Abstract
Recently, the Tei-index, a noninvasive index that combines systolic and diastolic time intervals, has been proposed to assess global cardiac performance. However, the effects of isoflurane on the Tei-index have not been characterized. This study aimed at studying the effects of 1.0 minimal alveolar concentration isoflurane anesthesia on the pre-ejection period (PEP), left ventricular ejection time (LVET), PEP/LVET ratio, isovolumic relaxation time (IVRT), stroke index (SI), cardiac index (CI), heart rate (HR), and the Tei-index in healthy unpre-medicated dogs. We observed significant increases in PEP, PEP/LVET ratio, IVRT, and TEI, whose maximal increases obtained throughout the study were 47%, 48%, 78%, and 56%, respectively. The LVET and HR did not change significantly, whereas the SI and CI decreased during anesthesia (29% and 26%, respectively). In conclusion, isoflurane produced direct effects on the Tei-index. The changes in systolic and diastolic parameters were supportive of this finding and were consistent with an overall impairment of left ventricular function during anesthesia.
Résumé
Effets de l’isoflurane sur la fonction myocardique évaluée par la détermination de l’index-Tei chez des chiens normaux. La détermination de l’index-Tei, un outil non invasif combinant les intervalles systolique et diastolique, a récemment été proposée dans l’évaluation de la fonction cardiaque globale. Toutefois, les effets de l’isoflurane sur cet index n’ont pas été caractérisés. L’objectif de cette étude est d’établir les effets de l’anesthésie à l’isoflurane utilisant une concentration alvéolaire minimale de 1,0 sur la période de pré-éjection (PEP), la période d’éjection ventriculaire (LVET), le ratio PEP/LVET, la période de relaxation isovolumétrique (IVRT), l’index d’éjection (SI), l’index cardiaque (CI), la fréquence cardiaque (HR) et l’index-Tei chez des chiens normaux non-prémédiqués. Une élévation significative de PEP, ratio PEP/LVET, IVRT et index-Tei a été observée et l’augmentation maximale enregistrée pour ces paramètres était de 47 %, 48 %, 78 % et 56 % respectivement. Les LVET et HR n’ont pas changé significativement alors que les SI et CI ont diminué durant l’anesthésie (29 % et 26 % respectivement). En conclusion, l’isoflurane a des effets significatifs sur l’index-Tei. Les changements notés au niveau des paramètres systoliques et diastoliques suggèrent un effet négatif sur la fonction ventriculaire gauche durant l’anesthésie à l’isoflurane.
(Traduit par Docteure Marie-Claude Bélanger)
Introduction
Isoflurane is an inhalation anesthetic that can cause dose-dependent depression of cardiac function (1,2). Isoflurane has been associated with a reduced cardiac output, stroke volume, and ejection fraction in infants, resulting in a decreased systolic function (3). Although not extensively studied, diastolic function also has been shown to be affected during isoflurane anesthesia (4). In dogs, isoflurane was shown to cause dose-related impairment in cardiac function, including reductions in cardiac output, stroke volume, arterial pressure, myocardial contractility, and left ventricular systolic pressure and afterload (5,6)
Transthoracic echocardiography is a reliable and widely used tool for the noninvasive assessment of cardiac function in veterinary patients. Several echocardiographic indices have been proposed for the evaluation of systolic and diastolic function, such as fractional shortening, ejection fraction, cardiac output, and mitral early to late ventricular filling ratio (7). However, some of the latter parameters may be affected by numerous factors, including loading conditions, heart rate, blood pressure, age, and rate of myocardial relaxation, to name a few (8–10). Recently, a Doppler-derived index that combines systolic and diastolic time intervals has been developed to assess global cardiac function (11,12). This index, often referred to as the Tei-index of myocardial performance, is defined as the sum of isovolumic contraction time and isovolumic relaxation time divided by ventricular ejection time (10). Although simple, several investigators have considered the Tei-index as a reliable and potential indicator of ventricular function in humans (13), because it is not significantly affected by heart rate (14), blood pressure (12), and ventricular loading conditions (15). The index has been calculated in normal conscious dogs (16) and in humans with several cardiac diseases (13). To the authors’ knowledge, however, the Tei-index has not been studied during volatile anesthesia to assess systolic and diastolic function in either veterinary or human patients.
