Abstract
Drug resistance resulting from emergence of imatinib-resistant BCR-ABL point mutations is a significant problem in advanced-stage chronic myelogenous leukemia (CML). The BCR-ABL inhibitor, nilotinib (AMN107), is significantly more potent against BCR-ABL than imatinib, and is active against many imatinib-resistant BCR-ABL mutants. Phase 1/2 clinical trials show that nilotinib can induce remissions in patients who have previously failed imatinib, indicating that sequential therapy with these 2 agents has clinical value. However, simultaneous, rather than sequential, administration of 2 BCR-ABL kinase inhibitors is attractive for many reasons, including the theoretical possibility that this could reduce emergence of drug-resistant clones. Here, we show that exposure of a variety of BCR-ABL+ cell lines to imatinib and nilotinib results in additive or synergistic cytotoxicity, including testing of a large panel of cells expressing BCR-ABL point mutations causing resistance to imatinib in patients. Further, using a highly quantifiable bioluminescent in vivo model, drug combinations were at least additive in antileukemic activity, compared with each drug alone. These results suggest that despite binding to the same site in the same target kinase, the combination of imatinib and nilotinib is highly efficacious in these models, indicating that clinical testing of combinations of BCR-ABL kinase inhibitors is warranted.
Introduction
The BCR-ABL tyrosine kinase oncogene, which results from a reciprocal t(9;22) chromosome translocation in a hematopoietic stem cell,1 causes chronic myelogenous leukemia (CML) and Philadelphia chromosome–positive (Ph+) acute lymphoblastic leukemia (ALL). The 210-kDa BCR-ABL protein is expressed in CML patients, and the p190-kDa BCR-ABL protein occurs in Ph+ ALL patients and stems from a different breakpoint in the BCR gene.2,3
CML, which occurs with a frequency of about 1 in 100 000 people per year, when left untreated progresses in 3 phases: The initial chronic phase is a clonal myeloproliferative disorder that is marked by excessive production of mature granulocytes and immature myeloid cells in tissues including bone marrow, spleen, and peripheral blood. An intermediate accelerated phase, characterized by the appearance of undifferentiated blast cells in blood and bone marrow, is followed by a terminal blast-crisis phase, in which median survival is 18 weeks, and more than 30% of the blood and bone marrow cells are blasts.4,5
Imatinib mesylate (Gleevec, STI571; Novartis Pharma, Basel, Switzerland) is an effective, frontline therapy for early, chronic-phase CML that acts by targeting the tyrosine kinase activity of BCR-ABL.6,7 Newly diagnosed patients treated for a median of 19 months show an estimated 76% cytogenetic response (CCR) and 97% complete hematologic response (CHR).8 However, after initially responding to treatment most ALL patients and many CML patients in the accelerated or blastic phases relapse under treatment within one year.9,10 Resistance to imatinib also occurs in a small subset of early, chronic-phase CML patients, with relapse occurring following months or years of treatment. Relapse is frequently due to point mutations in BCR-ABL that reduce the binding affinity of imatinib to the protein, or occasionally with amplification of the BCR-ABL gene.11–18
The novel, selective Abl inhibitor, nilotinib (AMN107), was designed to interact with the ATP-binding site of BCR-ABL with a higher affinity than imatinib. In addition to being significantly more potent compared with imatinib (IC50 < 30 nM), nilotinib also maintains activity against most of the BCR-ABL point mutants that confer imatinib resistance.19,20 The in vitro cellular efficacy of nilotinib translates into activity in vivo, as demonstrated by activity against a variety of imatinib-resistant BCR-ABL point mutants in animal models of myeloproliferative disease.20 In phase 1/2 clinical trials, nilotinib is producing cytogenetic and hematologic responses in imatinib-refractory CML patients.21
If nilotinib continues to show promise in further clinical trials, it could either be used as a single agent in patients who are refractory to imatinib, or it could be used in conjunction with imatinib to achieve a higher degree of patient responsiveness by suppressing the emergence of drug-resistant BCR-ABL mutations. We have previously observed a synergistic interaction between nilotinib and imatinib in mouse hematopoietic cells expressing p210BCR-ABL or p190BCR-ABL.20 Here, we show positive cooperative effects of combinations of nilotinib with imatinib in a panel of imatinib-sensitive and imatinib-resistant BCR-ABL–expressing cells in vitro, which translate into additive effects in a mouse leukemia model.
Materials and methods
Chemical compounds and biologic reagents
Nilotinib free-base (AMN107; NVP-AMN107-NX; 4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl) phenyl]-3-[4-(3-pyridinyl)-2-pyrimidinyl]amino]-benzamide) and imatinib (Novartis Pharma AG) were dissolved in DMSO to make 10-mM stock solutions. Serial dilutions were made in DMSO to obtain final dilutions for cellular assays.
Antibodies
Anti-pTyr (clone 4G10; Upstate Biotechnology, Lake Placid, NY) was used at a dilution of 1:1000 for immunoblotting. The Abl antibody (C19; Santa Cruz Biotechnology, Santa Cruz, CA) was used at a dilution of 1:500 for immunoblotting. Monoclonal anti–beta-actin (Clone AC-15) was purchased from Sigma (St Louis, MO) and used at a dilution of 1:1000 for immunoblotting.
Cell lines and cell culture
The erythroleukemia cell line K562, derived from a patient in blast-crisis CML, and the human CML cell line KU812 were purchased from American Type Culture Collection (Rockville, MD). Murine hematopoietic 32D cells were transduced with retrovirus to express p210 BCR-ABL (32D.p210 cells)22; this cell line is rapidly lethal in syngeneic, nonimmunosuppressed C3H mice. Imatinib-resistant BCR-ABL constructs (pCI-neo Mammalian Expression Vector [no. E1841]; Promega, Madison, WI) harboring the point mutations F317L, T315I, M351T, and F486S were stably transfected into Ba/F3 cells by electroporation. Similarly, 32D cells were transfected to express E255V BCR-ABL. Cells were selected for neomycin resistance and IL3-independent growth. The E255K-Ba/F3 and Y253H-Ba/F3 cell lines were developed as previously described.23
All cells were cultured in the presence of 5% CO2 at 37°C, at a concentration of 5 × 105 cells/mL, in cellgro RPMI 1640 medium (Mediatech, Herndon, VA), supplemented with 10% fetal calf serum (FCS; Harlan Bioproducts, Indianapolis, IN), 1% glutamine, and penicillin/streptomycin. Media for cells expressing BCR-ABL point mutants were supplemented with 1 mg/mL G418.
