Abstract
We previously reported that brief (1 hr), but not extended (6 hrs), daily access to cocaine results in a sensitized locomotor response to cocaine and in elevated c-Fos immunoreactivity and DAT binding in the nucleus accumbens (N.Acc) core. In order to better our understanding of the neural adaptations mediating the transition from controlled drug-use to addiction, the current experiments were set to further explore the neural adaptations resulting from these two access conditions. Rats received either brief daily access to saline or cocaine, or brief daily access followed by extended daily access, to cocaine. Subjects were then sacrificed either 20 minutes, or 14 or 60 days, after the last self-administration session. Samples of the ventral tegmental area (VTA), N.Acc core and shell, dorsal striatum, and medial prefrontal cortex (mPFC) were taken for analysis of D1 ([3H]SCH-23390), D2 ([3H]Spiperone), and NMDA ([3H]MK-801) receptor binding (using the method of receptor autoradiography). At 20 minutes into withdrawal D2 receptors were elevated and NMDA receptors were reduced in the mPFC of the brief access animals while D1 receptors were elevated in the N.Acc shell of the extended access animals, compared to saline controls. D2 receptors were reduced in the N.Acc shell of the brief access animals compared to saline controls after 14 days, and compared to extended access animals after 60 days of withdrawal. In summary, extended access to cocaine resulted in only transient changes in D1 receptors binding. These results suggest that the development of compulsive drug use is largely unrelated to changes in total binding of D2 or NMDA receptors.
Keywords: Cocaine, D1, D2, NMDA, Addiction, Self-administration, Drug-reinforcement
1. Introduction
In order to illuminate the neural mechanisms that underlie cocaine addiction, it's critical to understand and distinguish between the neural response to first drug challenge, the neural adaptations occurring with repeated stable drug administration and finally those that mediate the transition to compulsive uncontrolled drug use. While research efforts so far have greatly advanced our understanding of the neural response to an acute cocaine challenge and the neuroadaptations that accompany stable drug use, our knowledge of the mechanisms that underlie the transition to addiction is still lacking. In order to study the neuroadaptations that accompany the transition to addiction, we adopted an animal model in which animals receive extended daily access to cocaine and as a result exhibit escalated patterns of drug use, reminiscent of the pattern of drug use seen in human addicts (Ahmed & Koob, 1998). More specifically, we employed three conditions in our studies: a control group that had daily access to IV saline (Sal group), a brief access group that had daily access to IV cocaine for one hour (Coc1h group), and an extended access group that had 6 hours of daily access to cocaine (Coc6h group). Similar to Ahmed & Koob (1998), we observed stable levels of cocaine self-administration in the brief access group, but an escalated consumption of cocaine in the extended access condition (Ben-Shahar et al., 2004, 2005, 2006).
We have previously reported that when challenged with one self-administered IV infusion of cocaine after 14 days of withdrawal, animals from the brief access group exhibited sensitized locomotor and c-Fos immunoreactive response. In contrast, extended access animals produced comparable levels of locomotion and c-Fos as those exhibited by saline control, in response to this same cocaine challenge (Ben-Shahar et al., 2004). After 60 days of withdrawal a tolerant locomotor response to an IP cocaine challenge was observed in the extended access, but not the brief access, group (Ben-Shahar et al., 2005). Finally, levels of the dopaminergic transporter (DAT) were found to be elevated in the nucleus accumbens core of the brief access, but not extended access, animals, after 14 days of withdrawal (Ben-Shahar et al., 2006). In short, increasing the amount of daily access to cocaine not only resulted in an escalated pattern of cocaine use, but also in a reduced response to cocaine challenge following withdrawal, and a return to baseline DAT levels. We concluded that the extended access condition leads to a different set of neuronal changes than those induced by the brief access condition, and that those changes associated with the extended access condition are the ones underlying the development of the addictive state.
Cocaine reinforcement and cocaine-induced behavioral sensitization depend upon the activation of dopaminergic and glutamatergic neurotransmission (especially via NMDA receptors) in the ventral tegmental area (VTA), nucleus accumbens (N.Acc), and prefrontal cortex (PFC) (Goeders & Smith, 1983,1993; Hurd et al., 1997; Pierce et al., 1997; Pulvirenti et al., 1992; Ritz et al., 1987, 1988; Roberts & Koob, 1982; Vanderschuren & Kalivas, 2000; Volkow et al., 1997a,1997b; Wolf, 1998; White et al., 1995; Zhang et al., 1997). The differences we previously observed between the brief and extended access group were also found within either the N.Acc and/or PFC (i.e. c-Fos activation and DAT levels), or were related to dopaminergic and glutamatergic function within the VTA, N.Acc, and PFC (i.e. locomotor activation). It is therefore reasonable to assume that the neuroadaptations resulting from the extended access condition involve changes in the function of dopamine and glutamate within the VTA, N.Acc and PFC. This conclusion is strengthen by the findings that while cocaine-induced dopamine release within the nucleus accumbens was similar in brief access and extended access animals, extended access animals worked to maintain significantly higher levels of synaptic dopamine as compared to brief access animals (Ahmed et al., 2003). In addition, much lower doses of the D1/D2 antagonist cis-Flupenthixol were needed to affect rates of cocaine self-administration in extended, compared to brief, access animals (Ahmed et al., 2003; Ahmed & Koob, 2004; Koob et al., 2004). Ahmed & Koob (2004) concluded that the higher sensitivity to cis-Flupenthixol observed in the extended access group reflects changes in number and/or function of post synaptic D1 and D2 receptors. In the current investigation we continued our exploration of the biological mechanisms underlying the development of addiction by monitoring levels of D1, D2, and NMDA receptors following 0, 14, or 60 days withdrawal from zero, brief, or extended access to cocaine.
