Abstract
Vacuole fusion occurs in three stages: priming, in which Sec18p mediates Sec17p release, LMA1 (low Mr activity 1) relocation, and cis-SNARE (soluble N-ethylmaleimide-sensitive factor attachment protein receptor) complex disassembly; docking, mediated by Ypt7p and trans-SNARE association; and fusion of docked vacuoles. Ca2+ and calmodulin regulate late stages of the reaction. We now show that the vacuole proton gradient, generated by the vacuolar proton ATPase, is needed for trans-SNARE complex formation during docking and hence for the subsequent LMA1 release. Though neither the vacuolar Pmc1p Ca2+-ATPase nor the Vcx1p Ca2+/H+ exchanger are needed for the fusion reaction, they participate in Ca2+ and ΔμH+ homeostasis. Fusion itself does not require the maintenance of trans-SNARE pairs.
Subcellular protein compartmentation requires vesicular traffic between organelles (1). Trafficking entails selective protein sorting into budding vesicles, directed movement of vesicles to the target organelle, and regulated fusion. Vesicles in many trafficking reactions use homologous GTPases, N-ethylmaleimide-sensitive factor (NSF), SNAREs (soluble NSF attachment protein receptors), SNAPs (soluble NSF attachment protein), and other proteins to catalyze “docking” to the target membrane, followed by membrane fusion (1–4). Similar proteins catalyze these events in yeast and human neurons, establishing their generality (5, 6).
Vacuoles (lysosomes) from Saccharomyces cerevisiae offer several advantages for studying membrane fusion. Vacuole fusion is the final step of the inheritance of this organelle and maintains it in low copy number. Because vacuoles are readily visualized (7, 8), mutations that block their fusion and allow them to remain fragmented can be easily scored. Vacuoles are readily isolable, and the in vitro fusion of vacuoles with biochemically complementary deficiencies yields simple, colorimetric, quantitative assays (9, 10). Soluble proteins that support vacuole fusion have been isolated (11), and detergent extracts of the vacuole membrane can be reconstituted to yield fusion-competent proteoliposomes (12), providing for the eventual isolation of the proteins needed for reconstitution of membrane fusion with all purified components.
Our studies of in vitro homotypic vacuole fusion have provided a broad outline of this reaction. Isolated vacuoles bear a multisubunit “cis-SNARE complex” consisting of the Vam3p t-SNARE, the Vam7p soluble N-ethylmaleimide-sensitive factor attachment protein (SNAP)-23/25 homolog (s-SNARE), the Nyv1p v-SNARE, and the v-SNAREs Vti1p and Ykt6p (13–16). In addition to these SNAREs, the Sec18p (N-ethylmaleimide-sensitive factor) ATPase, a chaperone, and its cochaperone Sec17p (α-SNAP) are part of this complex (13, 17). Furthermore, the cis-SNARE complex contains a novel heterodimeric protein termed LMA1 (low Mr activity 1) (18), which has thioredoxin and IB2 as subunits (19). The overall reaction occurs in three obligatorily ordered stages: priming, docking, and fusion itself. In priming, ATP hydrolysis by Sec18p drives four reactions: the release of Sec17p from the vacuoles, the disassembly of the cis-SNARE complex, the activation of the t-SNARE, and the transfer of LMA1 from its association with Sec18p to the activated t-SNARE, which it stabilizes (13, 17, 18). Primed vacuoles then undergo a two-part docking reaction (20). Their reversible association, termed tethering, is catalyzed by the Rab-like Ypt7p (20, 21). Tethered vacuoles are stabilized in their association by trans-SNARE pair formation (20) to complete docking. Docked vacuoles then undergo fusion. Though less is known of fusion per se, it requires neither ATP, Sec17p, or Sec18p nor the continued presence of trans-SNARE pairs (17, 20, 22). This final fusion stage needs calcium, calmodulin (CaM), and a Microcystin-LR-sensitive phosphatase, is sensitive to guanosine 5′-[γ-thio]triphosphate and mastoparan, and is accompanied by the release of bound LMA1 (10, 18, 22, 23). We now report that the latter stages of this reaction occur in separable steps, which require the vacuole acidification for trans-SNARE pairing, followed by membrane fusion and mixing of contents.
METHODS
Yeast Strains.
Deletions of VCX1 in yeast strains BJ3505 and DKY6281 were created by transformation with plasmid pKC72 (24), cutting with KpnI and SacI, and selection on SC minus uracil (25). Deletions of PMC1 in BJ3505 were by transformation with plasmid pKC59 (26), cutting with StuI and BglII, and selection on SC minus tryptophan. Deletions of PMC1 in DKY6281 were made by transformation by plasmid pKC52 (26), cutting with HindIII, and selection on SC minus leucine. PMC1 transformants were replica-plated on yeast extract/peptone/dextrose containing 200 mM CaCl2 to test for growth delay (26). Double deletions in PMC1 and VXC1 were made in the respective vcx1Δ strain by deleting PMC1 as described above.