Cell viability, cell cycle, and apoptosis analysis
The trypan blue exclusion assay has been previously described, and was used to determine proliferation of cells cultured in the presence and absence of nilotinib, imatinib, or a combination of the 2 agents. Cell viability is reported as percentage of control (untreated) cells. Apoptosis of drug-treated cells was measured using the Annexin-V-Fluos Staining Kit (Boehringer Mannheim, Indianapolis, IN), as previously described.24
Synergy studies
For synergy studies, imatinib and nilotinib were added simultaneously at fixed ratios to imatinib-sensitive and imatinib-resistant BCR-ABL–expressing cells according to the method of Chou and Talalay.25 Cell viability was determined using the trypan blue exclusion assay. ED50 values were determined from the dose-response curves using graphic extrapolation. Specifically, (Y2 − Y1)/(X2 − X1) = (50 − Y1)(X50 − X1), where X50 = X1+ [(50 − Y1) * (X2 − X1)/(Y2 − Y1)] for linear x-axes and X50 = 10 (LOG10(C1)+(X − E1) * (LOG10(C2) − LOG10(C1)/(E2 − E1) for logarithmic x-axes. For calculation of the combination index, the following formula was used: (ICXa in mix/ICXa alone) + (ICXb in mix/ICXb alone). For the ICX value (nM), X is set to 25, 50, 75, or 90.
Immunoprecipitation
Protein lysis preparation and immunoprecipitation were carried out as previously described.24
Bone marrow colony assay and hematology profile assessment
Normal murine bone marrow cells were flushed from the femur of a male NCR nude mouse. Normal human bone marrow cells were obtained from to-be-discarded bone marrow harvest collection bags, under a protocol approved by the Dana Farber Cancer Institute institutional review board. Cells were lysed in ammonium chloride buffer to remove erythrocytes and washed. Plates were prepared containing 60 000 mouse bone marrow cells each in mouse MethoCult (GF M3434, methylcellulose medium with recombinant cytokines, cat no. 03434; StemCell Technologies, Vancouver, BC). Similarly, plates were prepared containing 60 000 CD34+ selected human bone marrow cells each in human MethoCult (GF H4434, “complete” methylcellulose medium containing recombinant cytokines, cat no. 04434; StemCell Technologies). These plates also contained varying concentrations of imatinib alone, nilotinib alone, and a combination of the two compared with vehicle (DMSO) control. The plates containing murine bone marrow cells alone and human bone marrow cells alone, with or without the drug combinations, were incubated at 37°C in 5% CO2 for up to 12 days, at which time myeloid and erythroid colonies were counted on an inverted microscope.
The peripheral blood of 5 male NCR nude mice (2 NMP-PEG300 vehicle–treated mice, and 3 imatinib [75 mg/kg] + nilotinib [20 mg/kg]–treated mice) was obtained through tail bleeds. Blood samples were analyzed by the DF/HCC Research Pathology Core Facility (Boston, MA).
Bioluminescent BCR-ABL model of CML
Cells were transduced with a retrovirus encoding firefly luciferase (MSCV-Luc), and selected with G418 at a concentration of 1 mg/mL to produce the 32D.p210-luciferase (luc+) cell line. 32D.p210-luc+ cells free of Mycoplasma and viral contamination were washed once with Hanks balanced salt solution (HBSS; Mediatech), and resuspended in HBSS prior to administration to mice. Solutions of nilotinib were prepared just prior to administration, by dissolving 100 mg in 1.0 mL NMP to give a clear solution and diluting with 9.0 mL PEG300.
Male NCR-nude mice (5-6 weeks of age; Taconic, Germantown, NY) were sublethally irradiated with a single fraction of 3 Gy, and approximately 3 hours later, a total of 800 000 cells was administered by tail-vein injection. Anesthetized mice were imaged and total body luminescence was measured as previously described.26 Baseline imaging 2 days after tumor cell inoculation was used to establish treatment cohorts with matched tumor burden. Cohorts of mice were treated with oral administration of vehicle (10% NMP, 90% PEG300), osmotic pump administration of 75 mg/kg imatinib, oral administration of 20 mg/kg per day nilotinib (diluted in 10% NMP, 90% PEG 300), or a combination of imatinib (75 mg/kg; osmotic pump) and nilotinib (20 mg/kg; oral gavage). Due to the significantly shorter half-life of imatinib in mice compared with humans, an alternative to continuous drug administration via the osmotic pump would entail twice daily intraperitoneal administration of imatinib, which has proved in our hands to be inefficient in terms of achieving maximum efficacy in mice. Treatment with vehicle and nilotinib was carried out for a total of 8 days; osmotic pumps were loaded with enough imatinib to allow up to 8 full days of treatment. Images were taken on days 2, 4, 5, and 7 after intravenous injection of 32D.p210-luc+ cells. On day 7 after intravenous injection, mice had received a total of 5 days of treatment with vehicle, nilotinib alone, imatinib alone, or the combination of nilotinib and imatinib. At the planned end of this study (9 days following the final imaging day), any remaining mice were killed, body and spleen weights were recorded, and tissues were preserved in 10% formalin for histopathologic analysis.
Additional in vivo imaging studies were performed that included a variety of combinations of doses of nilotinib and imatinib, each administered alone and in combination to male NCR-nude mice (5-6 weeks of age; Taconic). Drug formulations, treatments, and imaging were carried out as described above with some variations in experimental design (described in figure legends for Figures 6–7). Mice were administered the doses of nilotinib and imatinib, alone or in combination, at 20 mg/kg ± 50 mg/kg, 15 mg/kg ± 50 mg/kg, and then 15 mg/kg ± 75 mg/kg. Histopathologic analysis was then carried out.
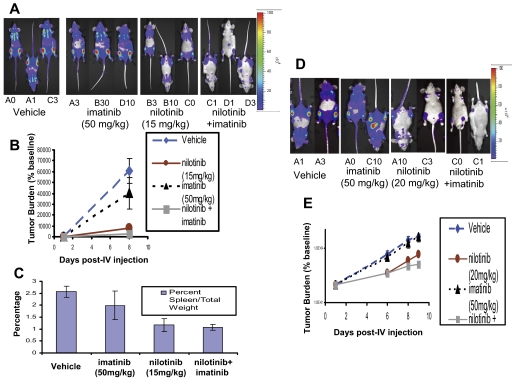
Figure 6.
In vivo effects of the combination of nilotinib (15-20 mg/kg) and imatinib (50 mg/kg) on BCR-ABL–expressing cells in a murine leukemia model. (A) Effects of vehicle, nilotinib alone (15 mg/kg), imatinib alone (50 mg/kg), or a combination of nilotinib and imatinib on growth of 32D.p210-luc+ cells in NCR nude mice. Mice were intravenously injected via tail vein with 800 000 cells/mouse (with no prior sublethal irradiation). Images were taken on day 1 and day 8 after intravenous injection. On day 8 after intravenous injection, mice had received a total of 7 days of treatment with vehicle or drug. Mice were killed, weighed, and preserved for histopathologic analysis approximately 7 days after the last imaging day. Photo images show bioluminescence as seen on the last imaging day. (B) Bioluminescence values plotted as percent of baseline for mice treated with vehicle, nilotinib alone (15 mg/kg), imatinib alone (50 mg/kg), or a combination of nilotinib and imatinib. Vehicle (n = 3), nilotinib alone (n = 3), imatinib alone (n = 3), nilotinib + imatinib (n = 3). (C) Percent spleen weights for 32D.p210-luciferase–injected NCR nude mice treated with nilotinib alone (15 mg/kg), imatinib alone (50 mg/kg), or a combination of nilotinib and imatinib. (D) Effects of nilotinib (20 mg/kg) and imatinib (50 mg/kg), alone and combined, on growth of 32D.p210-luc+ cells in NCR nude mice. Mice for this experiment were injected with 32D.p210-luc+ cells via tail-vein injection (with no prior sublethal irradiation). Images were taken on day 1, day 6, day 8, and day 9 after intravenous injection. By day 9 after intravenous injection, mice had received a total of 8 days of treatment with vehicle or drug. Mice were killed and preserved for histopathologic analysis on day 9 after intravenous injection. Photo images show bioluminescence as seen on the last imaging day. (E) Bioluminescence values are plotted as percent of baseline. Vehicle (n = 2), nilotinib only (n = 2), imatinib only (n = 2), nilotinib + imatinib (n = 2). Where shown, error bars indicate SEM.