2. Results
Self-Administration
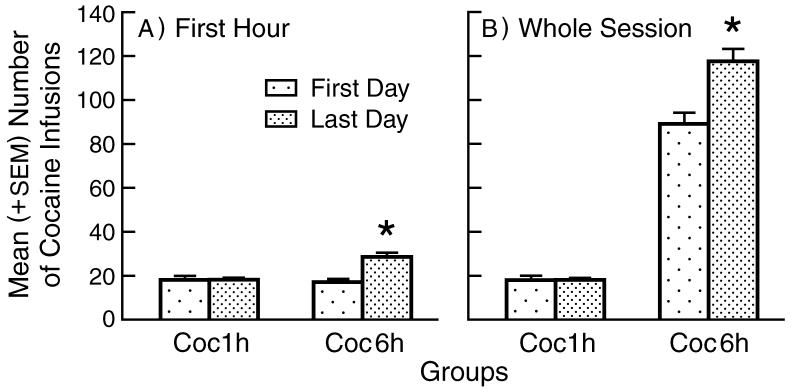
As expected, saline control animals (n=19) showed low levels of responding (6 lever-presses per session on average). Coc1h (n=18) animals exhibited stable self-administration patterns and showed no change in self-administration responding between the first and last day of the eight-day period (Figure 1). Coc6h (n=16) animals showed increased rates of self-administration (i.e., escalation) from the first to the last day of the eight-day period. This was true for levels of self-administration during the first hour of the session (See Figure 1 Panel A) where a two-way ANOVA yielded a significant main effect for Day (F(1,32)=38.271, p<.0001), a significant main effect for Group (F(1,32)=6.557, p<.015), and a significant Day X Group interaction (F(1,32)=42.291, p<.0001). One-way ANOVA's conducted on each cocaine group separately revealed no difference between day 1 and day 8 for the Coc1h group, but a significant such difference for the Coc6h group (F(1,15)=60.651, p<.0001). Increase in self-administration responding from first to last session was also seen in total infusions taken by the Coc6h animals during the whole session (See Figure 1 Panel B). Thus a two way ANOVA revealed a significant main effect for Day (F(1,32)=82.283, p<0.0001), a significant main effect for Group (F(1,32)=279.292, p<0.0001), and significant interaction for Day X Group (F(1,32)=85.497, p<0.0001). For the Coc1h group the first hour comprised the whole session, therefore there was no need to repeat the simple effect analysis for this group. However, one-way ANOVA for the Coc6h group revealed significant effect for day (F(1,15)=85.021, p<.0001).
1.
Self-Administration Patterns – Group mean (+SEM) number of self-administered cocaine infusions during the first hour (Panel A) and throughout the session (Panel B) of the first and last day of self-administration post-training. * signifies a significant difference between first and last day (p<.0001).
D1, D2, and NMDA Densities
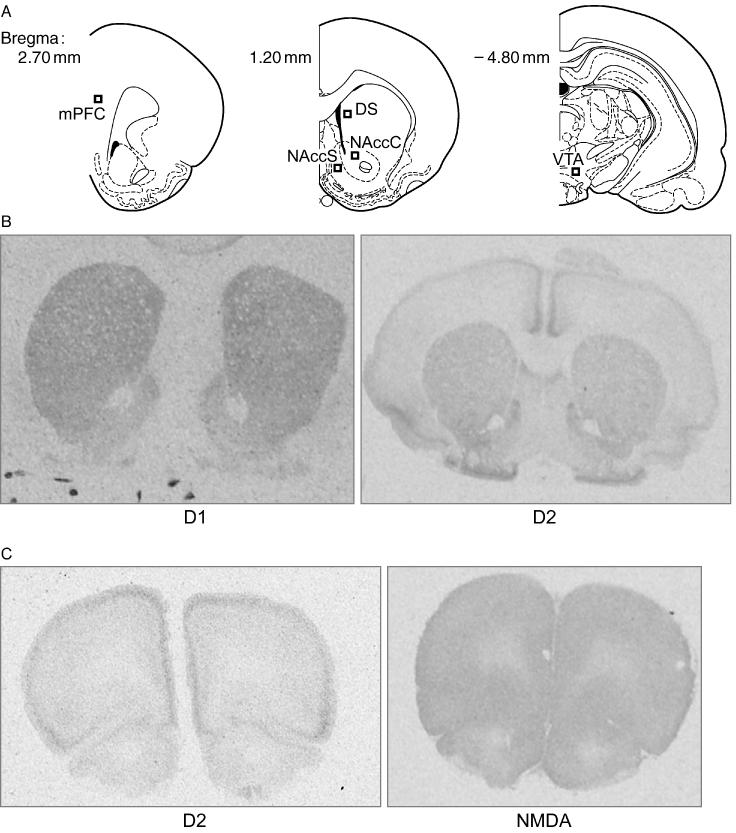
One Way ANOVA's followed by Tukey Post Hoc comparisons were utilized to compare D1, D2, and NMDA densities in the VTA, N.Acc core and shell, dorsal striatum, and mPFC of animals from the Sal, Coc1h, and Coc6h groups. Schematics of the coordinates for the these brain areas are included in Figure 2 Panel A. Sample sections of the N.Acc and striatum stained for D1 or D2 dopamine receptors are presented in Figure 2 Panel B. Sample sections of the mPFC stained for D2 dopamine receptors or NMDA receptors are presented in Figure 2 Panel C.
2.
Sample Autoradiographs – Schematics of all the brain areas sampled and their coordinates are illustrated in Panel A. Panel B includes sample autoradiographs of D1 and D2 staining in the nucleus accumbens and striatum. Panel C includes D2 and NMDA staining in the mPFC.