Biochemical Methods.
Reagents were as described (17, 27, 28). SDS/PAGE, immunoblotting using ECL (29), purification of IgGs, His6-tagged Sec18p, and Sec17p (28), purification of CaM (23) and LMA1 (11), and coimmunoprecipitation to detect trans-SNARE pairs (20) were as described. Salt-washed vacuoles were made as described (11).
Vacuole Fusion.
Vacuoles (27) were used immediately after isolation or salt wash (11). Standard fusion reactions (30 μl) contained 3 μg of each vacuole type (BJ3505 and DKY6281) in reaction buffer (10 mM Pipes/KOH, pH 6.8/200 mM sorbitol/150 mM KCl/0.5 mM MgCl2/0.5 mM MnCl2), 0.5 mM ATP, 3 mg/ml cytosol, 3.5 units/ml creatine kinase, 20 mM creatine phosphate, and protease inhibitors (11): 3.3 μM Pefabloc SC, 0.1 ng/ml leupeptin, 16.6 μM o-phenanthroline, and 16.6 ng/ml pepstatin. Cytosol was as described (27). Vacuoles lacking Nyv1p or Vam3p were used at 4 μg each in the fusion reaction (20). One unit of fusion activity is defined as 1 μmol p-nitrophenyl phosphate hydrolyzed per min and μg BJ3505.
RESULTS
To assay homotypic vacuole fusion, we purify vacuoles from two strains of yeast. One strain (BJ3505) has a deletion in the gene encoding the major vacuolar proteases and therefore accumulates catalytically inactive pro-alkaline phosphatase. The other strain, DKY6281, is deleted for the gene for this phosphatase but has normal vacuolar proteases. Though neither population of purified vacuoles has phosphatase activity, the fusion of these vacuoles in vitro allows the proteases to process the pro-phosphatase to its catalytically active form that can be measured colorimetrically (10).
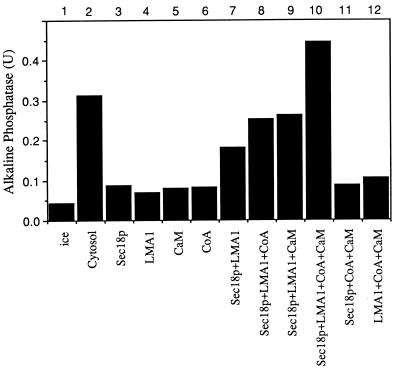
Though we previously have reported that purified vacuoles can fuse in a minimal reconstituted reaction with ATP, purified LMA1, and Sec18p (19), it also has been reported that CoA, calcium, and CaM have important roles in this reaction (23, 28). Consistent with these findings, salt-washed vacuoles show little stimulation of fusion over the ice control by the addition of either Sec18p, LMA1, CaM, or CoA alone (Fig. 1, lanes 3–6) whereas substantial fusion is seen with Sec18p and LMA1 together (lane 7). However, the further addition of CoA (lane 8), CaM (lane 9), or both (lane 10) stimulates the fusion reaction, until the fusion supported by ATP, Sec18p, LMA1, CoA, and CaM (lane 10) is greater than that supported by ATP and the starting cytosol (lane 2). The identification of other soluble factors may further optimize this reaction.
Figure 1.
Salt-washed vacuoles require Sec18p, LMA1, CoA, and CaM for optimal fusion. Vacuoles (11) were incubated for 100 min at 27°C with ATP and Sec18p (0.33 μg/ml), LMA1 (0.33 μg/ml), CoA (20 μM), and CaM (7.5 μM) as indicated. Alkaline phosphatase activity was measured (27).