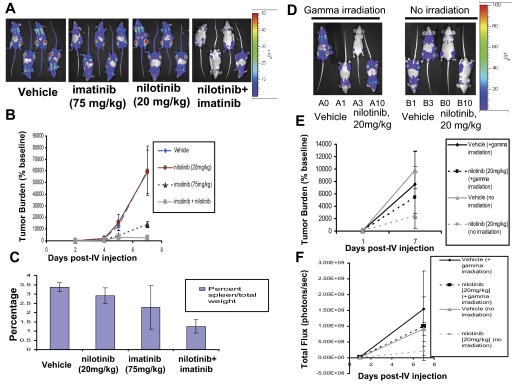
Figure 7.
In vivo effects of the combination of nilotinib (20 mg/kg) and imatinib (75 mg/kg) on BCR-ABL–expressing cells in a murine leukemia model. Influence of preirradiation on mouse responsivity to nilotinib. (A) Effects of vehicle, nilotinib alone (20 mg/kg), imatinib alone (75 mg/kg), or a combination of nilotinib and imatinib on growth of 32D.p210-luc+ cells in NCR nude mice. Mice were sublethally irradiated with a single fraction of 3 Gy followed by tail-vein injection of 800 000 cells/mouse. Images were taken on day 2, day 4, day 5, and day 7 after intravenous injection. On day 7 after intravenous injection, mice had received a total of 5 days of treatment with vehicle or drug. Mice were killed, weighed, and preserved for histopathologic analysis approximately 9 days after the last imaging day. Photo images show bioluminescence as seen on the last imaging day for representative mice. (B) Bioluminescence values plotted as percent of baseline for mice treated with vehicle, nilotinib alone (20 mg/kg), imatinib alone (75 mg/kg), or a combination of nilotinib and imatinib. Vehicle (n = 6), nilotinib alone (n = 4), imatinib alone (n = 6), nilotinib + imatinib (n = 5). (C) Percent spleen weights for 32D.p210-luciferase–injected NCR nude mice treated with nilotinib alone (20 mg/kg), imatinib alone (75 mg/kg), or a combination of nilotinib and imatinib. (D) Investigation of effects of gamma irradiation prior to intravenous injection of 32D.p210-luc+ cells on leukemia growth and nilotinib responsiveness. Mice were divided into 2 groups of 4: One group was sublethally irradiated with a single fraction of 3 Gy prior to being intravenously injected with 600 000 32D.p210-luc+ cells. The other group was not irradiated prior to cell injection. Both groups were then treated for a total of 6 days with either vehicle or nilotinib (20 mg/kg); final images were performed on postinjection day 7. Photo images show bioluminescence as seen on the last imaging day. (E) Bioluminescence values plotted as percent of baseline for mice treated with vehicle or nilotinib alone (20 mg/kg). Vehicle (n = 4), nilotinib alone (n = 4). (F) Raw bioluminescence values. Error bars indicate SEM.
For all in vivo imaging studies, we estimated doses of first nilotinib and then imatinib that would result in subcurative, partial suppression of tumor burden in mice with each agent used alone. The ideal subcurative doses in vivo were determined to be 15 to 20 mg/kg nilotinib and 50 to 75 mg/kg imatinib. The partial inhibition of tumor burden by each agent observed in vivo is analogous to the partial inhibition of BCR-ABL+ cell proliferation by each agent observed in vitro.
Results
Drug combination studies: imatinib and nilotinib against imatinib-sensitive, BCR-ABL–expressing cell lines
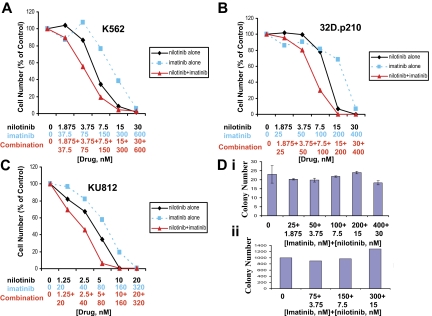
Nilotinib was tested in combination with imatinib across a range of doses and against a panel of imatinib-sensitive BCR-ABL–expressing cells. Combination indices calculated for K562, 32D.p210, and KU812 cell lines treated with nilotinib alone, imatinib alone, and nilotinib combined with imatinib suggest overall additive to slightly synergistic effects between the 2 BCR-ABL inhibitors when combined, with no evidence for antagonism across a range of doses (Figure 1; Table 1).
Figure 1.
Drug combination studies: imatinib and nilotinib against imatinib-sensitive, BCR-ABL–expressing cell lines. Proliferation studies showing 3-day treatments of (A) K562 cells, (B) 32D.p210 cells, and (C) KU812 cells with nilotinib, imatinib, or a combination of nilotinib and imatinib. (D, i) Normal murine bone marrow colony assay. Colony count following 12 days of treatment. (ii) Normal human bone marrow colony assay. Colony count following 19 days of treatment. Error bars indicate SEM.
Table 1.
Combination indices for synergy experiments
| Cell lines | CI at IC25 | CI at IC50 | CI at IC75 | CI at IC90 |
|---|---|---|---|---|
| K562 | 0.89 | 1.02 | 1.03 | 1.22 |
| KU812F | 0.95 | 1.00 | 0.99 | 0.90 |
| 32D.p210 | 0.90 | 0.88 | 1.01 | 1.23 |
| F317L-Ba/F3 | ND* | 0.50 | 0.49 | 0.52 |
| M351T-Ba/F3 | ND* | 0.64 | 0.91 | 0.94 |
| F486S-Ba/F3 | 1.03 | 1.15 | 1.14 | 1.28 |
| E255K-Ba/F3 | 0.74 | 0.78 | 0.88 | 0.90 |
Combination indices calculated for dose-response curves shown in Figures 1 and 2. These data are representative of 2 independent studies.
Missing IC25 values could not be calculated since the percentage of inhibition started at around 30% for imatinib alone and around 60% or 30% for the drug combination.