This study was aimed at evaluating the Tei-index in dogs undergoing 1.0 minimal alveolar concentration (MAC) iso-flurane anesthesia to determine whether it may be a reliable measurement of global myocardial depression.
Materials and methods
Animals
After approval by the Commission on the Ethics and Welfare in Animal Experimentation of the College of Agricultural and Veterinarian Sciences-São Paulo State University, 16 mature mixed breed dogs of either sex were enrolled in the study. The mean body weight ± standard deviation (s) was 11.06, s = 2.72 kg. The dogs were housed in individual cages, given free access to water, and provided with commercially available dog food, q12h, during the entire experimental period. The use of animals for this study complied with the guidelines outlined in the United States’ National Institutes of Health Guide for the Care and Use of Laboratory Animals. The animals were determined to be healthy, based on results of physical, laboratorial, electrocardiographic, and echocardiographic examinations prior to the beginning of the experiment.
Before the experiment actually started, the dogs were acclimatized to the procedure by daily contact with people involved in the research and visits to the experimental room twice a week. Every time dogs were brought to the experimental room, they were restrained on the echocardiography table and underwent complete routine transthoracic echocardiography.
Anesthesia
After performing a baseline transthoracic echocardiogram with the patient breathing room air, anesthesia was induced by increasing the concentration of inspired isoflurane (Forane; Abbott Laboratórios do Brasil Ltda, Rio de Janeiro, Brazil) in 100% oxygen via face mask, until the animal allowed tracheal intubation. After orotracheal intubation, the dog was positioned in left lateral recumbency on an echocardiographic table and the tracheal tube was connected to a circle breathing system. Anesthesia was then maintained during spontaneous breathing with a 1.4% end-expired concentration of isoflurane in 100% oxygen (17). Although the animals were allowed to breath spontaneously at most times, individual adjustments were done whenever necessary to maintain end-tidal carbon dioxide (ETCO2) at 35–45 mmHg throughout anesthesia, as determined by a multiparametric monitor (DX2010; Dixtal, Manaus, Brazil), which was calibrated for the monitored gases. A thermal mattress (Brasmed; Paulínia, Brazil) was used to avoid steep decreases in body temperature, which was measured with an esophageal temperature probe inserted through the mouth into the esophagus of the anesthetized dogs.
Echocardiography
Every dog was examined with a Doppler transthoracic echocardio-graph (Pandion S300; PieMedical, Maastricht, The Netherlands) and a 5.0 MHz mechanical sector transducer. Echocardiographic images were recorded on videotape with a simultaneous lead II electrocardiogram for offline measurements.
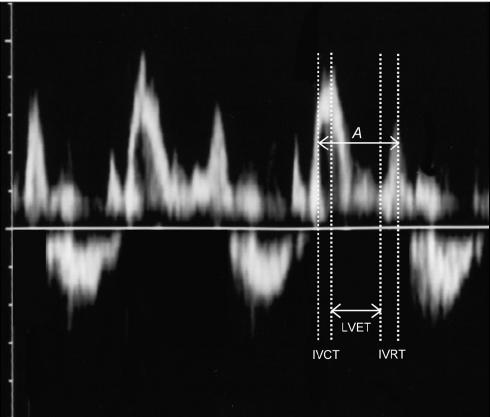
Prior to the induction of anesthesia, hair was clipped between the left 2nd and 7th intercostal spaces. Coupling gel was applied to this area of the thorax just before the echocardiographic examination. With the dog still positioned in left lateral recumbency, apical 5-chamber view images were acquired. Gain and filter settings were adjusted individually to reduce background noise and result in clear flow signals. To acquire inflow and outflow velocity spectra during the same cardiac cycle, the Doppler sample volume was placed midway between mitral inflow and left ventricular outflow in the apical 5-chamber view. This image was used to measure the mitral closing-to-opening time, which was named interval A (13). The left ventricular ejection time (LVET) was determined as the duration of the left ventricular outflow profile (13). Therefore, the Tei-index was calculated as (A - LVET)/LVET (Figure 1) (13,18). The latter image was also used to measure the pre-ejection period (PEP) from the electrocardiogram Q-wave to the onset of the left ventricular outflow (7). Still using the latter image, aortic cross-sectional area (AA) was estimated from the aortic diameter measured at the level of the valves, and aortic flow velocity integral (FVI) was determined by tracing the aortic flow profile. The stroke index (SI) was then calculated as (FVI × AA)/body surface area (7). Isovolumic relaxation time (IVRT) was measured as the interval between aortic valve closure and the onset of mitral inflow (7). Heart rate (HR) was calculated from the simultaneous lead II electrocardiogram. Also, the PEP-to-LVET ratio (PEP/LVET) and the cardiac index (CI = SI × HR) were calculated (7).