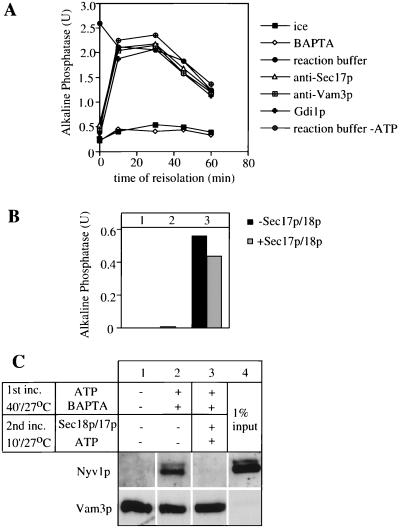
Recent studies (23) have shown that calcium and CaM are required for a late stage of the reaction. We find that 1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate (BAPTA), a specific chelator of calcium, reversibly inhibits the fusion stage of the reaction while allowing priming and docking to proceed. Vacuoles incubated with BAPTA did not fuse for up to 60 min (Fig. 2A, ⋄). Aliquots that were sedimented to remove BAPTA and resuspended without chelator recovered the capacity for fusion (Fig. 2A, ●), though there was a gradual decay of vacuole fusion capacity. Initial resuspension in the presence of anti-Sec17p, an inhibitor of priming, blocked the reaction (Fig. 2A, ▵), but full resistance to anti-Sec17p was rapidly attained, indicating that BAPTA allowed priming to proceed. Similarly, the vacuoles rapidly gained resistance to anti-Vam3p (Fig. 2A, □) or Gdi1p (Fig. 2A, crosses), inhibitors of docking, during incubation with ATP and BAPTA. BAPTA thus serves as a reversible inhibitor with a known target that specifically blocks vacuole fusion after docking.
Figure 2.
BAPTA allows priming and docking of vacuoles but is a reversible inhibitor of the fusion reaction. (A) Time course of inhibitor addition. Isolated vacuoles (27) were incubated at 27°C in the presence of ATP and 5 mM BAPTA. At the indicated times, 30-μl aliquots were removed, diluted with 300 μl of buffer D (10 mM Pipes/KOH, pH 6.8/200 mM sorbitol/150 mM KCl), and centrifuged (4 min, 8,000 × g, 4°C). Vacuoles were resuspended in the presence of cytosol, ATP, and the indicated inhibitor and incubated for the remaining time. Fusion was measured after 90 min. (B and C) Trans-SNARE pairs are not needed at the fusion stage of the reaction. Vacuoles (100 μg) from the two tester strains (DKY vam3Δ and BJ nyv1Δ) were incubated in the presence of BAPTA (5 mM), ATP, and cytosol for 40 min at 27°C (lanes 2 and 3) or left on ice in the absence of ATP (lane 1). Vacuoles were diluted 10-fold in buffer D, reisolated (4 min, 16,000 × g, 4°C), and resuspended in reaction buffer containing cytosol, ATP, and (where indicated) His6-Sec17p (2.5 μg/ml) and His6-Sec18p (26 μg/ml). After 10 min at 27°C, a 30-μl aliquot of each was placed on ice (B, lane 2) and another was incubated for additional 140 min (B, lane 3) and each was assayed for phosphatase. After the 10-min second incubation, the remainder of the reactions were centrifuged (5 min, 16,000 × g, 4°C), vacuoles were washed with PS buffer (10 mM Pipes/KOH, pH 6.8/200 mM sorbitol), solubilized in 0.5% Triton X-100, 2 mM EDTA, PBS, pH 7.4 (43), 1 mM PMSF, and 1× PIC (11) and analyzed for Nyv1p cross-precipitation with Vam3p antibodies (C) as described (20).
We recently have reported that the addition of high levels of purified Sec17p and Sec18p to docked vacuoles will efficiently disrupt the trans-SNARE pairs without affecting the subsequent fusion reaction (20). However, because of imperfect reaction synchrony in these experiments, approximately 35% of the final fusion already had occurred by the time of maximal trans-SNARE pairing when further incubations were performed with (or without) added Sec17p and Sec18p. To achieve better fusion synchrony, we incubated BJ3505 vacuoles deleted for the v-SNARE Nyv1p with DKY6281 vacuoles deleted for the t-SNARE Vam3p in the presence of BAPTA. After 40 min, almost no fusion had occurred (Fig. 2B, lane 2), yet Nyv1p had formed a trans-SNARE complex with Vam3p, as judged by cross-immunoprecipitation (Fig. 2C, lane 2). As previously reported (20), only a small percent of SNAREs form a complex in trans. Vacuoles then were briefly incubated with, or without, added Sec17p and Sec18p and sedimented and resuspended to remove the BAPTA. Incubation with ATP, Sec17p, and Sec18p disrupted trans-SNARE pairs (Fig. 2C, lane 3). Removal of the BAPTA block allowed fusion to proceed whether or not the trans-SNARE pairs had been disrupted (Fig. 2B, lane 3), in agreement with our earlier studies (20).