To determine if the combination of nilotinib plus imatinib is more toxic against normal bone marrow than individual drugs, we performed a mouse bone marrow colony assay, measuring granulocyte-macrophage–colony-forming units (CFU-GMs) and erythroid–burst-forming units (BFU-Es), using the same concentrations of imatinib and nilotinib as were used in combination in proliferation studies, and bone marrow cells obtained from the flushed femur of an untreated male NCR nude mouse. The highest concentration of imatinib (400 nM) combined with nilotinib (30 nM) appeared to lead to a delay in colony formation, since after 7 days the overall number of colonies was lower for this treatment group (Figure S7, available on the Blood website; see the Supplemental Figures link at the top of the online article). However, by the 12th day of observation, colony numbers for the highest dose group were more similar to the other treatments, suggesting minimal toxicity of the combination of imatinib and nilotinib against mouse bone marrow (Figure 1D upper panel). Similarly, a colony assay using CD34+ human bone marrow cells showed no decline in the number of colonies formed on imatinib + nilotinib–treated plates compared with vehicle (DMSO)–treated plates (Figure 1 lower panel).
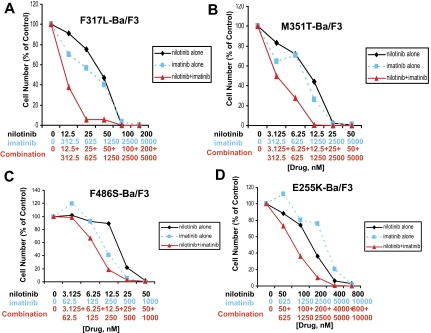
Drug combination studies: imatinib and nilotinib against imatinib-resistant, BCR-ABL–expressing cell lines
A panel of imatinib-resistant BCR-ABL–expressing cell lines was tested with either BCR-ABL inhibitor alone, or both combined. For the imatinib-resistant BCR-ABL point mutants, F317L and M351T, results ranged from synergistic to additive effects across a range of doses (Figure 2; Table 1). For the imatinib-resistant BCR-ABL point mutant, F486S, results ranged from nearly additive effects to slight to moderate antagonism (Figure 2; Table 1). Overall, results observed for both imatinib-sensitive and imatinib-resistant BCR-ABL–expressing cell lines suggest positive enhancement of the activity of nilotinib and imatinib when the 2 inhibitors are combined.
Figure 2.
Drug combination studies: imatinib and nilotinib against imatinib-resistant, BCR-ABL–expressing cell lines. Proliferation studies showing 3-day treatments of (A) F317L-Ba/F3 cells, (B) M351T-Ba/F3 cells, and (C) F486S-Ba/F3 cells with nilotinib, imatinib, or a combination of nilotinib and imatinib. (D) Proliferation study showing a 2-day treatment of E255K-Ba/F3 cells with nilotinib, imatinib, or a combination of nilotinib and imatinib.
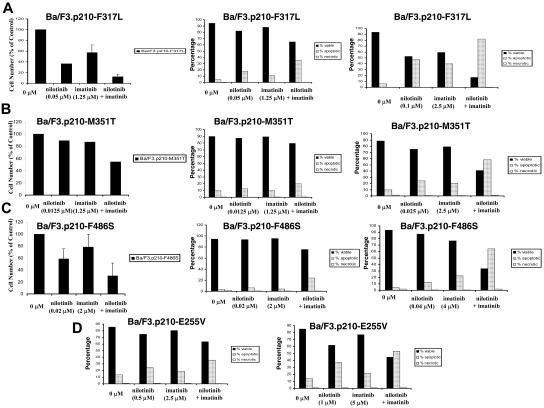
Nilotinib + imatinib combination experiments were performed using Ba/F3 cells expressing the E255K and Y253H BCR-ABL point mutants, both of which were identified as conferring resistance to nilotinib in a mutagenesis assay.23 A 2-day treatment of E255K-Ba/F3 cells with nilotinib only, imatinib only, or a combination of the 2 agents showed an increase in cell killing when both drugs were used together (Figure 2D; Table 1). This experiment suggests positive drug enhancement (effects that range from slight-to-moderate synergy to nearly additive) when nilotinib is combined with imatinib in the treatment of E255K-expressing cells, compared with each drug alone. In contrast, for the Y253H mutant, it did not appear that the addition of nilotinib enhanced the effects of imatinib against this mutant (Figure S6).
The highly imatinib-resistant T315I-BCR-ABL point mutant was investigated for responsiveness to the combination of nilotinib and imatinib (Figure S1). The addition of 10 μM imatinib, which does not significantly inhibit the growth of T315I-BCR-ABL–expressing cells, to a range of concentrations of nilotinib, resulted in only a modest increase in inhibition of cellular proliferation (Figure S1). Inhibitory effects on cell growth seen with nilotinib and imatinib against the T315I mutant were far less pronounced than those seen for more imatinib-sensitive BCR-ABL point mutants, such as F317L (Figure S1).
Induction of apoptosis and inhibition of proliferation of nonmutated and imatinib-resistant BCR-ABL–expressing cells by nilotinib and imatinib
Nilotinib was tested in combination with imatinib for induction of apoptosis at select concentrations. In imatinib-sensitive and imatinib-resistant BCR-ABL–expressing cell lines, the combination of nilotinib and imatinib resulted in a higher degree of induction of apoptosis and inhibition of cellular proliferation compared with each inhibitor alone (Figures 3–4). A modest increase in induction of apoptosis was observed for the nilotinib and imatinib combination in the K562 cell line (Figure S2).
Figure 3.
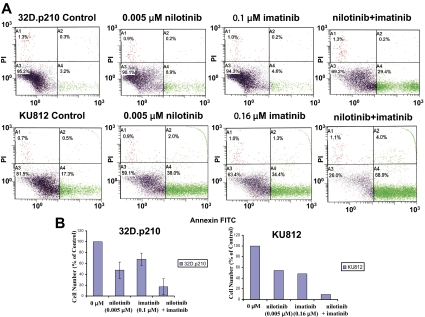
Induction of apoptosis and inhibition of proliferation of nonmutated BCR-ABL–expressing cells by nilotinib and imatinib. (A) Effects of nilotinib and imatinib, alone and combined, on induction of apoptosis of nonmutated BCR-ABL–expressing cells following 2 days of treatment. (B) Corresponding effects of nilotinib and imatinib, alone and combined, on proliferation of nonmutated BCR-ABL–expressing cells following 2 days of treatment (n = 2 for 32D.p210; n = 1 for KU812). Error bars indicate SEM.
Figure 4.
Induction of apoptosis and inhibition of proliferation of imatinib-resistant BCR-ABL–expressing cells by nilotinib and imatinib. (A) Effects of nilotinib and imatinib, alone and combined, on proliferation (left panel; n = 2) and induction of apoptosis (middle and right panels; n = 1) of imatinib-resistant BCR-ABL–expressing cells: F317L. (B) Effects of nilotinib and imatinib, alone and combined, on proliferation (left panel; n = 1) and induction of apoptosis (middle and right panels; n = 1) of imatinib-resistant BCR-ABL–expressing cells: M351T. (C) Effects of nilotinib and imatinib, alone and combined, on proliferation (left panel; n = 2) and induction of apoptosis (middle and right panels; n = 1) of imatinib-resistant BCR-ABL–expressing cells: F486S. (D) Effects of nilotinib and imatinib, alone and combined, on induction of apoptosis of imatinib-resistant BCR-ABL–expressing cells: E255V (n = 1). Where shown, error bars indicate SEM.