Figure 1.
Mitral and left ventricular outflow spectra obtained by pulsed-wave Doppler representing the intervals used for calculation of the Tei-index. Interval A extends from the cessation to the onset of mitral inflow, and comprises isovolumic contraction time (IVCT), left ventricular ejection time (LVET), and isovolumic relaxation time (IVRT).
All echocardiographic measurements were recorded immediately before (M0), and at 25 (M1), 40 (M2), and 55 (M3) min after isoflurane anesthesia was induced. Every dog was allowed a 20-minute interval after induction of anesthesia to equilibrate end-tidal isoflurane at 1.0 MAC before M1, M2, and M3 were actually recorded. At least 3 consecutive beats were measured and averaged for each parameter. All Doppler parameters were recorded by using pulsed-wave Doppler. Care was taken to perform these measurements with the Doppler beam adjusted as parallel as possible to the presumed direction of blood flow.
Statistical analyses
The mean and s of echocardiographic measurements were calculated. A repeated measures analysis of variance was applied to the various echocardiographic measurements to investigate interactions between anesthesia and the time course. When the differences were determined by the analysis of variance to be significant, the post hoc Tukey-Kramer multiple comparisons test was used to further investigate differences. Pearson’s correlation coefficient was calculated to detect correlations between the Tei-index and heart rate, Tei-index and SI, and Tei-index and CI at baseline and during anesthesia. For all analyses, significance was set at P < 0.05.
Results
The results of baseline and isoflurane echocardiographic parameters are presented in Table 1. Significant differences among moments are reported.
Table 1.
Echocardiographic parameters in 16 healthy dogs before (M0) and during anesthesia (M1, M2, M3) with isoflurane (1.0 MAC) (mean values, s)
| M0 Baseline | M1 25 minutes | M2 40 minutes | M3 55 minutes | P ANOVA | |
|---|---|---|---|---|---|
| HR (bpm) | 98.91, s = 23.56 | 113.81, s = 17.45 | 110.75, s = 11.51 | 112.75, s = 13.81 | 0.0632 |
| PEP (msec) | 50.31, s = 15.90 | 74.00, s = 21.27a | 72.43, s = 14.90a | 73.93, s = 16.56a | 0.0003 |
| LVET (msec) | 216.00, s = 21.68 | 217.43, s = 26.07 | 218.81, s = 30.14 | 221.56, s = 21.15 | 0.9335 |
| PEP/LVET | 0.23, s = 0.08 | 0.34, s = 0.10a | 0.33, s = 0.06a | 0.33, s = 0.06a | 0.0010 |
| IVRT (msec) | 42.81, s = 13.01 | 66.81, s = 13.65a | 69.31, s = 15.67a | 76.06, s = 21.26a | < 0.0001 |
| Tei-index | 0.43, s = 0.10 | 0.65, s = 0.11a | 0.65, s = 0.09a | 0.67, s = 0.11a | < 0.0001 |
HR — heart rate; PEP — e-ejection period; LVET — left ventricular ejection time; PEP/LVET — PEP-to-LVET ratio; IVRT — isovolumic relaxation time; ANOVA — analysis of variance; s — standard deviation; bpm — beat per minute; msec — millisecond