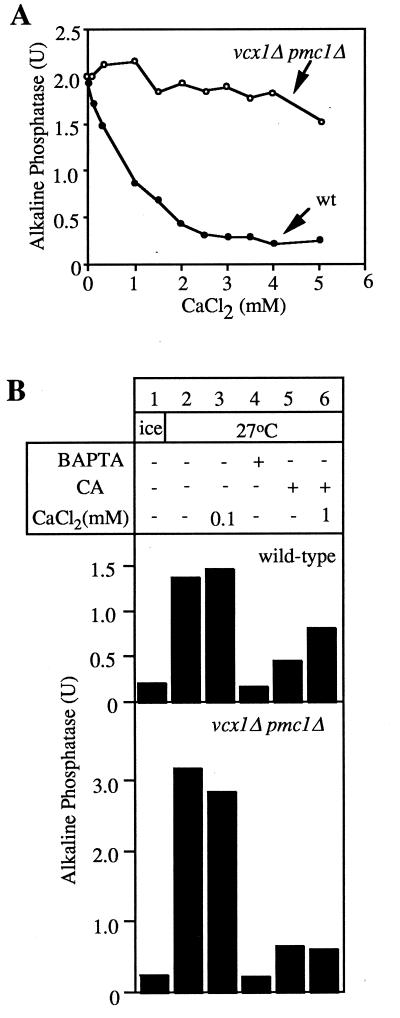
The central role of calcium in the fusion reaction and Ca2+ release from the vacuoles upon docking (23) suggests that Ca2+ release may occur by the well-characterized Pmc1p, a Ca2+-ATPase (26), or Vcx1p, a H+/Ca2+ exchanger (24), and that these may be the targets of cyclopiazonic acid (CA), a calcium channel blocker (30, 31) that inhibits vacuole fusion (23). To examine the role of the known vacuolar calcium transporters, we deleted the genes for either the Pmc1p Ca2+-ATPase, the Vcx1p H+/Ca2+ exchanger, or both from our fusion tester strains. Wild-type vacuole fusion (Fig. 3A, ●) was sensitive to calcium. Vacuoles lacking both calcium transporters (Fig. 3A, ○) or even lacking Vcx1p alone (data not shown) are fully active for fusion but are no longer sensitive to calcium inhibition. These observations suggest that calcium may inhibit by being exchanged for vacuole luminal protons by Vcx1p, thereby dissipating the vacuole luminal acidity. The CA inhibition of fusion (23), seen with wild-type vacuoles (Fig. 3B, Upper, lane 5), is partially relieved by Ca2+ (Fig. 3B, Upper, lane 6) but this relief depends on Vxc1p and Pmc1p (Fig. 3B, Lower, lanes 5 and 6). Ca2+ may function by being pumped into the vacuoles by Pmc1p at the expense of ATP hydrolysis and then being exchanged for protons by the Vcx1p. In this model, CA would block a regulator of the membrane proton gradient, and calcium would cycle through the vacuole via Pmc1p and Vcx1p to partially restore the membrane electrochemical potential at the expense of ATP. Excess external calcium then can function in two ways, to dissipate the vacuole proton gradient via the Vcx1p exchanger and to generate a proton gradient via Pmc1p and Vcx1p.
Figure 3.
Deletion of both Vxc1p and Pmc1p results in low sensitivity to Ca2+ but does not impair fusion activity. (A) Vacuoles were isolated from our normal tester strains and pmc1Δ vcx1Δ derivatives, then incubated for 90 min at 27°C in the presence of ATP and CaCl2, and phosphatase was assayed. (B) Fusion of wild-type and pmc1Δ vcx1Δ vacuoles. Vacuoles were incubated in reaction buffer and ATP for 90 min at 27°C in the presence of CaM (7.5 μM), CA (250 μM), BAPTA (5 mM), and CaCl2, and alkaline phosphatase activity was measured.