Inhibition of cellular tyrosine phosphorylation in BCR-ABL–expressing cells by imatinib and nilotinib, combined
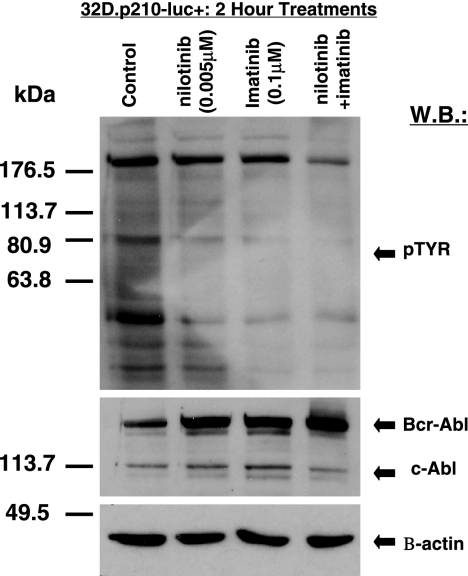
Treatment of 32D.p210-luc+ cells for 2 hours with a combination of both nilotinib (0.005 μM) and imatinib (0.1 μM) resulted in more pronounced inhibition of cellular tyrosine phosphorylation in these cells, compared with either 0.005 μM nilotinib or 0.1 μM imatinib alone (Figure 5). In contrast, cells treated with either compound alone or in combination led to an apparent increase in BCR-ABL expression (Figure 5). This is a previously observed phenomenon that may be related to BCR-ABL protein stabilization due to drug-protein complex formation.
Figure 5.
Inhibition of cellular tyrosine phosphorylation in BCR-ABL–expressing cells by imatinib and nilotinib, combined. Immunoblot showing inhibitory effects of nilotinib and imatinib (alone and combined) on total cellular tyrosine phosphorylation and BCR-ABL expression in nonmutated BCR-ABL–expressing cells. Immunoblot was hybridized with a beta-actin antibody as a loading control.
In vivo effects of the combination of nilotinib and imatinib on BCR-ABL–expressing cells in a murine leukemia model
To directly assess the in vivo antitumor efficacy of nilotinib alone, imatinib alone, and the combination of nilotinib and imatinib, we used a mouse model of CML in which tumor burden was quantified by noninvasive imaging of the luminescent tumor cells (Figures 6–7). Murine 32D.p210 cells were engineered to stably express firefly luciferase, and NCR nude mice were then inoculated with these cells. Noninvasive imaging was used to serially assess tumor burden, and mice with established leukemia were divided into cohorts with similar tumor burden. Nilotinib was then administered via oral gavage, as was vehicle. Osmotic pumps were surgically implanted into mice receiving imatinib.
In the first series of studies, nilotinib was tested at doses of 15 and 20 mg/kg, alone or in combination with 50 mg/kg imatinib. In the first study, mice were administered vehicle, nilotinib (15 mg/kg), imatinib (50 mg/kg), or a combination of both imatinib and nilotinib at their respective doses (Figure 6). The lowest tumor burden as assessed by bioluminescence was observed to be in the drug combination group on day 8 after intravenous injection of 32D.p210-luc+ cells (Figure 6A-B). The Student t test was used for statistical evaluation of this experiment and yielded P ≤ .007 (vehicle versus drug combination on day 8 after intravenous injection); P ≤ .01 (vehicle versus nilotinib on day 8 after intravenous injection); and P ≤ .319 (vehicle versus imatinib on day 8 after intravenous injection). Lowest percent spleen weights were observed in mice treated with either nilotinib alone or both BCR-ABL inhibitors together, compared with vehicle and imatinib alone, following death 7 days after the last imaging day (Figure 6C). The Student t test (type 2 analysis, 2 tails) was performed for statistical evaluation of these results and yielded P ≤ .002 (vehicle versus nilotinib); P ≤ .001 (vehicle versus drug combination); and P ≤ .068 (drug combination versus imatinib). Differences between percent spleen weights for vehicle versus imatinib, and drug combination versus nilotinib, were not statistically significant (P ≤ .214 and P ≤ .631, respectively).
In the second study, 20 mg/kg nilotinib was tested alone and in combination with 50 mg/kg imatinib (Figure 6). In mice treated for a total of 8 days with vehicle, nilotinib (20 mg/kg), imatinib (50 mg/kg), or a combination of nilotinib and imatinib, lowest tumor burden as measured by bioluminescence was observed to be in the drug combination group (Figure 6D-E). Imatinib as a single agent at 50 mg/kg did not display notable efficacy, whereas nilotinib treatment alone at 20 mg/kg resulted in substantial tumor suppression in this study (Figure 6D-E).
Additional in vivo imaging experiments were carried out in which mice were first sublethally irradiated with 3 Gy prior to tail-vein injection of 32D.p210-luc+ cells in an attempt to increase engraftment and aggressiveness of tumor burden. In the first of these studies, following 5 days of daily treatment, tumor burden remained lower in mice treated with the combination of imatinib (75 mg/kg) and nilotinib (20 mg/kg), compared with mice treated with either vehicle, imatinib alone, or nilotinib alone (Figure 7A-B). Similarly, lower percent spleen weights were observed in mice treated with both BCR-ABL inhibitors, compared with vehicle and either agent alone, following death 10 days after the last imaging day (Figure 7C). The Student t test (type 2 analysis, 2 tails) was performed for statistical evaluation of these results and yielded P ≤ .079 (vehicle versus nilotinib); P ≤ .074 (vehicle versus imatinib); P ≤ .001 (vehicle versus drug combination); P ≤ .095 (drug combination versus imatinib); and P ≤ .001 (drug combination versus nilotinib). Histopathologic analysis of mice used in this experiment showed an apparent absence of leukemic cells in the spleens of several mice treated with both nilotinib and imatinib. These results reflect the positive drug combination effects between nilotinib and imatinib observed in BCR-ABL–expressing cell lines tested in vitro. The Student t test was used for statistical evaluation of this experiment and yielded P ≤ .026 (vehicle versus drug combination on day 7 after intravenous injection); P ≤ .028 (vehicle versus imatinib only on day 7 after intravenous injection); P ≤ .057 (vehicle versus drug combination on day 5 after intravenous injection); and P ≤ .042 (vehicle versus imatinib only on day 5 after intravenous injection).
In another study involving sublethal irradiation of mice prior to cell injection, mice were treated for a total of 5 days with vehicle, nilotinib (15 mg/kg), imatinib (75 mg/kg), or nilotinib combined with imatinib at their respective doses (Figure S4). Again, the lowest tumor burden as assessed by bioluminescence was observed to be in the drug combination group (Figure S4).