Twenty minutes of withdrawal
Twenty minutes after the end of the last self-administration session NMDA receptor densities were lower in the mPFC of the Coc1h (n=4) animals in comparison to the Sal (n=5), but not the Coc6h (n=4), animals. This was evident from a significant main effect for Group (F(2,12)=5.195, p<.028) resulting from a significant difference between Coc1h and Sal groups (Post Hoc comparison: p<.023). Levels of D2 dopamine receptors in the mPFC were higher in the Coc1h group as compared to the Sal, but not the Coc6h, group, at this time point. A One Way ANOVA confirmed a significant effect for Group (F(2,12)=7.985, p<.008). Subsequent Post Hoc tests revealed a significant difference between the Coc1h and the Sal groups (p<.007). Finally, at 20 minutes of withdrawal, densities of D1 dopamine receptors were significantly higher in the N.Acc shell of the Coc6h condition compared to the Sal, but not the Coc1h, condition (F(2,12)=4.509, p<.04). Post Hoc tests yielded a significant different between the Coc6h and the Sal conditions (p<.04). Densities of NMDA and D2 receptors in the VTA, N.Acc and dorsal striatum, and densities of D1 in the VTA, N.Acc core, dorsal striatum, and mPFC were similar for the three experimental groups at 20 minutes of withdrawal (See Table 1).
Table 1.
Receptor Density After 20 Minutes of Withdrawal – Mean (+SEM) density (fmol/mg) of D1, D2, and NMDA receptors in the VTA, N.Acc Shell, N.Acc Core, Dorsal Striatum, and mPFC in the Sal, Coc1h, and Coc6h groups 20 minutes after the last self-administration session.
| Saline | Coc1h | Coc6h | |
|---|---|---|---|
| D1 | |||
| mPFC | 13 ± 4 | 17 ± 2 | 22 ± 3 |
| N.Acc Core | 34 ± 6 | 38 ± 2 | 46 ± 6 |
| N.Acc Shell | 15 ± 3 | 25 ± 4 | 31 ± 7 ** |
| Striatum | 62 ± 10 | 59 ± 8 | 73 ± 6 |
| VTA | 9 ± 3 | 6 ± 1 | 5 ± 2 |
| D2 | |||
| mPFC | 168 ± 25 | 308 ± 25 ** | 248 ± 26 |
| N.Acc Core | 211 ± 32 | 298 ± 28 | 299 ± 23 |
| N.Acc Shell | 252 ± 16 | 286 ± 21 | 278 ± 19 |
| Striatum | 174 ± 17 | 164 ± 29 | 207 ± 38 |
| VTA | 212 ± 19 | 152 ± 37 | 175 ± 39 |
| NMDA | |||
| mPFC | 395 ± 21 | 254 ± 43 ** | 356 ± 32 |
| N.Acc Core | 220 ± 43 | 234 ± 62 | 278 ± 42 |
| N.Acc Shell | 193 ± 40 | 179 ± 54 | 225 ± 48 |
| Striatum | 268 ± 46 | 240 ± 33 | 242 ± 35 |
| VTA | 59 ± 14 | 141 ± 34 | 106 ± 21 |
Significantly different than Saline
Fourteen Days of Withdrawal
Lower levels of D2 receptors within the N.Acc shell were found in the Coc1h group (n=6) relative to the Sal group (n=6), but not compared to the Coc6h group (n=5). This was evident from a significant One Way ANOVA (F(2,14)=4.048, p<.045) and a significant subsequent Post Hoc comparison between the Coc1h and the Sal groups (p<.054). Densities of D2 receptors in the VTA, N.Acc core, dorsal striatum, and mPFC, and densities of D1 and NMDA receptors in all the brain areas analyzed, were similar for the three experimental groups at 14 days of withdrawal (See Table 2).
Table 2.
Receptor Density After 14 Days of Withdrawal – Mean (+SEM) density (fmol/mg) of D1, D2, and NMDA receptors in the VTA, N.Acc Shell, N.Acc Core, Dorsal Striatum, and mPFC in the Sal, Coc1h, and Coc6h groups 14 days after the last self-administration session.
| Saline | Coc1h | Coc6h | |
|---|---|---|---|
| D1 | |||
| mPFC | 21 ± 4 | 25 ± 1 | 19 ± 4 |
| N.Acc Core | 156 ± 9 | 151 ± 11 | 167 ± 10 |
| N.Acc Shell | 131 ± 6 | 121 ± 19 | 135 ± 8 |
| Striatum | 164 ± 10 | 171 ± 10 | 179 ± 17 |
| VTA | 13 ± 1 | 9 ± 2 | 9 ± 1 |
| D2 | |||
| mPFC | 143 ± 15 | 163 ± 12 | 178 ± 13 |
| N.Acc Core | 163 ± 13 | 115 ± 11 | 154 ± 14 |
| N.Acc Shell | 240 ± 29 | 153 ± 19 ** | 174 ± 13 |
| Striatum | 121 ± 18 | 84 ± 18 | 83 ± 11 |
| VTA | 33 ± 14 | 47 ± 7 | 50 ± 22 |
| NMDA | |||
| mPFC | 260 ± 21 | 273 ± 42 | 258 ± 32 |
| N.Acc Core | 126 ± 18 | 122 ± 18 | 124 ± 19 |
| N.Acc Shell | 111 ± 25 | 134 ± 21 | 113 ± 26 |
| Striatum | 112 ± 17 | 124 ± 12 | 118 ± 14 |
| VTA | 84 ± 20 | 76 ± 16 | 115 ± 19 |
Significantly different than Saline
Sixty Days of Withdrawal
A One Way ANOVA analyzing the D2 receptor densities data within the N.Acc shell revealed a significant main effect for Group (F(2,22)=3.714, p<.042) that resulted from significantly lower levels of D2 receptors in this brain area in the Coc1h (n=8) condition compared to the Coc6h (n=7), but not the Sal (n=8), condition (p< .038). Densities of D2 receptors in the VTA, N.Acc core, dorsal striatum, and mPFC, and densities of D1 and NMDA receptors in all the brain areas analyzed, were similar for the three experimental groups at 60 days of withdrawal (See Table 3).
Table 3.
