid levels were not elevated in white-coat-hypertensive subjects despite a similar degree of obesity. Finally, decreased renal blood flow, which is a characteristic finding in subjects with hypertension, might result in increased urate reabsorption and an elevation in serum uric acid.37 Hence, one might postulate that an elevated uric acid level might simply reflect the severity of hypertension, rather than contribute to it pathogenetically.
There are, however, several lines of evidence that suggest serum uric acid contributes to hypertension. First, an elevated uric acid is known to independently predict the development of hypertension7,8,34 and hence, precede hypertension. Second, infusion of uric acid in human subjects causes endothelial dysfunction and impaired vasodilation,38 known features of essential hypertension. Third, if uric acid were simply a marker, then a similar degree of hyperuricemia in the children with secondary hypertension would be expected, and this was not observed.
Recent experimental studies provide a plausible physiologic mechanism by which increases in serum uric acid might cause hypertension.9,10,29,39 Uric acid enters vascular smooth muscle cells, where it stimulates mitogen-activated protein kinases, cyclooxygenase 2, and platelet-derived growth factor to induce vascular smooth muscle proliferation and preglomerular arteriolopathy.10,11,39 Increased serum uric acid further causes an increase in juxtaglomerular renin production and a decrease in macula densa neuronal nitric oxide synthase expression, and these 2 processes lead directly to increased BP.9 Once the vascular lesion is established, the rats show salt sensitivity despite correction of their uric acid levels.11 This is consistent with studies in other experimental models, which show that induction of preglomerular microvascular disease, regardless of etiology, leads to salt-sensitive hypertension.40,41 The mechanism for the persistent salt sensitivity is thought to be due to the development of renal ischemia, activation of the renin-angiotensin system, leading to renal vasoconstriction, decreased sodium filtration, and increased sodium reabsorption.42 Other factors, such as a congenital reduction in nephron number,43 can also enter into this pathway.44
Perspective
Serum uric acid is strongly correlated with BP in childhood primary hypertension. An elevated or high-normal serum uric acid value >5.5 mg/dL in a child being evaluated for hypertension strongly supports the presence of primary hypertension and essentially excludes white-coat hypertension. The remarkable association of uric acid with BP in childhood is consistent with recent animal model data and the hypothesis that uric acid might have a pathogenic role in the development of hypertension.
Acknowledgments
This study was supported by National Institutes of Health (Bethesda, Md) grants RR-17665, HL-68607, and DK-52121 and the George O'Brien Center grant 1P50DK-064233-01.
References
- 1.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Ford CE, Shulman NB, Stamler J. Blood pressure and end-stage renal disease in men. N Engl J Med. 1996;334:13–18. doi: 10.1056/NEJM199601043340103. [DOI] [PubMed] [Google Scholar]
- 2.Coresh J, Wei GL, McQuillan G, Brancati FL, Levey AS, Jones C, Klag MJ. Prevalence of high blood pressure and elevated serum creatinine level in the United States: findings from the third National Health and Nutrition Examination Survey (1988–1994) Arch Intern Med. 2001;161:1207–1216. doi: 10.1001/archinte.161.9.1207. [DOI] [PubMed] [Google Scholar]