The apparent lack of effect of nilotinib observed alone at 15 to 20 mg/kg in experiments involving preirradiation may be due to the influence of sublethal irradiation of mice prior to cell injection, as a correlation was observed in all experiments between the potency of nilotinib as a single agent and the inclusion/exclusion of preirradiation. To investigate the effects of gamma irradiation prior to intravenous injection of 32D.p210-luc+ cells on leukemia growth and nilotinib responsiveness, we sublethally irradiated a group of 4 mice with a single fraction of 3 Gy prior to being intravenously injected with 600 000 32D.p210-luc+ cells. Another group of 4 mice was not irradiated prior to cell injection. Both groups were then treated for a total of 6 days with either vehicle or nilotinib (20 mg/kg); final imaging was performed on postinjection day 7. As shown in Figure 7D-E, there was greater nilotinib responsiveness and less variable final tumor burden within the treatment groups for mice that were not sublethally irradiated prior to cell injections, compared with mice that were sublethally irradiated. Raw bioluminescence values shown in Figure 7F suggest higher tumor engraftment and aggressive tumor growth in mice that were sublethally irradiated prior to cell injection, compared with mice that were not irradiated. These results suggest that the inclusion of irradiation may influence the aggressiveness and pattern of leukemia growth in mice, which may consequently influence measurement of antitumor effects of nilotinib as a single agent.
Histopathologic study of vital organs did not suggest any evidence of gross organ toxicity in any of the imatinib + nilotinib combination studies performed in vivo. We performed additional assays, including investigation of 5 athymic nude mice that were injected with 32D.p210-luc+ cells and treated for a total of 1 week with either vehicle (NMP-PEG300) or a combination of imatinib (75 mg/kg) + nilotinib (20 mg/kg). Two mice received vehicle via oral gavage, and 3 mice received imatinib administered via osmotic pump + nilotinib administered via oral gavage. The hematology profiles between the 2 groups of mice were similar (Figure S5).
Discussion
Imatinib remains a highly effective, frontline therapy for CML. However, the discovery of BCR-ABL point mutations that impede imatinib from effectively inhibiting the activity of BCR-ABL led to the development of second-generation BCR-ABL inhibitors, including nilotinib20 and the dual Src/Abl inhibitor, dasatinib (BMS-354825).27 Nilotinib, a novel aminopyrimidine ATP-competitive inhibitor of BCR-ABL, has been shown to be at least 20-fold more potent than imatinib in the killing of nonmutated BCR-ABL–expressing cells, and has been demonstrated to inhibit the activity of more than 30 mutant forms of BCR-ABL occurring in imatinib-resistant patients.20,28 Nilotinib is currently in phase 2 clinical trials for imatinib-resistant CML. In recent phase 1 studies performed in parallel at 3 American and European centers that involved nilotinib treatment of mostly advanced-staged, imatinib-resistant CML patients, significant activity was observed.21
Since it is expected that, similar to imatinib, resistance to the second-generation inhibitors will also present a challenge in the treatment of CML and Ph+ ALL, mutagenesis screens designed to identify drug-resistant BCR-ABL point mutations have been carried out.23,29–31 Such assays have been performed in an attempt to predict resistance mechanisms for nilotinib and dasatinib, as well as to establish the resistance profiles of the available BCR-ABL inhibitors.23,29–31 Although these studies did not consistently identify the same drug-resistant BCR-ABL point mutations for imatinib, nilotinib, and dasatinib, it was clear from the results of these studies that all 3 compounds display different mutagenicity profiles.
A combination between any 2 of the 3 inhibitors might be expected to impart significant clinical benefit, as each agent could be effective in suppressing the emergence of BCR-ABL point mutants conferring resistance to the other BCR-ABL inhibitors. In the case of imatinib-resistant leukemia, because of the nature of resistance mechanisms, such as emergence of drug-resistant clones, it will likely be of significant clinical benefit to simultaneously administer more than one BCR-ABL inhibitor to patients as a way to suppress the development of these drug-resistant mutants.
The distinct binding properties of imatinib and the second-generation BCR-ABL inhibitors add to the potential of combination therapy. Crystallographic structural studies show that one molecule of BCR/ABL could bind only one molecule of imatinib, nilotinib, or dasatinib at a time. Imatinib and nilotinib preferentially bind the kinase in its inactive conformation, while dasatinib can bind to either the active or inactive conformation.
Combinations of different BCR-ABL inhibitors, including dasatinib and imatinib, have been effective in reducing the occurrence of drug-resistant mutants.32,33 Similarly, the combination of imatinib with the dual Scr/Abl inhibitors AP23848 and dasatinib, respectively, has shown promise in preclinical in vitro studies involving treatment of nonmutated BCR-ABL–expressing cells, as well as imatinib-resistant BCR-ABL point mutant–expressing cells.34 Imatinib combined with each Src/Abl inhibitor led to enhanced drug effects against nonmutated BCR-ABL–expressing cells and the imatinib-resistant BCR-ABL point mutant, M351T, which has been found in patients and is located in the SH2 contact region of BCR-ABL35; however only slight enhancement of drug effects was observed against more highly imatinib-resistant BCR-ABL point mutants such as Y253H and E255K.34 The apparent lack of antagonism observed between imatinib and the 2 Src/Abl inhibitors against these BCR-ABL–expressing cell lines was similar to the overall positive cooperation observed between imatinib and nilotinib against many of the imatinib-sensitive and -resistant cell lines that we investigated in the present study. Here, the combination of nilotinib and imatinib yielded additive to synergistic effects against the F317L and M351T mutants across a wide range of concentrations, whereas the F486S mutant was inhibited in a nearly additive fashion by the 2 inhibitors across a more limited range of concentrations. Some enhancement of activity was also observed between nilotinib and imatinib against the highly imatinib-resistant point mutant, E255V.
BCR-ABL point mutations occurring in the nucleotide-binding (P) loop of the Abl kinase domain, such as Y253H and E255K, confer imatinib resistance by disrupting the induced-fit binding of imatinib to Abl.36 We examined the combined effects of nilotinib and imatinib against the E255K and Y253H mutants, which in addition to being resistant to imatinib, were also uncovered in a random mutagenesis screen designed to identify nilotinib-resistant mutants.23 Whereas the combination of both Abl inhibitors was nearly additive to synergistic against the E255K mutant using concentrations of imatinib lower than or equal to 10 μM, the combination of the 2 agents using the same therapeutically relevant range of concentrations of imatinib did not display additive-to-synergistic effects against the Y253H mutant.
The T315I mutation, which is believed to cause resistance by sterically inhibiting imatinib, is located within the Abl kinase domain of BCR-ABL, and is positioned at the periphery of the Abl nucleotide binding site where it forms a direct and critical contact point between BCR-ABL and imatinib.37 As has been observed in other studies investigating combination effects of different BCR-ABL inhibitors,32–34 the combination of nilotinib and imatinib in the present study was ineffective against the highly imatinib-resistant T315I mutant. These results suggest that combination of nilotinib and imatinib may be most effective against BCR-ABL point mutants that respond to physiologically achievable levels of imatinib, and less effective against mutants such as T315I and Y253H that confer high imatinib resistance.