Receptor Density After 60 Days of Withdrawal – Mean (+SEM) density (fmol/mg) of D1, D2, and NMDA receptors in the VTA, N.Acc Shell, N.Acc Core, Dorsal Striatum, and mPFC in the Sal, Coc1h, and Coc6h groups 60 days after the last self-administration session.
| Saline | Coc1h | Coc6h | |
|---|---|---|---|
| D1 | |||
| mPFC | 15 ± 2 | 18 ± 2 | 12 ± 2 |
| N.Acc Core | 37 ± 4 | 42 ± 3 | 42 ± 3 |
| N.Acc Shell | 27 ± 6 | 27 ± 3 | 31 ± 6 |
| Striatum | 62 ± 6 | 65 ± 3 | 72 ± 5 |
| VTA | 4 ± 1 | 6 ± 1 | 5 ± 1 |
| D2 | |||
| mPFC | 242 ± 15 | 253 ± 24 | 261 ± 23 |
| N.Acc Core | 242 ± 25 | 242 ± 25 | 251 ± 8 |
| N.Acc Shell | 278 ± 30 | 220 ± 17 * | 297 ± 18 |
| Striatum | 173 ± 23 | 184 ± 14 | 175 ± 20 |
| VTA | 207 ± 17 | 193 ± 31 | 190 ± 23 |
| NMDA | |||
| mPFC | 357 ± 37 | 298 ± 49 | 311 ± 37 |
| N.Acc Core | 217 ± 22 | 229 ± 32 | 227 ± 31 |
| N.Acc Shell | 190 ± 28 | 205 ± 27 | 190 ± 27 |
| Striatum | 246 ± 19 | 206 ± 27 | 255 ± 23 |
| VTA | 70 ± 9 | 107 ± 14 | 73 ± 15 |
Significantly different than Coc6h
3. Discussion
The main findings of the current investigation were that after 20 minutes of withdrawal, brief, but not extended, daily access to cocaine resulted in decreased NMDA receptor binding and increased D2 receptors binding in the mPFC relative to saline controls. In addition, extended, but not brief, daily access to cocaine resulted in increased D1 binding in the N.Acc shell, also at 20 minutes of withdrawal. Brief daily access also resulted in decreased D2 receptors binding in the N.Acc shell, relative to saline controls after 14 days of withdrawal, and as compared to the extended access treatment, at 60 days of withdrawal. These data extend our previous results showing that the brief access condition accompanied by stable drug use is associated with different neuroadaptations than those associated with the extended access condition and escalated drug use.
D2 dopamine receptors in the mPFC mostly serve as autoreceptors, the stimulation of which results in decreased dopamine (DA)-release in this brain area (Sesack & Bunney 1989). This inhibitory role of D2 receptors in the mPFC is modulatory in that it does not inhibit basal DA release, but only stimulated dopamine release (Pehek, 1999). Increased numbers of D2 receptors in the mPFC can therefore result in decreased release of DA and therefore diminish the action of cocaine (i.e. cocaine-induced increase of DA levels in the synapse) in this brain area. Blockade of NMDA receptors in the mPFC stimulates DA release (Nishijima et al., 1994, 1996). Thus reduced number of NMDA receptors in the mPFC may lead to increased DA release in this brain area and therefore facilitate the action of cocaine. It seems then, that the inhibition of cocaine action in the mPFC of the Coc1h animals produced by increased number of D2 receptors, at 20 minutes of withdrawal, could be counteracted by the decreased number of NMDA receptors in this brain area. Since DA levels in the mPFC are inversely related to DA levels in the N.Acc (Carter & Pycock, 1980 ; Lecesse & Lyness, 1987; Louilot et al., 1989; Pycock et al., 1980a,b), it follows that the net effects of these adaptations on N.Acc DA may be negligible. This hypothesis is consistent with our previous findings (Ben-Shahar et al., 2004) showing similar c-Fos immunoreactive response to cocaine challenge given after 3 days of withdrawal, in the Sal and Coc1h groups. These changes were not present in the Coc6h group, suggesting that either they never developed in this condition or were counteracted by other neuronal changes occurring while these subjects were allowed longer daily access to cocaine.
Levels of D2 receptors were reduced in the N.Acc shell of the brief access animals, as compared to saline animals after 14 days of withdrawal, and as compared to extended access animals after 60 days of withdrawal. Similarly, Mantsch et al., (2004) observed higher levels of mRNA for D2 receptors, in rats experiencing 10 hours of daily access to cocaine as compared to animals receiving only 3 hours of daily access to the drug. If there were no accompanying changes in the sensitivity or coupling of these receptors, lower levels of D2 receptors could lead to lesser autoreceptor-induced inhibition of dopamine release, or increased dopamine release in the N.Acc shell. Such a conclusion is again consistent with our previous results (Ben-Shahar et al., 2004, 2005) showing a heightened locomotor response to cocaine challenge in the brief access animals, as compared to saline animals after 14 days of withdrawal and as compared to extended access animals after 60 days of withdrawal.
It is difficult to further compare our results to the existing literature, since most of the studies exploring changes in glutamate and dopamine function after chronic administration of cocaine used experimenter-delivered IP administration protocols (as opposed to IV self-administration) and even fewer used extended access conditions. Bearing this in mind, the most appropriate comparison of our findings concerning the brief access condition is with those found after treatments inducing behavioral sensitization to cocaine, since the brief access condition also resulted in behavioral sensitization (Ben-Shahar et al., 2004). It has been reported that treatments inducing behavioral sensitization to cocaine (all of which used experimenter administered IP injections) produce no change in total levels of D1 or D2 receptors in the VTA, N.Acc, striatum or PFC (For reviews see: Pierce & Kalivas, 1997; Vanderschuren & Kalivas, 2000; Wolf, 1998). Consistent with these negative findings, brief access animals that exhibited behavioral sensitization (Ben-Shahar et al., 2004), exhibited no change in total D1 receptor levels. However, we did find an increase in D2 receptors levels at 20 minutes into withdrawal in the mPFC, and a decrease in N.Acc shell levels of D2 receptors at 14 days withdrawal. These differences may stem from the difference in procedure (IV self-administration vs. experimenter administered IP cocaine).