Statistically different from baseline (P < 0.05)
MAC — mean alveolar concentration
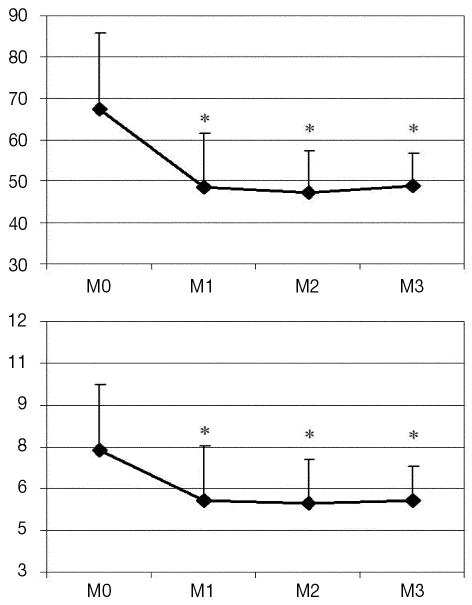
A significant increase was observed in PEP and IVRT from M1 to M3 with respect to baseline. A mild change was observed in LVET, which did not attain statistical significance in comparison with awake measurement. The magnitude of the maximal change in IVRT (78% at M3) was greater than that in PEP (47% at M1) and LVET (3% at M3). Although HR increased during anesthesia, the change was not significantly different from baseline value. Maximal increase was 15% at M1. From M1 to M3, HR remained relatively unchanged. The PEP/LVET ratio increased significantly throughout anesthesia (maximal magnitude of change was 48% at M1). Both SI and CI dropped significantly after isoflurane anesthesia was initiated (P = 0.0088 and P < 0.0001, respectively). Maximal decreases occurred at M2 and were 29% for SI and 26% for CI. Figure 2 shows the changes in SI and CI during the study.
Figure 2.
Variation in Doppler-derived stroke index (mL/beat · m2) (top) and cardiac index (L/min · m2) (bottom) in 16 healthy unpremedicated dogs. Data were acquired at baseline (M0) and at 25 (M1), 40 (M2), and 55 (M3) minutes after induction of isoflurane anesthesia (equilibrated at 1.0 minimal alveolar concentration at M1, M2, and M3). Statistical differences (P < 0.05) with respect to baseline measurement are indicated (*).
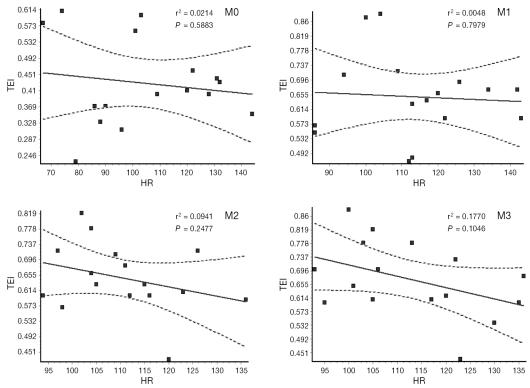
During isoflurane anesthesia, the Tei-index increased significantly in comparison with M0 (from 0.43, s = 0.10 to 0.67, s = 0.11 at M3). When the Tei-index and HR were compared, however, no statistical correlation was found to exist at both baseline and isoflurane measurements. Correlation coefficients and P values are shown in Figure 3. Interestingly, although CI, SI, and the Tei-index changed during isoflurane anesthesia, we found no linear correlation to exist between either CI or SI and the Tei-index.
Figure 3.
Correlation between heart rate and Tei-index in 16 healthy unpremedicated dogs. Data were acquired at baseline (M0) and at 25 (M1), 40 (M2), and 55 (M3) minutes after induction of isoflurane anesthesia (equilibrated at 1.0 minimal alveolar concentration at M1, M2, and M3).
Body temperature decreased significantly (P < 0.0001) from 38.53, s = 0.46 at M0 to 38.08, s = 0.57 at M1; 37.43, s = 0.55 at M2; and 37.20, s = 0.55 at M3.
Discussion
Our objective was not to validate the theoretical aspects of the index, but to investigate its changes during anesthesia. Because anesthesia was induced with increasing concentrations of inspired isoflurane using a face mask, the results do not reflect the additive depressant effects of sedative and induction agents, but only changes attributable to isoflurane.