The observed positive cooperativity between imatinib and dasatinib against BCR-ABL–expressing cells could be explained in part because dasatinib adds the capability of targeting both the active and inactive conformations of BCR-ABL, but since this is not true for nilotinib, other mechanisms are likely to apply. For example, recent studies suggest that synergy between nilotinib and imatinib might result from interactions with cell transporters, such as the multidrug efflux transporter ABCG2, which confers resistance to certain anticancer drugs,38 or the organic cation transporter Oct-1, which is important for imatinib influx but not for the uptake of nilotinib.39 Such differential cell transport mechanisms between nilotinib and imatinib could account for synergistic interactions between the 2 agents. It will be important to determine if one drug influences the intracellular concentration, the metabolism, or the affinity of the other drug in order to better understand these results.
While nilotinib could potentially be used as a single agent in selected patients intolerant or resistant to imatinib, alternatively, nilotinib and imatinib could be administered together to achieve higher patient responsiveness and potentially reduce the likelihood of emergence of some BCR-ABL mutations conferring resistance to imatinib. Combinations of BCR-ABL kinase inhibitors could theoretically be administered on either a sequential, rotating schedule, or administered simultaneously, provided the drugs did not interfere with each other.
Acknowledgments
J.D.G. is supported by National Institutes of Health (NIH) grant CA66996, and a Specialized Center of Research Award from the Leukemia and Lymphoma Society. J.D.G. is also supported by NIH grants CA36167 and DK50654.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: E.W. was responsible for generation of research findings reported in paper (design/performance of in vitro and in vivo imaging experiments), integrity and analysis of the data, and writing of the paper; L.C. was responsible for generation of research findings reported in paper (design/performance of in vitro and in vivo imaging experiments), and integrity and analysis of the data; R.D.W. assisted with technical aspects of in vivo imaging experiments (specifically, intravenous injections, gavage, and collection of bioluminescence values); D.M. assisted with technical aspects of in vivo imaging experiments (specifically, gavage); L.B. assisted with technical aspects of in vivo imaging experiments (specifically, collection of bioluminescence values); A.R. assisted with histopathologic analysis of mice used in in vivo imaging experiments; P.W.M. assisted with interpretation of research reported in paper and analysis of the data; J.M. assisted with calculations for combination indices pertaining to combination studies; D.F. assisted with interpretation of research reported in paper and analysis of the data; J.J. assisted with technical aspects of in vivo imaging experiments (specifically, drug preparations); E.H.-M. assisted with technical aspects of in vivo imaging experiments (specifically, gavage); L.C. assisted with technical aspects of in vivo imaging experiments (specifically, intravenous injections); J.L.D. assisted with technical aspects of in vivo imaging experiments (specifically, collection of bioluminescence values); A.L.K. assisted with interpretation of research reported in paper and analysis of the data; and J.D.G. is responsible for conception of research reported in paper, and integrity and analysis of the data.
Conflict-of-interest disclosure: Several of the authors (P.W.M., J.M., and D.F.) are employed by a company (Novartis Pharma AG, Basel, Switzerland) whose product was studied in the present work. One of the authors (J.D.G.) has declared a financial interest in a company (Novartis Pharma AG) whose product was studied in the present work.
E.W. and L.C. contributed equally to this work.
Correspondence: James D. Griffin, Department of Adult Oncology, Dana Farber Cancer Institute, 44 Binney St, Boston, MA 02115; e-mail: james_griffin@dfci.harvard.edu.
References
- 1.Deininger MWN, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. [PubMed] [Google Scholar]
- 2.Melo JV, Myint H, Galton DA, Goldman JM. P190BCR-ABL chronic myeloid leukaemia: the missing link with chronic myelomonocytic leukaemia? Leukemia. 1994;8:208–211. [PubMed] [Google Scholar]
- 3.Ravandi F, Cortes J, Albitar M, et al. Chronic myelogenous leukaemia with p185BCR-ABL expression: characteristics and clinical significance. Br J Haematol. 1999;107:581–586. doi: 10.1046/j.1365-2141.1999.01736.x. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian HM, Talpaz M. Definition of the accelerated phase of chronic myelogenous leukemia. J Clin Oncol. 1988;6:180–182. doi: 10.1200/JCO.1988.6.1.180. [DOI] [PubMed] [Google Scholar]
- 5.O'Dwyer ME, Mauro MJ, Kurilik G, et al. The impact of clonal evolution on response to imatinib mesylate (STI571) in accelerated phase CML. Blood. 2002;100:1628–1633. doi: 10.1182/blood-2002-03-0777. [DOI] [PubMed] [Google Scholar]
- 6.Druker BJ, Tamura S, Buchdunger E, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of BCR-ABL positive cells. Nat Med. 1996;2:561–566. doi: 10.1038/nm0596-561. [DOI] [PubMed] [Google Scholar]
- 7.Buchdunger E, Matter A, Druker BJ. BCR-ABL inhibition as a modality of CML therapeutics. Biochim Biophys Acta. 2001;1551:M11–M18. doi: 10.1016/s0304-419x(01)00022-1. [DOI] [PubMed] [Google Scholar]
- 8.O'Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 9.Sawyers CL, Hochhaus A, Feldman E, et al. Imatinib induces hematologic and cytogenetic responses in patients with chronic myelogenous leukemia in myeloid blast crisis: results of a phase II study. Blood. 2002;99:3530–3539. doi: 10.1182/blood.v99.10.3530. [DOI] [PubMed] [Google Scholar]
- 10.Ottmann OG, Druker BJ, Sawyers CL, et al. A phase 2 study of imatinib in patients with relapsed or refractory Philadelphia chromosome-positive acute lymphoid leukemias. Blood. 2002;100:1965–1971. doi: 10.1182/blood-2001-12-0181. [DOI] [PubMed] [Google Scholar]
- 11.Gorre ME, Mohammed M, Ellwood K, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293:876–880. doi: 10.1126/science.1062538. [DOI] [PubMed] [Google Scholar]
- 12.Cowan-Jacob SW, Guez V, Griffin JD, et al. Bcr-Abl kinase mutations and drug resistance to imatinib (STI571) in chronic myelogenous leukemia. Mini Rev Med Chem. 2004;4:285–299. doi: 10.2174/1389557043487321. [DOI] [PubMed] [Google Scholar]