Most studies exploring changes in NMDA levels after chronic administration of cocaine measured NMDA receptor subunit protein expression or levels of mRNA for NMDA, as opposed to binding of the receptor complex. Since, there is no direct or linear relationship between these different measures of NMDA receptor function (e.g. King et al., 1994; Tang et al, 2004) it is difficult to compare these studies to the current one. Our findings of decreased binding of NMDA receptors in the mPFC of the Coc1h group are consistent with those of Bhargava & Kumar (1999) showing decreased binding of NMDA receptors in the cortex of mice exhibiting behavioral sensitization to cocaine, immediately after the last cocaine challenge. Szumlinski et al., (2000) and Itzhak & Martin (2000) found no change in NMDA binding in the striatum of rats or the striatum and frontal cortex of mice, respectively, 14 or 10 days after the cessation of sensitization inducing regimen. This is consistent with the lack of change in NMDA binding we observed after 14 days of withdrawal in the striatum and mPFC of Coc1h rats.
The increase in levels of D1 dopamine receptors observed in the N.Acc shell of the 6-hr animals immediately after the last session of self-administration are not consistent with the results of Graziella De Montis et al., (1998) who reported decreased levels of D1 receptors in the N.Acc of similarly-treated rats. However, the 6-hrs animals (Fischer rats) in the Graziella De Montis et al., (1998) study were reinforced with 0.33mg of cocaine/infusion and exhibited stable and relatively low rates of cocaine self-administration consuming on average 4 mg/kg/day, while our 6-hr animals (Sprague-Dawley rats) were reinforced with 0.25mg cocaine/infusion, produced escalated drug consumption over trials, and responded with higher rates of self-administration consuming on average 10 mg/kg/day. These differences in cocaine consumption could explain the difference in D1 receptors levels found between the two studies. An increase in N.Acc shell levels of D1 receptors in the current experiment could suggest heightened function of dopaminergic receptors (sensitization) in the N.Acc shell, of the extended access animals. However, this conclusion is inconsistent with our previous observations of a lack of a sensitized c-Fos immunoreactive response to cocaine after 3 days of withdrawal (Ben-Shahar et al., 2004) especially since this c-Fos immunoreactive response to cocaine is mediated via D1 receptors (Graybriel et al., 1990; Young et al., 1991). This “sensitization” hypothesis is also inconsistent with the results of Ahmed, Koob and colleagues and who showed that extended access animals worked to maintain significantly higher levels of dopamine in the N.Acc (Ahmed et al., 2003), and were more susceptible to the effects of cis-Flupethixol (Ahmed & Koob, 2004) – data suggesting reduced dopamine receptor function in the N.Acc. Of course, it is possible that while there were more D1 receptors in the N.Acc shell of the extended access animals at 20 minutes of withdrawal, receptor coupling was still significantly reduced. More studies are needed to address these issues.
Finally, our extended access animals did not show reduced levels of D2 receptors in the N.Acc or striatum immediately after the last session of self-administration or after withdrawal (i.e., 14 or 60 days). Thus, we did not reproduce the reduction in D2 receptor availability reported in the ventral or dorsal striatum observed in human cocaine or methamphetamine addicts (Volkow et al., 1993, 2001). However, Volkow et al. (1999a, 1999b, but see Martinez et al., 2004) also showed that in normal humans, lower D2 levels were associated with higher methylphenidate –induced euphoria, which raises a “chicken and egg” kind of dilemma: i.e., are humans with lower D2 receptors more sensitive to cocaine reinforcement and therefore more likely to develop addiction to the drug, or is it the cocaine consumption itself that results in lower D2 levels (Volkow, 2004). A correlation between increased vulnerability to cocaine reinforcement and lower D2 availability was also reported by Morgan et al. (2002) who showed that social housing resulted in increased D2 receptor availability in dominant monkeys which was associated with significantly lower levels of cocaine self-administration. Interestingly, the same group of researchers (Czoty et al., 2004) reported that long term cocaine self-administration equalized D2 levels in these monkeys across social ranks. Such data are consistent with the hypothesis that the decrease in D2 receptor availability found in human cocaine addicts is not the result of excessive cocaine consumption, but rather a pre-existing condition leading to a higher vulnerability to addiction. If this is true, then it is not surprising that we did not observe this reduced D2 availability in our extended access animals; animals that at the start of the experiment were not different from controls. It is also the case that in studies using PET imaging to monitor receptor availability in vivo (the method used by Volkow et al., 1993, 2001) only receptors residing on the cell membrane are measured, whereas in studies using in vitro receptor autoradiography (the method used in the current project) receptors residing both on the cell membrane and those residing in the cytosol are measured, which can lead to contrasting results (e.g., see Maggos et al., 1998). Thus, the lack of any observed decrease in D2 availability in our extended access animals could have resulted from either a difference in the method for monitoring receptor levels and/or the fact that reduced D2 availability may be a preexisting condition to (as opposed to one resulting from) compulsive consumption of cocaine.
In summary, our current results further strengthen our views that extended access to cocaine leads to neural adaptations that are distinct from those observed after brief daily access to the drug. However, it seem that changes in total levels of D1 or D2 dopamine receptors, or NMDA receptors, are not a major component of these adaptations. It might be that changes in the coupling or sensitivity of these receptors are important components of the neural adaptations that mediate the development of addiction. Other neurotransmitter systems, such as serotonin and CRF, might also be involved (Koob et al., 2004).
4. Experimental Procedure
4.1. Subjects
The subjects were male albino Sprague-Dawley rats weighing 300-350 g at the beginning of the experiment obtained from Charles River Laboratories (Hollister, CA). Fifty-three animals completed the entire test protocol (described below). The animals were housed individually in wire-hanging cages located within a temperature-controlled (22°C), 12/12 h light/dark cycle (lights on at 0700) vivarium located in the Psychology Department at UCSB. Subjects had ad libitum access to food and water, except during operant training for food reinforcement (see Food Training below). All procedures were conducted in strict adherence to the NIH Guide for the Care and Use of Laboratory Animals and were reviewed and approved by the UCSB Institutional Animal Care and Use Committee.