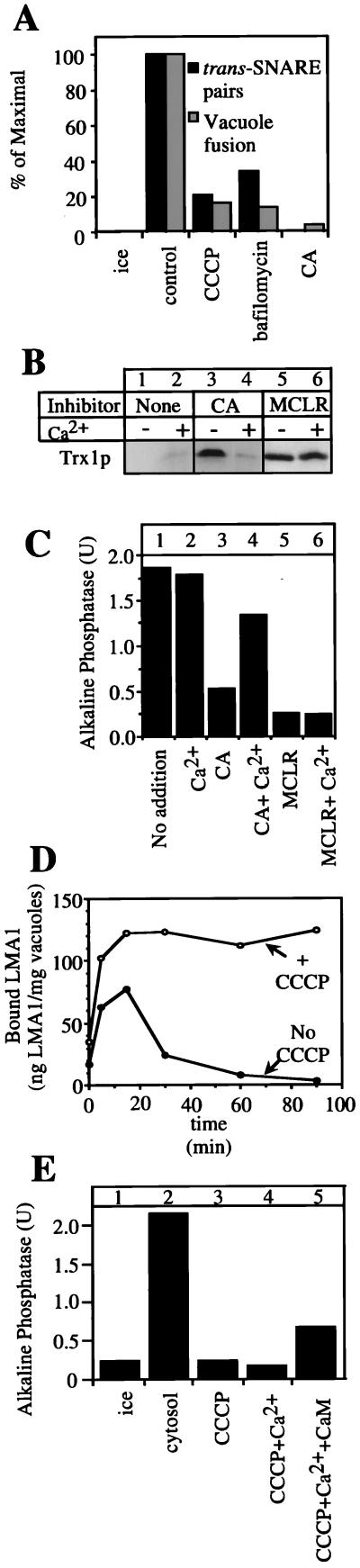
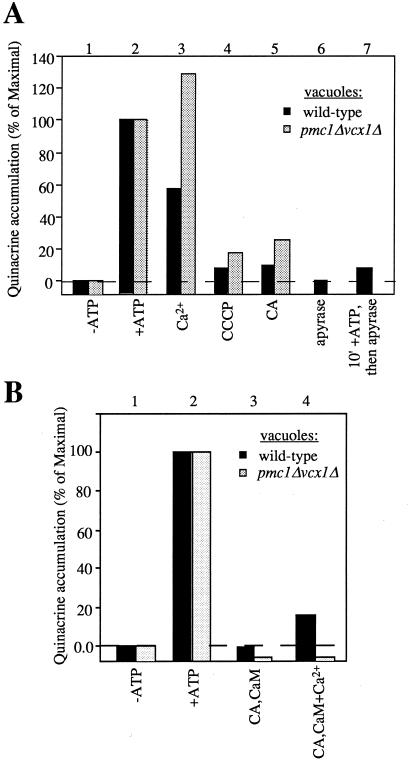
To test this concept, we studied the effects of CA and direct dissipation of the vacuole proton gradient by uncouplers or by inhibitors of the vacuolar ATPase on trans-SNARE pair formation and LMA1 release, the two known steps that precede membrane fusion. We find that either CA, bafilomycin, or carbonylcyanide m-chlorophenylhydrazone (CCCP) strongly inhibit trans-SNARE pair formation (Fig. 4A) and hence fusion. LMA1 normally has been released from vacuoles by the end of the fusion reaction as shown by its absence from an immunoblot of vacuoles at the end of a standard fusion reaction (Fig. 4B, lane 1; ref 18), and this release is not affected by calcium (Fig. 4B, lane 2). However, release is blocked by CA (Fig. 4B, lane 3), and this block is bypassed by added calcium (Fig. 4B, lane 4). An examination of the same incubations for fusion shows that calcium has little effect on fusion in the absence of the drugs (Fig. 4C, lanes 1 and 2) but restores fusion to CA-treated vacuoles (Fig. 4C, lane 4) as it restores LMA1 release (Fig. 4B, lane 4). Calcium does not relieve the inhibition of either fusion or LMA1 release by Microcystin-LR (Fig. 4 B and C, lanes 5 and 6). Salt-washed vacuoles appear to be less sensitive to exogenous Ca2+ than freshly isolated vacuoles (Figs. 3 and 4), possibly because of the removal of a Ca2+ sensor on the vacuole. Our postulate that these effects of CA and Ca2+ were mediated by the membrane potential was confirmed by the observation that CCCP efficiently blocks LMA1 release (Fig. 4D) and that the inhibition of the normal fusion of salt-washed vacuoles by CCCP (Fig. 4E, lanes 2 and 3) is partially reversed by the addition of both calcium and CaM (Fig. 4E, lane 5). Indeed, the ATP-dependent acidification of the vacuole, measured by luminal accumulation of quinacrine (7), is blocked by CA as efficiently as by CCCP (Fig. 5A, lanes 4 and 5). Furthermore, high concentrations of Ca2+ diminish the acidification of wild-type vacuoles, but not that of vacuoles deleted in the genes encoding the Ca2+ transporters (Fig. 5A, lanes 2 and 3), consistent with the finding that Ca2+ does not inhibit the efficient fusion of these vacuoles (Fig. 3A). Finally, the dissipation of vacuole acidification by CA is partially reversed by the addition of calcium and CaM (Fig. 5B, lane 4). This reversal is not seen in vacuoles from strains deleted for the two known Ca2+-transport proteins.
Figure 4.