- 3.Mohamed FA. On chronic Bright's disease, and its essential symptoms. Lancet. 1879;1:399–401. [Google Scholar]
- 4.Cannon PJ, Stason WB, Demartini FE, Sommers SC, Laragh JH. Hyper-uricemia in primary and renal hypertension. N Engl J Med. 1966;275:457–464. doi: 10.1056/NEJM196609012750902. [DOI] [PubMed] [Google Scholar]
- 5.Kinsey D, Walther R, Sise HS, Whitelaw G, Smithwick R. Incidence of hyperuricemia in 400 hypertensive subjects. Circulation. 1961;24:972–973. [Google Scholar]
- 6.Klein R, Klein BE, Cornoni JC, Maready J, Cassel JC, Tyroler HA. Serum uric acid: its relationship to coronary heart disease risk factors and cardiovascular disease, Evans County, Georgia. Arch Intern Med. 1973;132:401–410. doi: 10.1001/archinte.132.3.401. [DOI] [PubMed] [Google Scholar]
- 7.Selby JV, Friedman GD, Quesenberry CP., Jr. Precursors of essential hypertension: pulmonary function, heart rate, uric acid, serum cholesterol, and other serum chemistries. Am J Epidemiol. 1990;131:1017–1027. doi: 10.1093/oxfordjournals.aje.a115593. [DOI] [PubMed] [Google Scholar]
- 8.Jossa F, Farinaro E, Panico S, Krogh V, Celentano E, Galasso R, Mancini M, Trevisan M. Serum uric acid and hypertension: the Olivetti heart study. J Hum Hypertens. 1994;8:677–681. [PubMed] [Google Scholar]
- 9.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ. Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension. 2001;38:1101–1106. doi: 10.1161/hy1101.092839. [DOI] [PubMed] [Google Scholar]
- 10.Mazzali M, Kanellis J, Han L, Feng L, Xia YY, Chen Q, Kang DH, Gordon KL, Watanabe S, Nakagawa T, Lan HY, Johnson RJ. Hyper-uricemia induces a primary renal arteriolopathy in rats by a blood pressure-independent mechanism. Am J Physiol Renal Physiol. 2002;282:F991–F997. doi: 10.1152/ajprenal.00283.2001. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe S, Kang DH, Feng L, Nakagawa T, Kanellis J, Lan HY, Johnson RJ. Uric acid hominoid evolution and the pathogenesis of salt-sensitivity. Hypertension. 2002;40:355–360. doi: 10.1161/01.hyp.0000028589.66335.aa. [DOI] [PubMed] [Google Scholar]
- 12.Zinner SH, Rosner B, Oh W, Kass EH. Significance of blood pressure in infancy: familial aggregation and predictive effect on later blood pressure. Hypertension. 1985;7(pt 1):411–416. [PubMed] [Google Scholar]
- 13.Lauer RM, Clarke WR. Childhood risk factors for high adult blood pressure: the Muscatine Study. Pediatrics. 1989;84:633–641. [PubMed] [Google Scholar]
- 14.Berenson GS, Srinivasan SR, Hunter SM, Nicklas TA, Freedman DS, Shear CL, Webber LS. Risk factors in early life as predictors of adult heart disease: the Bogalusa Heart Study. Am J Med Sci. 1989;298:141–151. doi: 10.1097/00000441-198909000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Burke GL, Arcilla RA, Culpepper WS, Webber LS, Chiang YK, Berenson GS. Blood pressure and echocardiographic measures in children: the Bogalusa Heart Study. Circulation. 1987;75:106–114. doi: 10.1161/01.cir.75.1.106. [DOI] [PubMed] [Google Scholar]
- 16.Pontremoli R, Nicolella C, Viazzi F, Ravera M, Sofia A, Berruti V, Bezante GP, Del Sette M, Martinoli C, Sacchi G, Deferrari G. Microalbuminuria is an early marker of target organ damage in essential hyper-tension. Am J Hypertens. 1998;1(pt 1):430–438. doi: 10.1016/s0895-7061(97)00498-6. [DOI] [PubMed] [Google Scholar]
