Abstract
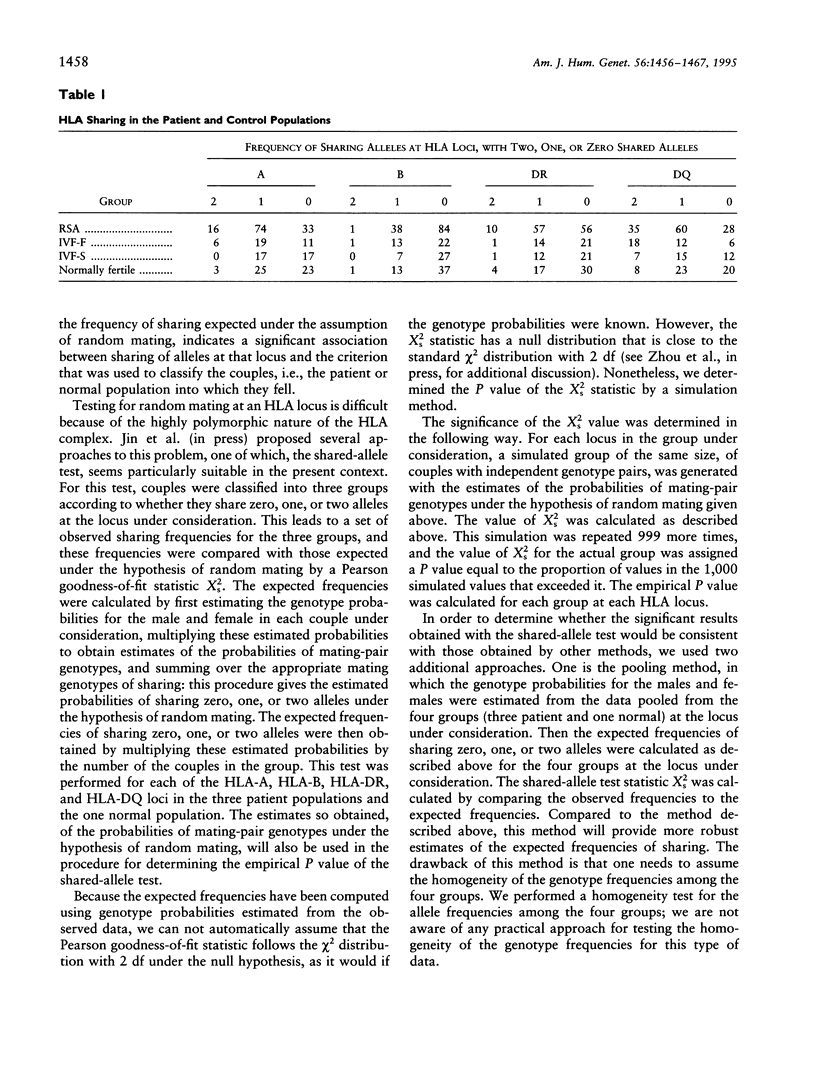
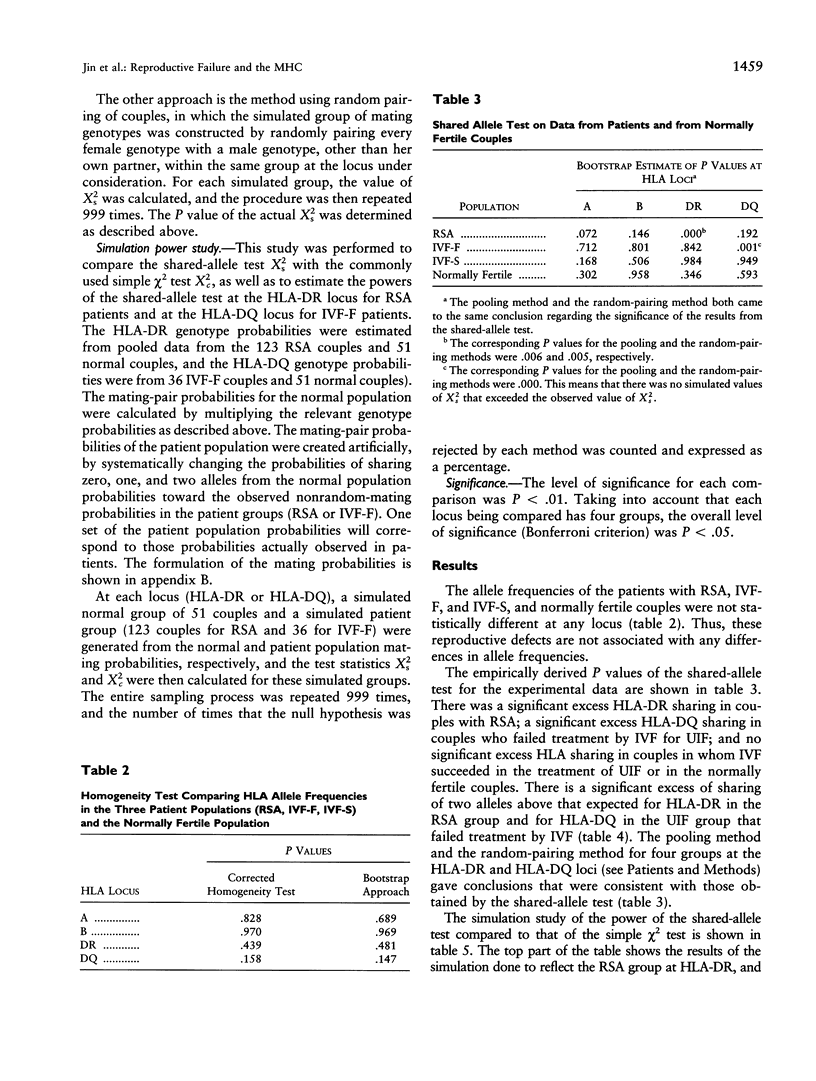
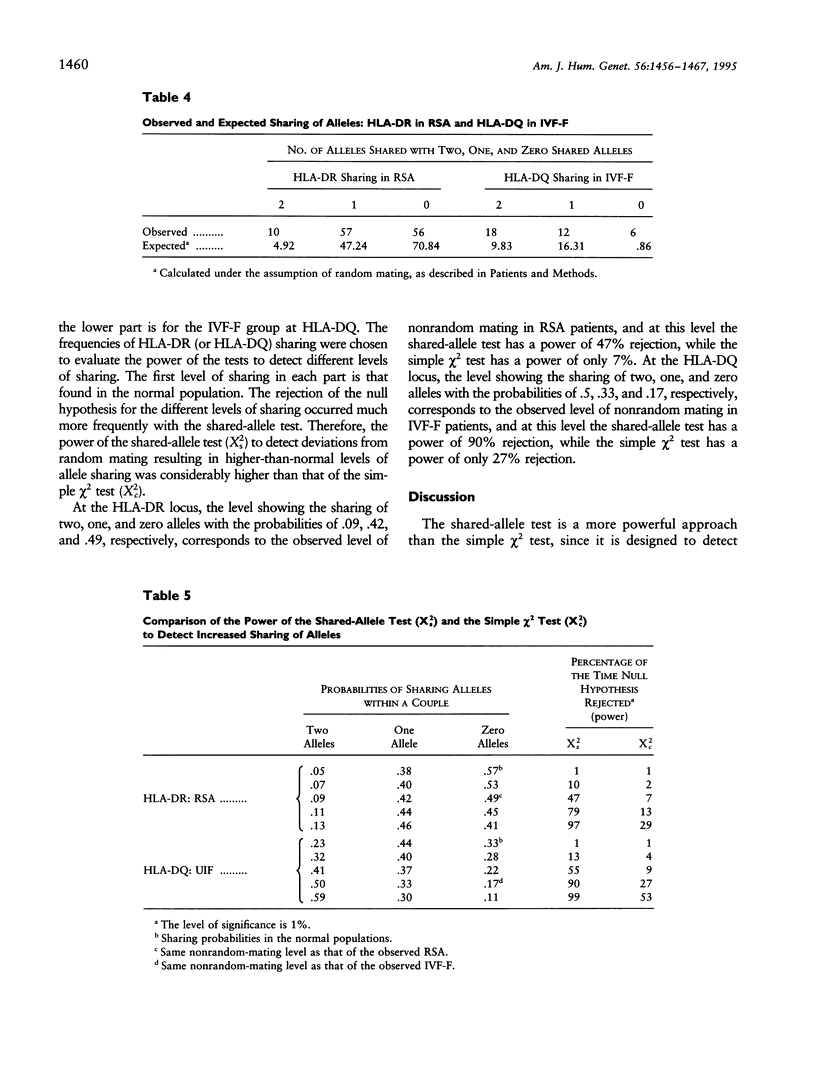
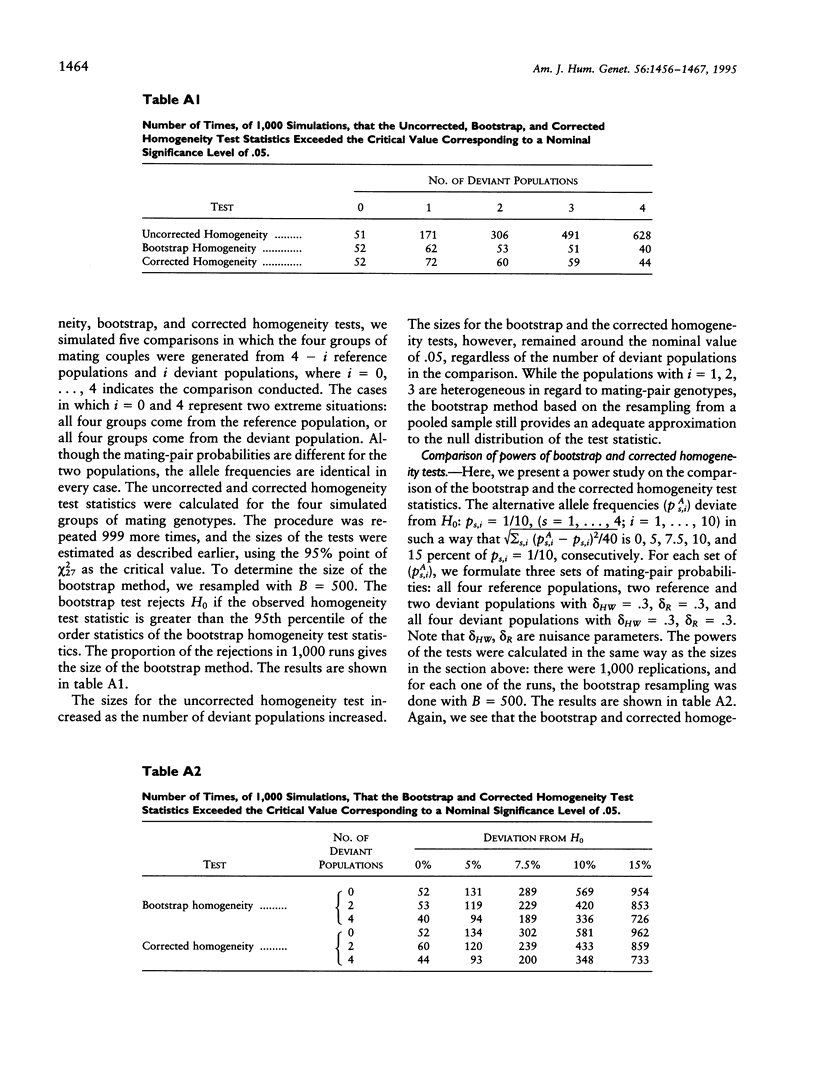
The association between HLA sharing and recurrent spontaneous abortion (RSA) was tested in 123 couples and the association between HLA sharing, and the outcome of treatment for unexplained infertility by in vitro fertilization (IVF) was tested in 76 couples, by using a new shared-allele test in order to identify more precisely the region of the major histocompatibility complex (MHC) influencing these reproductive defects. The shared-allele test circumvents the problem of rare alleles at HLA loci and at the same time provides a substantial gain in power over the simple chi 2 test. Two statistical methods, a corrected homogeneity test and a bootstrap approach, were developed to compare the allele frequencies at each of the HLA-A, HLA-B, HLA-DR, and HLA-DQ loci; they were not statistically different among the three patient groups and the control group. There was a significant excess of HLA-DR sharing in couples with RSA and a significant excess of HLA-DQ sharing in couples with unexplained infertility who failed treatment by IVF. These findings indicate that genes located in different parts of the class II region of the MHC affect different aspects of reproduction and strongly suggest that the sharing of HLA antigens per se is not the mechanism involved in the reproductive