4.2. Surgery
Each rat was implanted with a chronic intravenous silastic catheters in the right jugular vein under Isoflurane gas anesthesia (Abbott Laboratories, North Chicago, IL; 4% for induction; 2.0 – 2.5% for maintenance). A single dose of atropine (0.04 mg/kg IM) was administered to minimize respiratory congestion during anesthesia. Banamine (2 mg/kg SC), a non-opiate analgesic, was provided to treat post-surgical pain. Catheters were 13 cm long (0.3 mm inner diameter, 0.64mm outer diameter; Dow Corning Corporation, Midland, MI), and cemented to a 22 gauge guide cannula (Plastics One, Roanoke, VA) that was in turn secured with Bard Mesh (C.R. Bard Inc., Cranston, RI) to the animals' back. The other end of the catheter was passed subcutaneously around the shoulder to the neck where it was inserted into the jugular vein and secured in place by suture. Animals were allowed 10 days for recovery. Catheter patency was maintained by flushing the IV system with a solution of 30 units heparin in 0.1 ml sterile 0.9% physiological saline, each day. Catheter patency was confirmed in all animals with the fast acting anesthetic Brevital (1mg/0.1 ml saline), once a week and at the end of the last session of cocaine self-administration.
4.3. Apparatus
Eleven (29 cm wide × 25 cm long × 30 cm high) operant chambers (Med Associates Inc., St. Albans, VT) were used for all behavioral training and testing. Each chamber was equipped with a non-retractable (fixed) lever and a retractable lever, each positioned 7.0 cm above the grid floor on either side of a food pellet trough that was situated 2 cm above the grid floor. Food dispensers were located outside the chambers. A center house light (2.8 W) was situated 28 cm above the grid floor in the center of the back panel. Two cue lights (2.8 W) were located 6-7 cm above each lever. In the current study, only the right cue light was used. All behavioral testing equipment and data acquisition were controlled by a desktop personal computer running Med Associates software (MED-PC for Windows, Version 1.17). A custom made liquid swivel was located above the center of each operant chamber permitting the animals to freely move about the chamber without strain on the PE tubing. The inlet of the liquid swivel was connected with polyethylene tubing (Plastics One; outer diameter 0.127 cm, inner diameter 0.058 cm) to a 10-ml syringe containing the self-administration solutions and seated in a syringe pump (Med Associates Inc., St. Albans, VT). An additional length of PE tubing passed through a cannula connector (C313CT Plastic One) from the swivel overhead to the animal where it was connected to the external cannula on the animal's back. Intravenous infusions were administered by activation of the syringe pump.
4.4. Drugs
Cocaine hydrochloride (provided by the National Institute on Drug Abuse) was dissolved in 0.9% physiological saline. The concentration used for intravenous (IV) administration was 0.25mg/0.1 ml that was infused at a volume of 0.1 ml over a 4 s period.
4.5. Procedure
The procedure was the same as described previously (Ben-Shahar et al., 2004). Briefly, to facilitate acquisition of operant responding for cocaine, rats were initially trained to lever press for food (45 mg Noyes pellets) prior to catheter implantation. Rats were trained on an FR-1 schedule followed by a time-out (TO) period. The TO period lasted 1 sec initially and then was lengthened to 10 s, and finally to 20 s. Surgical implantation of catheters was performed one to two days after a rat completed the food-training regimen. Ten days after surgery, cocaine self-administration training began. Training consisted of 1-h daily sessions on an FR-1 TO 20 schedule. The reinforcer was either 0.1 ml physiological saline or 0.25 mg cocaine in 0.1 ml physiological saline. Once a rat exhibited a stable response rate for cocaine (i.e., no more than 15% variability over 3 consecutive days) and had experienced at least seven self-administration sessions, it was assigned to either the Coc1h group or the Coc6h group for the next 8 days. Saline animals (Sal group) continued to have access to IV saline for 1-h each day.
At the end of this eight-day period, rats were given 20 minutes, or 14 or 60 days of withdrawal during which they had no access to cocaine (or saline) and were never placed in the operant boxes. At the end of the withdrawal period subjects were administered the fast-acting anesthetic Brevital (2 mg/kg IV) to confirm catheter patency. The subjects were then decapitated, their brains removed, rapidly frozen in isopentane on dry ice, and then transferred to dry ice. Brains were then stored at −80°C until processing. Coronal sections of brain tissue (16 μm) were cut on a Leica cryostat and immediately mounted on 1.5% gelatin-coated slides. Using the Paxinos and Watson atlas (1986) as a guide, the mPFC, N.Acc Core, N.Acc Shell, dorsal striatum, and VTA were sampled.
4.6. Quantitative receptor autoradiography
For D1 labeling brain sections were pre-incubated at room temperature for 20min in 50mM Tris HCl buffer (pH 7.4) containing 120mM NaCl, 5mM KCl, 0.1% ascorbic acid, and 0.1% Bovine Serum Albumin (BSA). Total binding was measured from sections that had been incubated for 60min in the same buffer with 2nM [3H]SCH23390 and 30nM ketanserine. Non-specific binding was determined from adjacent sections by adding 10μM cis-Flupenthixol to the binding buffer. Sections were subsequently washed in ice-cold buffer (3 × 5 min), rinsed in ice-cold distilled water, and left to dry overnight. Slides were exposed to Kodak BioMax Film for 1 month.
For D2 labeling brain sections were pre-incubated at room temperature for 5min in 50mM Tris HCl buffer (pH 7.4) containing 120mM NaCl, 5mM KCl, 1mM MgCl2, and 0.1% Bovine Serum Albumin (BSA). Total binding was measured from sections that had been incubated for 60min in the same buffer with 7nM [3H]Spiperone, 1mM ascorbic acid, and 1.6μM ketanserine. Non-specific binding was determined from adjacent sections by adding 20μM (+)butaclamol to the binding buffer. Sections were subsequently washed in ice-cold buffer (2 × 20sec), rinsed in ice-cold distilled water, and left to dry overnight. Slides were exposed to Kodak BioMax Film for 21 days.