The membrane potential is required for trans-SNARE pairing and LMA1 release and can be partially restored by Ca2+. (A) Trans-SNARE complex formation requires the vacuolar membrane potential. Vacuoles (100 μg) from the two tester strains (DKY vam3Δ and BJ nyv1Δ) were incubated in the presence of ATP, cytosol, and CCCP (20 μM), Bafilomycin (10 μM), or CA (250 μM) where indicated for 40 min at 27°C and analyzed for trans-SNARE complex (20). An aliquot was further incubated for 140 min to measure fusion. (B and C) Release of LMA1 is blocked by CA and rescued by added Ca2+. Salt-washed vacuoles (equivalent to six standard reactions) were incubated with ATP, Sec18p, LMA1, CoA, and CaM (see Fig. 1) and 20 μM MCLR or 1 mM CA with or without 3 mM Ca2+. After 90 min, vacuoles equivalent to one fusion reaction were transferred to another tube to measure fusion activity (C). The remaining vacuoles were assayed for bound LMA1 (B) as described (18). (D) CCCP blocks LMA1 release. Samples equivalent to five standard reactions were incubated at 25°C with (○) or without (●) CCCP (30 μM). At the indicated times, vacuoles were reisolated (5 min, 8,000 × g, 4°C), resuspended in 300 μl of PS buffer with 1× PIC and 0.5 mM PMSF, reisolated, resuspended in 25 μl of PS buffer, and transferred to a fresh tube. The samples then were analyzed by “High-Tris” SDS/PAGE and immunoblot with anti-Trx1p antibodies (11). Bound LMA1 was quantified by densitometry and normalized to a standard of pure LMA1 (18). (E) CCCP inhibition of vacuole fusion is partially rescued by addition of Ca2+ and CaM. Salt-washed vacuoles were incubated for 100 min in the presence of ATP and cytosol (1 mg/ml) on ice or at 25°C. CCCP (30 μM), Ca2+ (1 mM), and CaM (7.5 μM) were added where indicated. Alkaline phosphatase activity was measured as described in Methods.
Figure 5.
Vacuolar acidification. (A) Sensitivity of the acidification of wild-type and mutant vacuoles to CA, CCCP, and Ca2+. Vacuoles (20 μg) from BJ3505 wild-type or pmc1Δvcx1Δ strains were incubated at 27°C for 30 min in the presence (lanes 2–7) or absence of ATP (lane 1). CA (250 μM), CCCP (50 μM), apyrase (40 units/ml), or Ca2+ (5 mM) were added where indicated. Quinacrine (7) was added at 200 μM to all samples just before the start of the incubation. At the end of the incubation, vacuoles were reisolated (3 min, 8,000 × g, 4°C), resuspended in 900 μl of 0.4% Triton X-100 in water, and assayed for OD430. (B) Partial reversal of CA-mediated block in acidification by Ca2+ and CaM. The experiment was performed as described in A. Ca2+ (1.5 mM) and CaM (10 μM) were added where indicated.
DISCUSSION
Vacuole fusion is sensitive to the same inhibitors and needs similar proteins as other membrane trafficking reactions. Calcium is needed at or near the membrane fusion event in neural transmission (32, 33) and endoplasmic reticulum to Golgi vesicular trafficking (34), as well as for homotypic vacuole fusion (23). Thus studies of the role of calcium and CaM in vacuole fusion may provide insights about membrane fusion in other organelles and organisms as well.
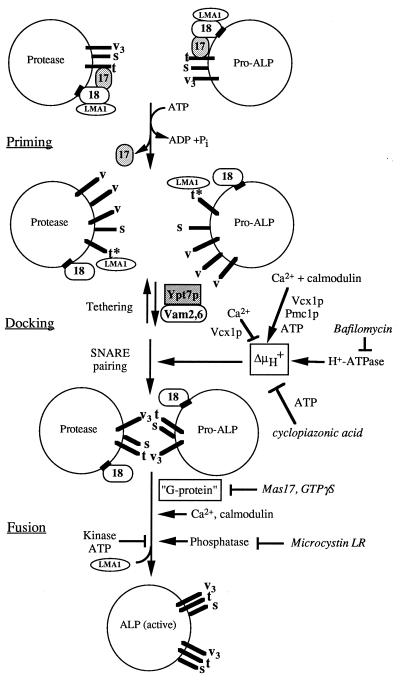
The need for calcium and CaM for a late stage of the reaction reveals the inherent complexity of trans-SNARE pairing and the ensuing steps that lead to fusion. These are summarized in a working model (Fig. 6). Trans-SNARE pairing, and hence LMA1 release and fusion, are regulated by the vacuole proton gradient (ΔμH+). In the presence of ATP, the maintenance of this gradient is sensitive to CA. The requirement for the vacuole membrane potential for trans-SNARE pairing is without precedent, as the distinction between the more abundant cis-SNARE pairs and the few trans-SNARE pairs has not been as experimentally accessible in other systems. The requirement for membrane potential could be direct, or may be indirect if the potential is required only to maintain essential ion gradients. Our data do not allow us to distinguish whether the membrane potential is required only for trans-SNARE pairing or whether it also has a function later in the fusion reaction. Further studies will be necessary to resolve this question.