defects. The segment of the MHC that has genes affecting reproduction also has genes associated with different autoimmune diseases, and this juxtaposition may explain the association between reproductive defects and autoimmune diseases.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M., Sandberg-Wollheim M., Sjögren K., Erlich H. A., Petterson U., Gyllensten U. Association of susceptibility to multiple sclerosis in Sweden with HLA class II DRB1 and DQB1 alleles. Hum Immunol. 1994 Jan;39(1):41–48. doi: 10.1016/0198-8859(94)90099-x. [DOI] [PubMed] [Google Scholar]
- Beer A. E., Quebbeman J. F., Ayers J. W., Haines R. F. Major histocompatibility complex antigens, maternal and paternal immune responses, and chronic habitual abortions in humans. Am J Obstet Gynecol. 1981 Dec 15;141(8):987–999. doi: 10.1016/s0002-9378(16)32690-4. [DOI] [PubMed] [Google Scholar]
- Benjamin R., Parham P. Guilt by association: HLA-B27 and ankylosing spondylitis. Immunol Today. 1990 Apr;11(4):137–142. doi: 10.1016/0167-5699(90)90051-a. [DOI] [PubMed] [Google Scholar]
- Campbell R. D., Trowsdale J. Map of the human MHC. Immunol Today. 1993 Jul;14(7):349–352. doi: 10.1016/0167-5699(93)90234-C. [DOI] [PubMed] [Google Scholar]
- Clark D. A. Controversies in reproductive immunology. Crit Rev Immunol. 1991;11(3-4):215–247. [PubMed] [Google Scholar]
- Clerget-Darpoux F., Babron M. C., Deschamps I., Hors J. Complementation and maternal effect in insulin-dependent diabetes. Am J Hum Genet. 1991 Jul;49(1):42–48. [PMC free article] [PubMed] [Google Scholar]