For NMDA labeling brain sections were pre-incubated at room temperature for 60min in 50mM Tris HCl buffer (pH 7.4). Total binding was measured from sections that had been incubated for 20min in the same buffer with 40nM [3H]MK-801. Non-specific binding was determined from adjacent sections by adding 100μM MK-801 to the binding buffer. Sections were subsequently rinsed briefly in fresh buffer, then washed in ice-cold buffer (2 × 30 seconds), and left to dry overnight. Slides were then exposed to Kodak BioMax Film for one month.
Autoradiograms of the three ligands were scanned using Epson900 Flatbed Scanner and were subsequently analyzed for receptor density using a computerized image-analysis system (ImageJ, National Institute of Health, USA). Optical densities were calibrated using co-exposed standardized 3H-microscales (Amersham, England) and were expressed as fmol/mg of tissue.
ACKNOWLEDGMENTS
The authors wish to acknowledge the following high school students: Frances Vernon, Allison Atwill, Dominique Fenton, and Jaclyn Kanners, for their assistance in running the experiments and Dr. Karen Szumlinski for her editorial comments. This work was supported by National Institute of Drug Abuse grant DA017104 awarded to OBS and by grant DA05041 awarded to AE
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Scope: Neurophysiology, Neuropharmacology and other forms of Intercellular Communication
REFERENCES
- Ahmed SH, Koob GF. Transition from moderate to excessive drug intake: change in hedonic set-point. Science. 1998;282:298–300. doi: 10.1126/science.282.5387.298. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Koob GF. Changes in response to a dopamine receptor antagonist in rats with escalating cocaine intake. Psychopharmacology. 2004;172:450–454. doi: 10.1007/s00213-003-1682-9. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. J Neurochem. 2003;86:102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A. The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res. 2004;995:46–54. doi: 10.1016/j.brainres.2003.09.053. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Jacob B, Roarty MP, Ettenberg A. Prolonged daily exposure to i.v. cocaine results in tolerance to its stimulant effects. Pharmacol Biochem Behav. 2005;82:411–416. doi: 10.1016/j.pbb.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Ben-Shahar O, Moscarello JM, Ettenberg A. One hour, but not 6 hours, of daily access to self-administered cocaine results in elevated levels of the dopamine transporter. Brain Research. 2006 doi: 10.1016/j.brainres.2006.04.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhargava HN, Kumar S. Sensitization to the locomotor stimulant effect of cocaine modifies the binding of [3H]MK-801 to brain regions and spinal cord of the mouse. Gen Pharmacol. 1999;32:359–363. doi: 10.1016/s0306-3623(98)00107-4. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Pycock CJ. Behavioural and biochemical effects of dopamine and noradrenaline depletion within the medial prefrontal cortex of the rat. Brain Res. 1980;192:163–176. doi: 10.1016/0006-8993(80)91016-1. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology. 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Graziella De Montis M, Co. C, Dworkin SI, Smith JE. Modifications of dopamine D1 receptor complex in rats self-administering cocaine. Eur J Pharmacol. 1998;362:9–15. doi: 10.1016/s0014-2999(98)00731-6. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- Goeders NE, Smith JE. Intracranial cocaine self-adminstration into the medial prefrontal cortex increases dopamine turnover in the nucleus accumbens. J Pharmacol.Exp. Ther. 1993;265:592–600. [PubMed] [Google Scholar]
- Graybiel AM, Moratalla R, Robertson HA. Amphetamine and cocaine induce drug-specific activation of the c-fos gene in striosome-matrix compartments and limbic subdivisions of the striatum. Proc Natl. Acad. Sci. U S A. 1990;87:6912–6916. doi: 10.1073/pnas.87.17.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurd YL, McGregor A, Ponten M. In vivo amygdala dopamine levels modulate cocaine self-administration behavior in the rat: D1 dopamine receptor involvement. Eur J Neurosci. 1997;9:2541–2548. doi: 10.1111/j.1460-9568.1997.tb01683.x. [DOI] [PubMed] [Google Scholar]
- Itzhak Y, Martin JL. Cocaine-induced kindling is associated with elevated NMDA receptor binding in discrete mouse brain regions. Neuropharmacology. 2000;39:32–39. doi: 10.1016/s0028-3908(99)00073-8. [DOI] [PubMed] [Google Scholar]
- King GR, Ellinwood EH, Jr, Silvia C, Joyner CM, Xue Z, Caron MG, Lee TH. Withdrawal from continuous or intermittent cocaine administration: changes in D2 receptor function. J Pharmacol Exp Ther. 1994;269:743–749. [PubMed] [Google Scholar]
- Koob GF, Ahmed SH, Boutrel B, Chen SA, Kenny PJ, Markou A, O'Dell LE, Parsons LH, Sanna PP. Neurobiological mechanisms in the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2004;27:739–749. doi: 10.1016/j.neubiorev.2003.11.007. [DOI] [PubMed] [Google Scholar]
- Leccese AP, Lyness WH. Lesions of dopamine neurons in the medial prefrontal cortex: effects on self-administration of amphetamine and dopamine synthesis in the brain of the rat. Neuropharmacology. 1987;26:1303–1308. doi: 10.1016/0028-3908(87)90091-8. [DOI] [PubMed] [Google Scholar]
- Louilot A, Le Moal M, Simon H. Opposite influences of dopaminergic pathways to the prefrontal cortex or the septum on the dopaminergic transmission in the nucleus accumbens. An in vivo voltammetric study. Neuroscience. 1989;29:45–56. doi: 10.1016/0306-4522(89)90331-x. [DOI] [PubMed] [Google Scholar]