Figure 6.
Working model for vacuole to vacuole fusion and its regulation. Abbreviations: Sec17p (17), Sec18p (18), Nyv1p, Ykt6p, and Vti1 (v [individually] or v3), Vam3p (t), Vam7p (s), pro-alkaline phosphatase (Pro-ALP), alkaline phosphatase (ALP), GTPγs, guanosine 5′-[γ-thio]triphosphate. ∗ on the t-SNARE indicates the activated state of the protein after priming. Inhibitors are written in italics. Arrows indicate activators, bars indicate inhibitors. See text for details.
It is interesting that deletions in the vacuolar ATPase subunit VMA1 (35, 36) or addition of bafilomycin to cell culture systems (37) causes a delay in protein transport to the vacuole/lysosome. This delay may be caused by a reduction of the electrochemical potential across the vacuole membrane and hence an inhibition of the docking of endosome-derived vesicles. In addition to this need for ΔμH+, both homotypic vacuole fusion and biosynthetic traffic to the vacuole share a need for many catalytic proteins (38–41).
The later stages of the homotypic vacuole fusion reaction do not require either of the known vacuolar calcium transporters (Fig. 3), suggesting that the docking-dependent calcium flux (23) occurs via a novel mechanism. Nevertheless (Fig. 6), these transporters can work with added Ca2+ to partially dissipate ΔμH+ (Fig. 5A). CA does not directly inhibit fusion through blocking these transporters, as it still fully inhibits in their absence (Fig. 3B). Ca2+ may partially overcome CA inhibition (Figs. 3B and 4C) by being pumped into the vacuole by Pmc1p at the expense of ATP, then being exchanged for protons by Vcx1p. This model (Fig. 6) is supported by the finding that Ca2+ and CaM can partially restore fusion and acidification to CA-treated vacuoles (Figs. 4C and 5B). Synaptic membrane fusion also is triggered by calcium flux gated by an electrical signal (32, 33). However, regulated exocytosis also occurs in the absence of a membrane potential as long as Ca2+ is provided to trigger fusion (42). It will be important to determine whether calcium must interact with CaM at the neural synapse, as has been shown for vacuole fusion (23).
LMA1 release is the last biochemically defined event that precedes fusion (18). Each of the late-stage inhibitors (CA, BAPTA, guanosine 5′-[γ-thio]triphosphate, mastoparan, and Microcystin-LR) act upstream of LMA1 release (18). Thus, the molecular dissection of LMA1 release may provide a critical assay for the dissection of late fusion events. The recent reconstitution of the entire vacuole fusion reaction with proteoliposomes from detergent-solubilized vacuoles (12) and new techniques for large-scale vacuole isolation (N. Margolis and W.W., unpublished work) suggest that this may be a practical approach.
Acknowledgments
We thank Drs. Kyle Cunningham and Andreas Mayer for plasmids, strains, and discussions. This work was supported by a grant from the National Institute of General Medical Sciences (to W.W.) and a fellowship from the Deutsche Forschungsgemeinschaft to C.U.
