Abstract
Six healthy horses were examined by using transabdominal ultrasonography, as described (1–3), to evaluate activity and size of the large colon and cecum at various locations.
Using size and number of sacculations, activity patterns and contractile frequency; significant differences that would allow ultrasonographic identification of dorsal versus ventral colons, if they were displaced, were not found. The cecum had significantly greater activity than the colon, and a trend was seen towards smaller sacculations in the cecum than in the large colon.
Résumé
Identification des paramètres normaux pour l’examen échographique du gros côlon et du caecum du cheval. Six chevaux en santé ont été examinés par échographie transabdominale tel que décrit (1–3) afin d’évaluer l’activité et la grosseur du gros côlon et du caecum à diverses localisations.
En utilisant la grosseur et le nombre des cellules coliques, le schéma d’activité et la fréquence des contractions, il n’a pas été possible de montrer de différences significatives qui auraient pu permettre l’identification par échographie du côlon dorsal et du côlon ventral, s’ils avaient été déplacés. Le caecum montrait une activité significativement plus grande que celle du côlon et une tendance à former des cellules plus petites que les cellules coliques.
(Traduit par Docteur André Blouin)
Introduction
Transcutaneous abdominal ultrasonography has become a valuable supplement to other diagnostic procedures in the examination of colic patients and is used as part of a standard diagnostic regimen in our clinic. The method is noninvasive, easy to perform, and gives immediate information on important parameters, such as the amount and location of abdominal fluid, patterns and frequency of small intestinal contractions, and the degree of small intestinal distention and intestinal content.
Abdominal ultrasonography can aid in the diagnosis of nephrosplenic entrapment of the large colon (1,2); small intestinal strangulating obstructions (3); enteritis (3–5); intussusceptions (6–8); and herniations, abscessations, and abdominal neoplasias (5,9).
Large colon displacement is a common cause of colic. The ultrasonographic changes that occur in displacement of the left dorsal colon have been well documented (1,2). Other displacements of the large colon can be a diagnostic challenge. Horses presented initially with mild to moderate colic often have normal heart rates and inconclusive findings on rectal palpation. If the different areas of the large colon could be identified consistently in the normal horse, abnormal positioning of the colon in displacements could potentially be diagnosed with ultrasonography. Although some authors have described the appearance of the dorsal versus the ventral large colon (3), no attempt has been made to define ultrasonographic criteria to positively identify the sections of the large colon.
The purpose of this study was to map the parts of the large colon by using transcutaneous ultrasonography.
We hypothesized that we could identify cecum and dorsal and ventral colon, based on differences in number and size of sacculations, number of taeniae, and contractility patterns.
Materials and methods
Six, healthy nonpregnant, adult quarter horse mares were used in this study. The horses were fed a diet of free choice grass hay and 1 kg sweet feed daily. They were kept in tie stalls with daily turn-out in a paddock. No sedatives were used during the study. For the examinations, the mares were placed in stocks and the abdomen was clipped bilaterally from the tuber coxa to the caudal border of the lung field, and ventrally from the udder to the xyphoid process. Abdominal ultrasonographic examination was performed by using a 5 MHz sector transducer and an ultrasound machine (Ausonics impact VS1; Universal Medical Systems, Solon, Ohio, USA).
Liberal amounts of alcohol were used to enhance contact with the probe. As a consequence of the normal in-situ positioning of the large colon, the transducer was oriented in a cranial to caudal direction to better visualize sacculations.
The examination started in the paralumbar fossa and proceeded cranially to the 10th intercostal space. Each side of the abdomen was divided into dorsal and ventral sections. Each intercostal space and the paralumbar fossa were evaluated with 2 recordings; the 1st dorsal at the level of the body of the cecum and dorsal part of the large colon, and the 2nd ventral at the level of the stifle, representing the apex of the cecum and the ventral part of the large colon. The ventral part of the abdomen was divided into 6 regions for evaluation: right and left inguinal areas, right and left areas lateral to the midline, on the midline (ventralmost aspect), and at the level of the xyphoid process. The right side represented the right ventral part of the large colon, the left side represented the left ventral part of the large colon, and the midline represented the apex of the cecum.
Identification of the large colon
The large colon is identified as a mobile structure outlined by a curvilinear, hypoechoic line overlying a hyperechoic gas shadow. The luminal contents and opposite wall were not visualized due to the acoustic gas shadow. The shape of the large colon was delineated by curved lines of different length, and we interpreted the measured length of each curved line as 1 sacculation (Figure 1).