- Maggos CE, Tsukada H, Kakiuchi T, Nishiyama S, Myers JE, Kreuter J, Schlussman SD, Unterwald EM, Ho A, Kreek MJ. Sustained withdrawal allows normalization of in vivo [11C]N-methylspiperone dopamine D2 receptor binding after chronic binge cocaine: a positron emission tomography study in rats. Neuropsychopharmacology. 1998;19:146–53. doi: 10.1016/S0893-133X(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Mantsch JR, Yuferov V, Mathieu-Kia AM, Ho A, Kreek MJ. Effects of extended access to high versus low cocaine doses on self-administration, cocaine-induced reinstatement and brain mRNA levels in rats. Psychopharmacology. 2004;175:26–36. doi: 10.1007/s00213-004-1778-x. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and d2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology. 2004;29:1190–202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Nishijima K, Kashiwa A, Nishikawa T. Preferential stimulation of extracellular release of dopamine in rat frontal cortex to striatum following competitive inhibition of the N-methyl-d-aspartate receptor. J. Neurochem. 1994;63:375–378. doi: 10.1046/j.1471-4159.1994.63010375.x. [DOI] [PubMed] [Google Scholar]
- Nishijima K, Kashiwa A, Hashimoto A, Iwama H, Umino A, Nishikawa T. Differential effects of phencyclidine and methamphetamine on dopamine metabolism in rat frontal cortex and striatum as revealed by in vivo dialysis. Synapse. 1996;22:304–312. doi: 10.1002/(SICI)1098-2396(199604)22:4<304::AID-SYN2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Pehek EA. Comparison of effects of haloperidol administration on amphetamine-stimulated dopamine release in the rat medial prefrontal cortex and dorsal striatum. J. Pharmacol. Exp. Ther. 1999;289:14–23. [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW. A circuitry model of the expression of behavioral sensitization to amphetamine-like psychostimulants. Brain Res Brain Res Rev. 1997;25:192–216. doi: 10.1016/s0165-0173(97)00021-0. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Meil WM, Kalivas PW. The NMDA antagonist, dizocilpine, enhances cocaine reinforcement without influencing mesoaccumbens dopamine transmission. Psychopharmacology. 1997;133:188–195. doi: 10.1007/s002130050390. [DOI] [PubMed] [Google Scholar]
- Pulvirenti L, Maldonado-Lopez R, Koob GF. NMDA receptors in the nucleus accumbens modulate intravenous cocaine but not heroin self-administration in the rat. Brain Res. 1992;594:327–330. doi: 10.1016/0006-8993(92)91145-5. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Carter CJ, Kerwin RW. Effect of 6-hydroxydopamine lesions of the medial prefrontal cortex on neurotransmitter systems in subcortical sites in the rat. J. Neurochem. 1980a;34:91–99. doi: 10.1111/j.1471-4159.1980.tb04625.x. [DOI] [PubMed] [Google Scholar]
- Pycock CJ, Kerwin RW, Carter CJ. Effect of lesion of cortical dopamine terminals on subcortical dopamine receptors in rats. Nature. 1980b;286:74–77. doi: 10.1038/286074a0. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine self-administration appears to be mediated by dopamine uptake inhibition. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:233–239. doi: 10.1016/0278-5846(88)90040-1. [DOI] [PubMed] [Google Scholar]
- Roberts D, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Bunney BS. Pharmacological characterization of the receptor mediating electrophysiological responses to dopamine in the rat medial prefrontal cortex: a microiontophoretic study. J. Pharmacol. Exp. Ther. 1989;248:1323–1333. [PubMed] [Google Scholar]
- Szumlinski KK, Herrick-Davis K, Teitler M, Maisonneuve IM, Glick SD. Behavioural sensitization to cocaine is dissociated from changes in striatal NMDA receptor levels. Neuroreport. 2000;11:2785–2788. doi: 10.1097/00001756-200008210-00035. [DOI] [PubMed] [Google Scholar]
- Tang W, Wesley M, Freeman WM, Liang B, Hemby SE. Alterations in ionotropic glutamate receptor subunits during binge cocaine self-administration and withdrawal in rats. J Neurochem. 2004;89:1021–1033. doi: 10.1111/j.1471-4159.2004.02392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Kalivas PW. Alterations in dopaminergic and glutamatergic transmission in the induction and expression of behavioral sensitization: a critical review of preclinical studies. Psychopharmacology. 2000;151:99–120. doi: 10.1007/s002130000493. [DOI] [PubMed] [Google Scholar]
- Volkow ND. Imaging the addicted brain: from molecules to behavior. J Nucl Med. 2004;45(11):13N–16N. 19N-20N, 22N. [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–77. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fischman MW, Foltin RW, Fowler JS, Abumrad NN, Vitkun S, Logan J, Gatley SJ, Pappas N, Hitzemann R, Shea CE. Relationship between subjective effects of cocaine and dopamine transporter occupancy. Nature. 1997a;386:827–830. doi: 10.1038/386827a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999a;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997b;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Wong C, Hitzemann R, Pappas NR. Reinforcing effects of psychostimulants in humans are associated with increases in brain dopamine and occupancy of D(2) receptors. J Pharmacol Exp Ther. 1999b;291:409–415. [PubMed] [Google Scholar]
- Wolf ME. The role of excitatory amino acids in behavioral sensitization to psychomotor stimulants. Prog Neurobiol. 1998;54:679–720. doi: 10.1016/s0301-0082(97)00090-7. [DOI] [PubMed] [Google Scholar]
- White FJ, Hu XT, Zhang XF, Wolf ME. Repeated administration of cocaine or amphetamine alters neuronal responses to glutamate in the mesoaccumbens dopamine system. J Pharmacol Exp Ther. 1995;273:445–454. [PubMed] [Google Scholar]
- Young ST, Porrino LJ, Iadarola MJ. Cocaine induces striatal c-fosimmunoreactive proteins via dopaminergic D1 receptors. Proc Natl Acad Sci U S A. 1991;88:1291–1295. doi: 10.1073/pnas.88.4.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XF, Hu XT, White FJ, Wolf ME. Increased responsiveness of ventral tegmental area dopamine neurons to glutamate after repeated administration of cocaine or amphetamine is transient and selectively involves AMPA receptors. J Pharmacol Exp Ther. 1997;281:699–706. [PubMed] [Google Scholar]