- 13.le Coutre P, Tassi E, Varella-Garcia M, et al. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood. 2000;95:1758–1766. [PubMed] [Google Scholar]
- 14.Weisberg E, Griffin JD. Mechanism of resistance to the ABL tyrosine kinase inhibitor STI571 in BCR-ABL-transformed hematopoietic cell lines. Blood. 2000;95:3498–3505. [PubMed] [Google Scholar]
- 15.Mahon FX, Deininger MV, Schultheis B, Chabrol Reiffers J, Goldman JM, Melo JV. Selection and characterization of BCR-ABL positive cell lines with differential sensitivity to the tyrosine kinase inhibitor STI571: diverse mechanisms of resistance. Blood. 2000;96:1070–1079. [PubMed] [Google Scholar]
- 16.Campbell LJ, Patsouris C, Rayeroux KC, Somana K, Januszewicz EH, Szer J. BCR-ABL amplification in chronic myelocytic leukemia blast crisis following imatinib mesylate administration. Cancer Genet Cytogenet. 2002;139:30–33. doi: 10.1016/s0165-4608(02)00615-5. [DOI] [PubMed] [Google Scholar]
- 17.Hochhaus A, Kreil S, Corbin AS, et al. Molecular and chromosomal mechanisms of resistance to imatinib (STI571) therapy. Leukemia. 2002;16:2190–2196. doi: 10.1038/sj.leu.2402741. [DOI] [PubMed] [Google Scholar]
- 18.Morel F, Bris MJ, Herry A, et al. Double minutes containing amplified bcr-abl fusion gene in a case of chronic myeloid leukemia treated by imatinib. Eur J Haematol. 2003;70:235–239. doi: 10.1034/j.1600-0609.2003.00046.x. [DOI] [PubMed] [Google Scholar]
- 19.Manley PW, Breitenstein W, Bruggen J, et al. Urea-derivatives of STI571 as inhibitors of Bcr-Abl and PDGFR kinases. Bioorg Med Chem Lett. 2004;14:5793–5797. doi: 10.1016/j.bmcl.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 20.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant BCR-ABL. Cancer Cell. 2005;7:129–141. doi: 10.1016/j.ccr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 21.Kantarjian H, Giles F, Wunderle L, et al. Nilotinib in imatinib-resistant CML and Philadelphia chromosome-positive ALL. N Eng J Med. 2006;354:2542–2551. doi: 10.1056/NEJMoa055104. [DOI] [PubMed] [Google Scholar]
- 22.Matulonis U, Salgia R, Okuda K, Druker B, Griffin JD. IL-3 and p210 BCR-ABL activate both unique and overlapping pathways of signal transduction in a factor-dependent myeloid cell line. Exp Hematol. 1993;21:1460–1466. [PubMed] [Google Scholar]
- 23.Ray A, Cowan-Jacob S, Manley PW, Mestan J, Griffin JD. Identification of BCR-ABL point mutations conferring resistance to the Abl kinase inhibitor AMN107 by a random mutagenesis study [abstract]. Blood. 2005;106:148a. doi: 10.1182/blood-2006-01-015347. [DOI] [PubMed] [Google Scholar]
- 24.Weisberg E, Boulton C, Kelly LM, et al. Inhibition of mutant FLT3 receptors in leukemia cells by the small molecule tyrosine kinase inhibitors PKC412. Cancer Cell. 2002;1:433–443. doi: 10.1016/s1535-6108(02)00069-7. [DOI] [PubMed] [Google Scholar]
- 25.Chou T-C, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enz Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 26.Armstrong SA, Kung AL, Mabon ME, et al. Validation of a therapeutic target identified by gene expression based classification. Cancer Cell. 2003;3:173–183. doi: 10.1016/s1535-6108(03)00003-5. [DOI] [PubMed] [Google Scholar]
- 27.Shah NP, Tran C, Lee FY, Chen P, Norris D, Sawyers CL. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 28.O'Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of BCR-ABL inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 29.Deininger MWN, Bradeen H, Jia T, et al. Comparison of imatinib, AMN107, and dasatinib in an accelerated cell-based mutagenesis screen [abstract]. Blood. 2005;106:204a. [Google Scholar]
- 30.Shah NP, Nicoll JM, Branford S, et al. Molecular analysis of dasatinib resistance mechanisms in CML patients identifies novel BCR-ABL mutations predicted to retain sensitivity to imatinib: rationale for combination tyrosine kinase inhibitor therapy [abstract]. Blood. 2005;106:318a. [Google Scholar]
- 31.Von Bubnoff N, Manley PW, Mestan J, Sanger J, Peschel C, Duyster J. BCR-ABL resistance screening predicts a limited spectrum of point mutations to be associated with clinical resistance to the Abl kinase inhibitor nilotinib (AMN107). Blood. 2006;108:1328–1333. doi: 10.1182/blood-2005-12-010132. [DOI] [PubMed] [Google Scholar]
- 32.Bradeen HA, Eide CA, O'Hare T, et al. Comparison of imatnib mesylate, dasatinib (BMS-354825), and nilotinib (AMN107) in an N-ethyl-N-nitrosourea (ENU)-based mutagenesis screen: high efficacy of drug combinations. doi: 10.1182/blood-2006-02-004580. Prepublished on June 13, 2006, as DOI 10.1182/blood-2006-02-004580. (Now available as Blood. 2006;108:2332-2338.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burgess MR, Skaggs BJ, Shah NP, Lee FY, Sawyers CL. Comparative analysis of two clinically active BCR-ABL kinase inhibitors reveals the role of conformation-specific binding in resistance. Proc Natl Acad Sci U S A. 2005;102:3395–3400. doi: 10.1073/pnas.0409770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Hare T, Walters DK, Stoffregen EP, et al. Combined Abl inhibitor therapy for minimizing drug resistance in chronic myeloid leukemia: Src/Abl inhibitors are compatible with imatinib. Clin Cancer Res. 2005;11:6987–6993. doi: 10.1158/1078-0432.CCR-05-0622. [DOI] [PubMed] [Google Scholar]
- 35.Azam M, Latek RR, Daley GQ. Mechanism of autoinhibition and STI-571/imatinib resistance revealed by mutagenesis of BCR-ABL. Cell. 2003;112:831–843. doi: 10.1016/s0092-8674(03)00190-9. [DOI] [PubMed] [Google Scholar]
- 36.Rourmiantsev S, Shah NP, Gorre ME, et al. Clinical resistance to the kinase inhibitor STI-571 in chronic myeloid leukemia by mutation of Tyr-253 in the Abl kinase domain P-loop. Proc Natl Acad Sci U S A. 2002;99:10700–10705. doi: 10.1073/pnas.162140299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schindler T, Bornmann W, Pellicena P, Miller WT, Clarkson B, Kurlyan J. Structural mechanism for STI-571 inhibition of Abelson Tyrosine Kinase. Science. 2000;289:1938–1942. doi: 10.1126/science.289.5486.1938. [DOI] [PubMed] [Google Scholar]
- 38.Jorgensen H, Allan E, Jordanides N, Hamilton A, Mountford T. AMN107 appears equipotent and may synergise with imatinib at the CML stem cell level through interaction with ABCG2 [abstract]. Blood. 2005;106:1080a. [Google Scholar]
- 39.White DL, Saunders VA, Dang P, et al. Oct-1 mediated influx is a key determinant of the intracellular uptake of imatinib but not nilotinib (AMN107): reduced OCT-1 activity is the cause of low in vitro sensitivity to imatinib. Blood. 2006;108:697–704. doi: 10.1182/blood-2005-11-4687. [DOI] [PubMed] [Google Scholar]