Although the inability to acquire mitral inflow and left ventricular outflow spectra at the same cardiac cycle is often referred to as a potential source of measurement error in the Tei index (13), we did not encounter difficulty in acquiring such images, because the dogs were anesthetized and remained quiet, making it possible to place the Doppler sample volume at the correct location. Because the dogs had been acclimatized to the procedure before initiation of the experimental period, the acquisition of good-quality echo images was not difficult, even during awake measurements.
Pre-ejection period represents the sum of the electromechanical delay and the isovolumic contraction times (7). In horses anesthetized with 1.2 MAC isoflurane, Yamanaka et al (19) found an almost constant PEP over time, which differed from our findings. Although no baseline values were described in horses receiving isoflurane, Raisis et al (2) reported that PEP increased linearly with time. Contrasting with our results, LVET was shown to increase significantly in horses during isoflurane anesthesia (19). In mice, however, isoflurane maintained LVET relatively stable over time (20). Gueugniaud et al (21) reported an unchanged rate-corrected LVET in humans receiving 0.6% end-tidal isoflurane. Heart rate-corrected left-ventricular ejection time has been used in humans as a noninvasive estimate of left ventricular preload (22). In our study, only a mild nonsignificant increase was observed in HR. Accordingly, the unchanged LVET might substantiate a stable preload. The PEP is a heart-dependent index of cardiac function, influenced by loading conditions and myocardial contractility (23). Myocardial contractility is known to be inversely correlated with PEP/LVET, when HR is constant (7). Therefore, it is likely that myocardial contractility was reduced in our study. However, the accuracy of this parameter could be suspect, because it was not possible to determine whether the prolongation of PEP resulted from either a reduced cardiac output or a prolonged opening of the aortic valve owing to an increased afterload, since these parameters were not controlled in this study.
The decrease in SI and CI in our study is probably attributable to an overall impairment in cardiac function during isoflurane anesthesia. In horses receiving 1.0 MAC isoflurane, CI was also shown to decrease (2). It is likely that the decrease in myocardial contractility, as demonstrated by the increase in PEP/LVET, played a role in this finding. Murray et al (3) found a decreased CI in children being given isoflurane, findings similar to those for our study. Conversely, Rivenes et al (24) found differing results from ours with respect to CI, which was preserved when up to 1.5 MAC isoflurane was used in children with congenital heart disease.
Isoflurane has been shown to cause a prolongation of IVRT in dogs (4); this finding is consistent with our own. Prolongation of IVRT has been associated with impaired ventricular relaxation and, accordingly, diastolic dysfunction. On the contrary, Oxorn et al (25) have shown that in human patients with normal cardiovascular function, isoflurane anesthesia is not associated with either prolonged left ventricular relaxation or increased myocardial restriction.
While our measure of the Tei-index in awake animals is within the 95% confidence interval proposed by Baumwart et al (16) for normal conscious dogs, the measures during isoflurane anesthesia resulted in an increased index that does not fit the confidence interval proposed in the latter study. Although the Tei-index has not previously been investigated in dogs undergoing volatile anesthesia, the significant increase observed during anesthesia was consistent with depressed systolic and diastolic function (13). Our findings of increased PEP/LVET, PEP, and IVRT are supportive of this finding. Although we did not find a relationship to exist between either echo-derived SI or CI and the Tei-index, the Tei-index fills in a gap for the noninvasive measurement of global cardiac function (18), because it is easily calculated from conventional Doppler echocardiographs, and several investigators have demonstrated that it has a good correlation with invasive measurements of cardiac function (inverse relationship with cardiac output, and ejection fraction; direct correlation with systolic peak +dP/dt, diastolic peak −dP/dt, and ventricular stiffness) (26,27). Pellett et al (13) compiled several studies in humans and reported that the Tei-index is increased with dilated cardiomyopathy, acute myocardial infarction with congestive heart failure, amyloidosis, and either isolated systolic or isolated diastolic dysfunction. Whether heart rate influences the Tei-index remains controversial: some studies have demonstrated that the Tei-index may be dependent on heart rate (14). In our study, however, we found no correlation between heart rate and the Tei-index in measurements from both awake and anesthetized subjects. The absence of correlation with heart rate was also demonstrated in humans.