Figure 1.
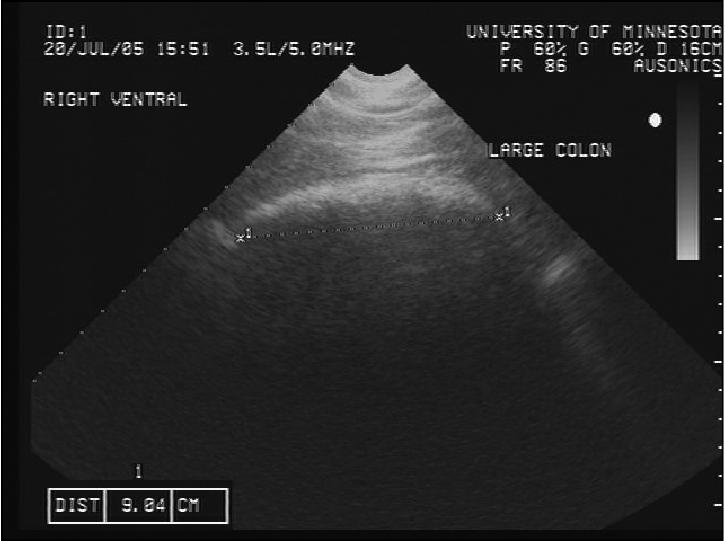
Transcutaneous abdominal ultrasonographic image of the large colon, measurement of width of sacculation.
Activity of the large colon was assessed, based on contractions per 30 s, and the movement in centimeters of each contraction away from the probe.
The horizontal activity of the large intestine was graded as mild, moderate, or continuous. Mild was defined as rare intermittent movement and continuous as constant movement.
Because the distinct layers of the colon wall could not be seen with the 5 MHz transducer, the wall was measured as the width of the hypoechoic line adjacent to the curved gas interface in the lumen of the colon.
Statistical analysis
Sacculation width, contractions per 30 s, and size of the contractions were measured in all recording spaces. A total of 40 measurements for each of these 3 variables were recorded per horse. The wall thickness was measured only in the dorsal and ventral parts of the paralumbar fossa and in the dorsal and ventral parts of the 15th intercostal space bilaterally. The reason for measuring wall thickness in only 2 sites was due to time-constraints during the examinations, difficulties in visualizing the colon wall, and the general impression that the measurements were not accurate. The sites picked pertained to a related study in pregnant mares (unpublished data).
Comparisons were made by using repeated measures ANOVA between all sites, and between sites of each main region (left, right, and ventral). A Wilcoxon rank-sum test was used for nonparametric variables. Significance was set at P ≤ 0.05. The results are described as a trend when P ≤ 0.1. Measurements are reported as the mean value (x¯) ± the standard error of the mean (Sx¯).
Results
Sacculation width
No significant differences in sacculation size and number were detected. However, a trend (P = 0.07) was seen towards smaller measurements in the cecal area (Table 1).
Table 1.
Ultrasonographic measurements comparing sacculation size between parts of the large colon and cecum
| Section | n | Width of sacculations in cm, Sx¯ |
|---|---|---|
| Right PLF | 12 | 6.11, Sx¯ = 0.40 |
| Right ventral | 48 | 7.65, Sx¯ = 0.28 |
| Right dorsal | 54 | 8.14, Sx¯ = 0.30 |
| Left ventral | 54 | 7.72, Sx¯ = 0.31 |
| Left dorsal | 53 | 7.97, Sx¯= 0.37 |
Sacculation size is expressed as mean and standard error of the mean (Sx¯). Measurements in the area of the cecum; Right paralumbar fossa (PLF) include the dorsal and ventral measurements. Measurements in the right ventral and right dorsal abdomen included rib spaces 17 through 10. Measurements on the left side also include the left paralumbar fossa.
No differences were seen in the size of sacculations between the dorsal and ventral parts of the large colon or between the right and left sides (Figures 2a and 2b). More sacculations were seen on the screen when the sacculations were smaller, but no significant difference was detected as to number of sacculations per screen image in the different areas.