- 17.Report of the Second Task Force on Blood Pressure Control in Children–1987. Task Force on Blood Pressure Control in Children: National Heart, Lung, and Blood Institute, Bethesda, Maryland. Pediatrics. 1987;79:1–25. [PubMed] [Google Scholar]
- 18.Update on the 1987 Task Force Report on High Blood Pressure in Children and Adolescents: a working group report from the National High Blood Pressure Education Program: National High Blood Pressure Education Program Working Group on Hypertension Control in Children and Adolescents. Pediatrics. 1996;98(pt 1):649–658. [PubMed] [Google Scholar]
- 19.Gruskin AB, Dabbagh S, Fleischmann LF, Apostol EL, Mattoo TK. Mechanisms of hypertension in childhood diseases. In: Barratt TM, Avner ED, Harmon W, editors. Pediatric Nephrology. 4th ed Williams & Wilkins; Baltimore, Md: 1999. pp. 987–1006. [Google Scholar]
- 20.Norwood VF. Hypertension. Pediatr Rev. 2002;23:197–208. doi: 10.1542/pir.23-6-197. [DOI] [PubMed] [Google Scholar]
- 21.Sorof JM, Poffenbarger T, Franco K, Portman R. Evaluation of white coat hypertension in children: importance of the definitions of normal ambulatory blood pressure and the severity of casual hypertension. Am J Hypertens. 2001;14(pt 1):855–860. doi: 10.1016/s0895-7061(01)02180-x. [DOI] [PubMed] [Google Scholar]
- 22.Kouidi E, Fahadidou-Tsiligiroglou A, Tassoulas E, Deligiannis A, Coats A. White coat hypertension detected during screening of male adolescent athletes. Am J Hypertens. 1999;12(pt 1):223–226. doi: 10.1016/s0895-7061(98)00186-1. [DOI] [PubMed] [Google Scholar]
- 23.Bald M. Ambulatory blood pressure monitoring in children and adolescents: current insights into a new technique. Minerva Pediatr. 2002;54:13–24. [PubMed] [Google Scholar]
- 24.Koch VH, Furusawa EA, Saito MI, Colli A, Ignes EC, Okay Y, Mion D., Jr. White coat hypertension in adolescents. Clin Nephrol. 1999;52:297–303. [PubMed] [Google Scholar]
- 25.Schettini C, Bianchi M, Nieto F, Sandoya E, Senra H. Ambulatory blood pressure: normality and comparison with other measurements: Hyper-tension Working Group. Hypertension. 1999;34(pt 2):818–825. doi: 10.1161/01.hyp.34.4.818. [DOI] [PubMed] [Google Scholar]
- 26.Soergel M, Kirschstein M, Busch C, Danne T, Gellermann J, Holl R, Krull F, Reichert H, Reusz GS, Rascher W. Oscillometric twenty-four-hour ambulatory blood pressure values in healthy children and adolescents: a multicenter trial including 1141 subjects. J Pediatr. 1997;130:178–184. doi: 10.1016/s0022-3476(97)70340-8. [DOI] [PubMed] [Google Scholar]
- 27.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971–1992. National Health and Nutrition Examination Survey. JAMA. 2000;283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 28.Franse LV, Pahor M, Di Bari M, Shorr RI, Wan JY, Somes GW, Applegate WB. Serum uric acid, diuretic treatment and risk of cardio-vascular events in the Systolic Hypertension in the Elderly Program (SHEP) J Hypertens. 2000;18:1149–1154. doi: 10.1097/00004872-200018080-00021. [DOI] [PubMed] [Google Scholar]
- 29.Sanchez-Lozada LG, Tapia E, Avila-Casado C, Soto V, Franco M, Santamaria J, Nakagawa T, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Mild hyperuricemia induces glomerular hypertension in normal rats. Am J Physiol Renal Physiol. 2002;283:F1105–F1110. doi: 10.1152/ajprenal.00170.2002. [DOI] [PubMed] [Google Scholar]