The Tei-index seems to be a sensitive and heart-rate-independent indicator of the depressant effects of isoflurane on global left ventricular function. Additional studies are needed to determine whether different inspired concentrations of isoflurane affect the Tei-index, as well as how the Tei-index performs in dogs with baseline impaired cardiac function. CVJ
Footnotes
This study was supported by a grant from the Graduation Program in Veterinary Surgery, College of Agricultural and Veterinarian Sciences, São Paulo State University, Campus of Jaboticabal, Brazil.
References
- 1.Pagel PS, Kampine JP, Schmeling WT, et al. Influence of volatile anesthetics on myocardial contractility in vivo: Desflurane versus isoflurane. Anesthesiology. 1991;74:900–907. doi: 10.1097/00000542-199105000-00016. [DOI] [PubMed] [Google Scholar]
- 2.Raisis AL, Blissitt KJ, Henley W. The effects of halothane and isoflurane on cardiovascular function in laterally recumbent horses. Br J Anaesth. 2005;95:317–325. doi: 10.1093/bja/aei180. [DOI] [PubMed] [Google Scholar]
- 3.Murray DJ, Forbes RB, Mahoney LT. Comparative hemodynamic depression of halothane versus isoflurane in neonates and infants: an echocardiographic study. Anesth Analg. 1992;74:329–337. doi: 10.1213/00000539-199203000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Pagel PS, Kampine JP, Schmeling WT, et al. Alteration of left ventricular diastolic function by desflurane, isoflurane, and halothane in the chronically instrumented dog with autonomic nervous system blockade. Anesthesiology. 1991;74:1103–1114. doi: 10.1097/00000542-199106000-00019. [DOI] [PubMed] [Google Scholar]
- 5.Hettrick DA, Pagel PS, Warltier DC. Desflurane, sevoflurane, and iso-flurane impair canine left ventricular-arterial coupling and mechanical efficiency. Anesthesiology. 1996;85:403–413. doi: 10.1097/00000542-199608000-00023. [DOI] [PubMed] [Google Scholar]
- 6.McMurphy RM, Hodgson DS, Bruyette DS, et al. Cardiovascular effects of 1.0, 1.5, and 2.0 minimum alveolar concentrations of isoflurane in experimentally induced hypothyroidism in dogs. Vet Surg. 1996;25:171–178. doi: 10.1111/j.1532-950x.1996.tb01394.x. [DOI] [PubMed] [Google Scholar]
- 7.Boon JA. Manual of Veterinary Echocardiography. Philadelphia: Williams & Wilkins, 1996:151–236.
- 8.Choong CY, Herrmann HC, Weymann AE, et al. Preload dependence of Doppler derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol. 1987;10:800–808. doi: 10.1016/s0735-1097(87)80273-5. [DOI] [PubMed] [Google Scholar]
- 9.Kuo LC, Quinones MA, Rokey R, et al. Quantification of atrial contribution to left ventricular filling by pulsed wave Doppler echocardiog-raphy and the effect of age in normal and diseased hearts. Am J Cardiol. 1987;59:1174–1178. doi: 10.1016/0002-9149(87)90870-8. [DOI] [PubMed] [Google Scholar]
- 10.Ärnlöv J, Lind L, Andrén B, et al. A Doppler-derived index of combined left ventricular systolic and diastolic function is an independent predictor of cardiovascular mortality in elderly men. Am Heart J. 2005;149:902–907. doi: 10.1016/j.ahj.2004.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Tei C. New non-invasive index for combined systolic and diastolic ventricular function. J Cardiol. 1995;26:135–136. [PubMed] [Google Scholar]
- 12.Tei C, Ling LH, Hodge DO, et al. New index of combined systolic and diastolic myocardial performance: A simple and reproducible measure of cardiac function — a study in normals and dilated cardiomyopathy. J Cardiol. 1995;26:357–366. [PubMed] [Google Scholar]
- 13.Pellett A, Tolar WG, Merwin BS, et al. The Tei index: methodology and disease state values. Echocardiography. 2004;21:669–672. doi: 10.1111/j.0742-2822.2004.04052.x. [DOI] [PubMed] [Google Scholar]