- Degli-Esposti M. A., Abraham L. J., McCann V., Spies T., Christiansen F. T., Dawkins R. L. Ancestral haplotypes reveal the role of the central MHC in the immunogenetics of IDDM. Immunogenetics. 1992;36(6):345–356. doi: 10.1007/BF00218041. [DOI] [PubMed] [Google Scholar]
- Degli-Esposti M. A., Andreas A., Christiansen F. T., Schalke B., Albert E., Dawkins R. L. An approach to the localization of the susceptibility genes for generalized myasthenia gravis by mapping recombinant ancestral haplotypes. Immunogenetics. 1992;35(6):355–364. doi: 10.1007/BF00179791. [DOI] [PubMed] [Google Scholar]
- Dizier M. H., Babron M. C., Clerget-Darpoux F. Interactive effect of two candidate genes in a disease: extension of the marker-association-segregation chi(2) method. Am J Hum Genet. 1994 Nov;55(5):1042–1049. [PMC free article] [PubMed] [Google Scholar]
- Dizier M. H., Eliaou J. F., Babron M. C., Combe B., Sany J., Clot J., Clerget-Darpoux F. Investigation of the HLA component involved in rheumatoid arthritis (RA) by using the marker association-segregation chi-square (MASC) method: rejection of the unifying-shared-epitope hypothesis. Am J Hum Genet. 1993 Sep;53(3):715–721. [PMC free article] [PubMed] [Google Scholar]