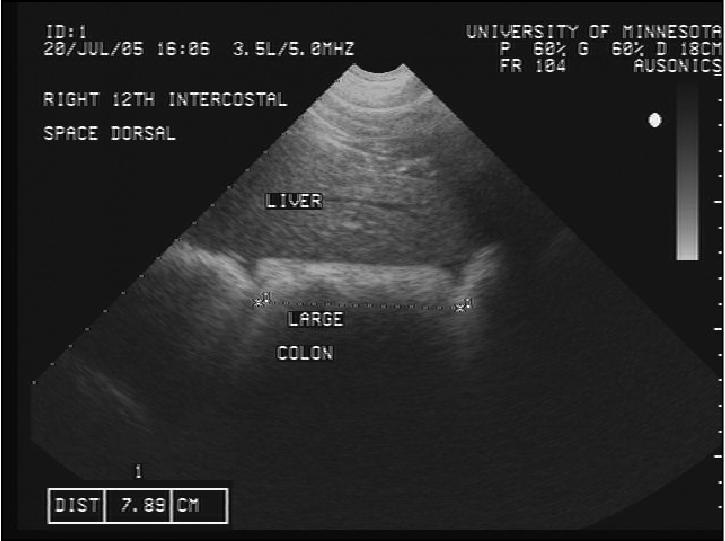
Figure 2a.
Transcutaneous abdominal ultrasonographic image of the large colon ventrally in the 12th intercostal space.
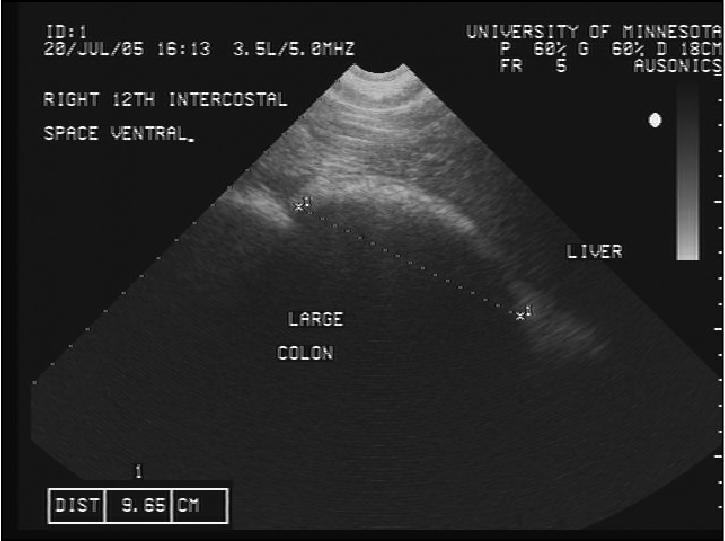
Figure 2b.
Transcutaneous abdominal ultrasonographic image of the large colon dorsally in the 12th intercostal space.
Contractility
Significantly larger contractions (P = 0.05) were detected in the area of the cecum than at the other sites measured. The mean size of the excursions in the right paralumbar fossa was 3.50, Sx¯ = 0.65 cm, versus an overall mean of 1.31 cm. The smallest excursions were found in the left caudodorsal part of the abdomen (intercostal spaces 14 to 17), where the mean measurements were from 0 to 0.4 cm.
Significant differences were not detected in the frequency of activity of the large colon and cecum at the various sites measured. The mean rate of contractions/min was 1.8, Sx¯ = 2.48.
No statistical significance or trend was seen between the different sites measured when evaluating the horizontal contractions. Continuous activity was most frequently seen in all areas.
Wall thickness
No significant differences were found in wall thickness between the sites measured. The mean wall thickness was 2.1, Sx¯ = 0.5 mm. The taeniae of the large colon and cecum could not be visualized.
Discussion
It has been reported that the dorsal and ventral parts of the large colon can be differentiated by the number of taeniae, the presence of sacculations in the ventral colon, and absence of sacculations in the dorsal colon (3,8,9), but no specific criteria have been established to validate this assertion. Using transrectal ultrasonography, Freeman (8) identified the main contractions of the cecum as being oriented vertically, and used this to distinguish the cecum from the horizontal movements of the large colon. Taeniae could be identified in the cecum by using a transcutaneous technique, but in the large colon only transrectally.
In this study, 1 curved line of the large colon measured in its entire visible length was designated as 1 sacculation.
The expectation, based on the absence of actual sacculations in the dorsal colon, was that the dorsal colon would have significantly longer curves than the ventral colon. The results of the present study did not verify this, and indicated that the dorsal and ventral parts of the large colon cannot be differentiated ultrasonographically by segmental size or number. One possible explanation for this outcome might be that the propulsive and retropulsive peristaltic activity of the dorsal colons lead to an appearance of smaller segmented areas ultrasonographically.