- 14.Poulsen SH, Nielsen JC, Andersen HR. The influence of heart rate on the Doppler-derived myocardial performance index. J Am Soc Echocardiogr. 2000;3:379–384. doi: 10.1016/s0894-7317(00)70007-1. [DOI] [PubMed] [Google Scholar]
- 15.Moller JE, Poulsen SH, Egstrup K. Effect of preload alterations on a new Doppler echocardiographic index of combined systolic and diastolic performance. J Am Soc Echocardiogr. 1999;135:1065–1072. doi: 10.1016/s0894-7317(99)70103-3. [DOI] [PubMed] [Google Scholar]
- 16.Baumwart RD, Meurs KM, Bonagura JD. Tei index of myocardial performance applied to the right ventricle in normal dogs. J Vet Intern Med. 2005;19:828–832. doi: 10.1892/0891-6640(2005)19[828:tiompa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 17.Deryck YLJM, Brimioulle S, Maggiorini M, et al. Systemic vascular effects of isoflurane versus propofol anesthesia in dogs. Anesth Analg. 1996;83:958–964. doi: 10.1097/00000539-199611000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Harada K, Tamura M, Toyono M, et al. Effect of dobutamine on a Doppler echocardiographic index of combined systolic and diastolic performance. Pediatr Cardiol. 2002;23:613–617. doi: 10.1007/s00246-002-0017-7. [DOI] [PubMed] [Google Scholar]
- 19.Yamanaka T, Oku K, Koyama H, et al. Time-related changes of the cardiovascular system during maintenance anesthesia with sevoflurane and isoflurane in horses. J Vet Med Sci. 2001;63:527–532. doi: 10.1292/jvms.63.527. [DOI] [PubMed] [Google Scholar]
- 20.Roth DM, Swaney JS, Dalton ND, et al. Impact of anesthesia on cardiac function during echocardiography in mice. Am J Physiol Heart Circ Physiol. 2002;282:2134–2140. doi: 10.1152/ajpheart.00845.2001. [DOI] [PubMed] [Google Scholar]
- 21.Gueugniaud PY, Vaudelin G, Bertin-Maghit M, et al. Comparison of the myocardial effects of desflurane and isoflurane in healthy patients: Assessment by continuous oesophageal aortic blood flow echo-Doppler. Br J Anaesth. 1998;81:844–849. doi: 10.1093/bja/81.6.844. [DOI] [PubMed] [Google Scholar]
- 22.Singer M, Bennett ED. Noninvasive optimization of left ventricular filling using esophageal Doppler. Crit Care Med. 1991;19:1132–1137. doi: 10.1097/00003246-199109000-00007. [DOI] [PubMed] [Google Scholar]
- 23.Borow KM. An integrated approach to the noninvasive assessment of left ventricular systolic and diastolic performance. In: St. John Sutton M, Oldershaw PJ, eds. Textbook of Adult and Paediatric Echocardiography and Doppler. Boston: Blackwell, 1989:97–168.
- 24.Rivenes SM, Lewin MB, Stephen A, et al. Cardiovascular effects of sevoflurane, isoflurane, halothane, and fentanyl-midazolam in children with congenital heart disease. Anesthesiology. 2001;94:223–229. doi: 10.1097/00000542-200102000-00010. [DOI] [PubMed] [Google Scholar]
- 25.Oxorn D, Edelist G, Harrington E, et al. Echocardiographic assessment of left ventricular filling during isoflurane anesthesia. Can J Anaesth. 1996;43:569–574. doi: 10.1007/BF03011768. [DOI] [PubMed] [Google Scholar]
- 26.Lacorte JC, Cabreriza SE, Rabkin DG, et al. Correlation of the Tei index with invasive measurements of ventricular function in a porcine model. J Am Soc Echocardiogr. 2003;16:442–447. doi: 10.1016/s0894-7317(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 27.Tei C, Nishimura RA, Seward JB, et al. Noninvasive Doppler-derived myocardial performance index: Correlation with simultaneous measurements of cardiac catheterization measurements. J Am Soc Echocardiogr. 1997;10:169–178. doi: 10.1016/s0894-7317(97)70090-7. [DOI] [PubMed] [Google Scholar]