- 30.Kannel WB. Metabolic risk factors for coronary heart disease in women: perspective from the Framingham Study. Am Heart J. 1987;114:413–419. doi: 10.1016/0002-8703(87)90511-4. [DOI] [PubMed] [Google Scholar]
- 31.Brand FN, McGee DL, Kannel WB, Stokes J, 3rd, Castelli WP. Hyper-uricemia as a risk factor of coronary heart disease: the Framingham Study. Am J Epidemiol. 1985;121:11–18. doi: 10.1093/oxfordjournals.aje.a113972. [DOI] [PubMed] [Google Scholar]
- 32.Rovda IuI, Kazakova LM, Plaksina EA. Parameters of uric acid metabolism in healthy children and in patients with arterial hypertension. Pediatriia. 1990;8:19–22. [PubMed] [Google Scholar]
- 33.Goldstein HS, Manowitz P. Relation between serum uric acid and blood pressure in adolescents. Ann Hum Biol. 1993;20:423–431. doi: 10.1080/03014469300002832. [DOI] [PubMed] [Google Scholar]
- 34.Torok E, Gyarfas I, Csukas M. Factors associated with stable high blood pressure in adolescents. J Hypertens Suppl. 1985;3(suppl 3):S389–S390. [PubMed] [Google Scholar]
- 35.Gruskin AB. The adolescent with essential hypertension. Am J Kidney Dis. 1985;6:86–90. doi: 10.1016/s0272-6386(85)80146-3. [DOI] [PubMed] [Google Scholar]
- 36.Reaven GM. The kidney: an unwilling accomplice in syndrome X. Am J Kidney Dis. 1997;30:928–931. doi: 10.1016/s0272-6386(97)90106-2. [DOI] [PubMed] [Google Scholar]
- 37.Messerli FH, Frohlich ED, Dreslinski GR, Suarez DH, Aristimuno GG. Serum uric acid in essential hypertension: an indicator of renal vascular involvement. Ann Intern Med. 1980;93:817–821. doi: 10.7326/0003-4819-93-6-817. [DOI] [PubMed] [Google Scholar]
- 38.Wang JG, Staessen JA, Fagard RH, Birkenhager WH, Gong L, Liu L. Prognostic significance of serum creatinine and uric acid in older Chinese patients with isolated systolic hypertension. Hypertension. 2001;37:1069–1074. doi: 10.1161/01.hyp.37.4.1069. [DOI] [PubMed] [Google Scholar]
- 39.Rao GN, Corson MA, Berk BC. Uric acid stimulates vascular smooth muscle cell proliferation by increasing platelet-derived growth factor A-chain expression. J Biol Chem. 1991;266:8604–8608. [PubMed] [Google Scholar]
- 40.Quiroz Y, Pons H, Gordon KL, Rincon J, Chavez M, Parra G, Herrera-Acosta J, Gomez-Garre D, Largo R, Egido J, Johnson RJ, Rodriguez-Iturbe B. Mycophenolate mofetil prevents salt-sensitive hypertension resulting from nitric oxide synthesis inhibition. Am J Physiol Renal Physiol. 2001;281:F38–F47. doi: 10.1152/ajprenal.2001.281.1.F38. [DOI] [PubMed] [Google Scholar]
- 41.Franco M, Tapia E, Santamaria J, Zafra I, Garcia-Torres R, Gordon KL, Pons H, Rodriguez-Iturbe B, Johnson RJ, Herrera-Acosta J. Renal cortical vasoconstriction contributes to development of salt-sensitive hyper-tension after angiotensin II exposure. J Am Soc Nephrol. 2001;12:2263–2271. doi: 10.1681/ASN.V12112263. [DOI] [PubMed] [Google Scholar]
- 42.Johnson RJ, Herrera-Acosta J, Schreiner GF, Rodriguez-Iturbe B. Subtle acquired renal injury as a mechanism of salt-sensitive hypertension. N Engl J Med. 2002;346:913–923. doi: 10.1056/NEJMra011078. [DOI] [PubMed] [Google Scholar]
- 43.Keller J, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–118. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- 44.Johnson RJ, Rodriguez-Iturbe B, Schreiner GF, Herrera-Acosta J. Hypertension: a microvascular and tubulointerstitial disease. J Hypertens. 2002;20(suppl 3):S1–S7. [PubMed] [Google Scholar]