Activity of the large colon as assessed by centimeters movement away from the probe, showed significantly greater movements in the right paralumbar fossa, corresponding to the area of the cecum, than in any other site measured.
Freeman (3) described the contractions of the cecum as being oriented vertically as opposed to horizontally in the rest of the large colon.
We found that the contractions of the cecum were of significantly greater amplitude than those of the large colon. However, the contractions of the large colon were oriented vertically, as well as horizontally.
No significant differences were found in frequency of contractions between any sites measured. The mean of 2 contractions per minute was in the low end of what has previously been reported for the large colon (2–6 contractions/min) (3).
The wall of the large colon consists of 4 layers: the serosa, muscularis, submucosa, and mucosa. Distinct ultrasonographic features have been described for the large colon wall, identifying each of these layers and adding a 5th layer, the mucosal interface (9). Normal values for the thickness of the large colon wall have also been reported, and abnormalities in wall thickness can be an indication of lesions in the colon (10,11).
We were unable to identify the separate layers of the large colon wall with the 5 MHz convex linear transducer. The range of the mean values for wall thickness in our study (1.6–2.7 mm) is less than that reported as normal values (2.0–3.75mm) (12). However, in our opinion, based on the limited number of measurements performed in this study, it is difficult to accurately measure wall thickness in the normal horse, and identification of a thinner than normal wall is likely to be imprecise. CVJ
Footnotes
Dr. Hendrickson’s current address is Daletoppen #18, 1387 Asker, Norway.
Reprints will not be available from the authors.
This project was supported by the University of Minnesota Equine Center with funds provided by the Minnesota Racing Commission, Minnesota Agricultural Experimental Station and contributions by private donors.
References
- 1.Santschi EM, Slone DE, Frank WM. Ultrasound diagnosis of renosplenic entrapment. Proc Am Assoc Equine Pract. 1992;38:467. [Google Scholar]
- 2.Santschi EM, Slone DE, Frank WM. Use of ultrasound for diagnosis of left dorsal displacement of the large colon and monitoring its nonsurgical correction. Vet Surg. 1993;22:281–284. doi: 10.1111/j.1532-950x.1993.tb00398.x. [DOI] [PubMed] [Google Scholar]
- 3.Freeman SL. Ultrasonography of the equine abdomen: Techniques and normal findings. In Pract. 2002 Apr;:204–211. [Google Scholar]
- 4.Klohnen A, Vachon AM, Fischer AT. Use of diagnostic ultrasonography in horses with signs of acute abdominal pain. J Am Vet Med Assoc. 1996;209:1597–1601. [PubMed] [Google Scholar]
- 5.Scharner D, Rötting A, Gerlach K, et al. Ultrasonography of the abdomen in the horse with colic. Clin Tech Equine Pract. 2002;1:118–124. [Google Scholar]
- 6.Bernard WV, Reef VB, Reimer JM, et al. Ultrasonographic diagnosis of small-intestinal intussusception in three foals. J Am Vet Med Assoc. 1989;194:395–397. [PubMed] [Google Scholar]
- 7.Hillyer MH. The use of ultrasonography in the diagnosis of abdominal tumors in the horse. Equine Vet Educ. 1994;6:273–278. [Google Scholar]
- 8.Freeman SL, England GCW. Effect of romifidine on gastrointestinal motility, assessed by transrectal ultrasonography. Equine Vet J. 2001;33:570–576. doi: 10.2746/042516401776563436. [DOI] [PubMed] [Google Scholar]
- 9.Freeman SL. Diagnostic ultrasonography of the mature equine abdomen. Equine Vet Educ. 2003;15:319–330. [Google Scholar]
- 10.Pease AP, Scrivani PV, Erb HN, Cook VL. Accuracy of increased large-intestinal wall thickness during ultrasonography for diagnosing large-colon torsion in 42 horses. Vet Radiol Ultrasound. 2004;45:220–224. doi: 10.1111/j.1740-8261.2004.04038.x. [DOI] [PubMed] [Google Scholar]
- 11.Jones SL, Davis J, Rowlingson K. Ultrasonographic findings in horses with right dorsal colitis: Five cases (2000–2001) J Am Vet Med Assoc. 2003;222:1248–1251. doi: 10.2460/javma.2003.222.1248. [DOI] [PubMed] [Google Scholar]
- 12.Freeman SL, Mason PJ, Stanley C. Comparison of ultrasonographic and histologic assessment of normal and strangulated intestine in the horse. Eur J Ultrasound (Supplement) 2000:S5.