ABBREVIATIONS
- SNARE
soluble N-ethylmaleimide-sensitive factor attachment protein receptor
- LMA1
low Mr activity 1
- CaM
calmodulin
- BAPTA
1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetate
- CA
cyclopiazonic acid
- CCCP
carbonylcyanide m-chlorophenylhydrazone
References
- 1.Rothman J E, Wieland F. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- 2.Rothman J E. Nature (London) 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- 3.Ferro-Novick S, Jahn R. Nature (London) 1994;370:191–193. doi: 10.1038/370191a0. [DOI] [PubMed] [Google Scholar]
- 4.Weis W I, Scheller R H. Nature (London) 1998;395:328–329. doi: 10.1038/26354. [DOI] [PubMed] [Google Scholar]
- 5.Bock J B, Scheller R. Nature (London) 1998;387:133–135. doi: 10.1038/387133a0. [DOI] [PubMed] [Google Scholar]
- 6.Schekman R. Nature (London) 1998;396:514–515. doi: 10.1038/24986. [DOI] [PubMed] [Google Scholar]
- 7.Weisman L S, Bacallao R, Wickner W. J Cell Biol. 1987;105:1539–1547. doi: 10.1083/jcb.105.4.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weisman L S, Wickner W T. Science. 1988;241:289–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- 9.Conradt B, Shaw J, Vida T, Emr S D, Wickner W. J Cell Biol. 1992;119:1469–1479. doi: 10.1083/jcb.119.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haas A, Conradt B, Wickner W. J Cell Biol. 1994;126:87–97. doi: 10.1083/jcb.126.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Z, Wickner W. J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato K, Wickner W. Science. 1998;281:700–702. doi: 10.1126/science.281.5377.700. [DOI] [PubMed] [Google Scholar]
- 13.Ungermann C, Nichols B J, Pelham H R B, Wickner W. J Cell Biol. 1998;140:61–69. doi: 10.1083/jcb.140.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichols B J, Ungermann C, Pelham H R B, Wickner W, Haas A. Nature (London) 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- 15.Ungermann C, Wickner W. EMBO J. 1998;17:3269–3276. doi: 10.1093/emboj/17.12.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungermann C, von Mollard G F, Jensen O N, Margolis N, Stevens T H, Wickner W. J Cell Biol. 1999;145:1435–1442. doi: 10.1083/jcb.145.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer A, Wickner W, Haas A. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z, Sato K, Wickner W. Cell. 1998;93:1125–1134. doi: 10.1016/s0092-8674(00)81457-9. [DOI] [PubMed] [Google Scholar]
- 19.Xu Z, Mayer A, Muller E, Wickner W. J Cell Biol. 1997;136:299–306. doi: 10.1083/jcb.136.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ungermann C, Sato K, Wickner W. Nature (London) 1998;396:543–548. doi: 10.1038/25069. [DOI] [PubMed] [Google Scholar]
- 21.Mayer A, Wickner W. J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Conradt B, Haas A, Wickner W. J Cell Biol. 1994;126:99–110. doi: 10.1083/jcb.126.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Peters C, Mayer A. Nature (London) 1998;398:575–580. doi: 10.1038/25133. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham K W, Fink G R. Mol Cell Biol. 1996;16:2226–2237. doi: 10.1128/mcb.16.5.2226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guthrie C, Fink G R, editors. Methods in Enzymology. San Diego: Academic; 1991. [Google Scholar]
- 26.Cunningham K W, Fink G R. J Cell Biol. 1994;124:351–363. doi: 10.1083/jcb.124.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haas A. Methods Cell Sci. 1995;17:283–294. [Google Scholar]
- 28.Haas A, Wickner W. EMBO J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- 29.Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. EMBO J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goeger D E, Riley R T, Dorner J W, Cole R J. Biochem Pharmacol. 1988;37:978–981. doi: 10.1016/0006-2952(88)90195-5. [DOI] [PubMed] [Google Scholar]
- 31.Seidler N W, Jona I, Vegh M, Martonosi A. J Biol Chem. 1989;264:17816–17823. [PubMed] [Google Scholar]
- 32.Südhof T C. Nature (London) 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 33.Goda Y, Südhof T C. Curr Opin Cell Biol. 1997;9:513–518. doi: 10.1016/s0955-0674(97)80027-0. [DOI] [PubMed] [Google Scholar]
- 34.Rexach M F, Schekman R. J Cell Biol. 1991;114:219–229. doi: 10.1083/jcb.114.2.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klionsky D J, Nelson H, Nelson N. J Biol Chem. 1992;267:3416–3422. [PubMed] [Google Scholar]
- 36.Yaver D S, Nelson H, Nelson N, Klionsky D J. J Biol Chem. 1993;268:10564–10572. [PubMed] [Google Scholar]
- 37.van Weert A W, Dunn K W, Gueze H J, Maxfield F R, Stoorvogel W. J Cell Biol. 1995;130:821–834. doi: 10.1083/jcb.130.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wichmann H, Hengst L, Gallwitz D. Cell. 1992;71:1131–1142. doi: 10.1016/s0092-8674(05)80062-5. [DOI] [PubMed] [Google Scholar]
- 39.Darsow T, Rieder S E, Emr S E. J Cell Biol. 1997;138:517–529. doi: 10.1083/jcb.138.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fischer von Mollard G, Notwehr S, Stevens T. J Cell Biol. 1997;137:1511–1524. doi: 10.1083/jcb.137.7.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sato K, Darsow T, Emr S E. Mol Cell Biol. 1998;18:5308–5319. doi: 10.1128/mcb.18.9.5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martin T F J. Trends Cell Biol. 1997;7:271–276. doi: 10.1016/S0962-8924(97)01060-X. [DOI] [PubMed] [Google Scholar]
- 43.Harlow E, Lane O. Antibodies. Plainview, NY: Cold Spring Harbor Lab. Press; 1988. [Google Scholar]