- Dorman J. S., LaPorte R. E., Stone R. A., Trucco M. Worldwide differences in the incidence of type I diabetes are associated with amino acid variation at position 57 of the HLA-DQ beta chain. Proc Natl Acad Sci U S A. 1990 Oct;87(19):7370–7374. doi: 10.1073/pnas.87.19.7370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duquesnoy R. J., Marrari M. Progress report on the ASHI/CAP Proficiency Survey Program in Histocompatibility Testing. II. HLA-DR, DQ serologic typing, antibody identification, and B-cell crossmatching. American Society for Histocompatibility of Immunogenetics. College of American Pathologists. Hum Immunol. 1994 Feb;39(2):96–105. doi: 10.1016/0198-8859(94)90107-4. [DOI] [PubMed] [Google Scholar]
- Francis D. A., Thompson A. J., Brookes P., Davey N., Lechler R. I., McDonald W. I., Batchelor J. R. Multiple sclerosis and HLA: is the susceptibility gene really HLA-DR or -DQ? Hum Immunol. 1991 Oct;32(2):119–124. doi: 10.1016/0198-8859(91)90108-l. [DOI] [PubMed] [Google Scholar]
- Gill T. J., 3rd Immunogenetics of spontaneous abortions in humans. Transplantation. 1983 Jan;35(1):1–6. [PubMed] [Google Scholar]
- Gill T. J., 3rd, Kunz H. W. Gene complex controlling growth and fertility linked to the major histocompatibility complex in the rat. Am J Pathol. 1979 Jul;96(1):185–206. [PMC free article] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Hassett A. L., Stranick K. S., Locker J., Kunz H. W., Gill T. J., 3rd Molecular analysis of the rat MHC. I. Delineation of the major regions in the MHC and in the grc. J Immunol. 1986 Jul 1;137(1):373–378. [PubMed] [Google Scholar]
- Ho H. N., Gill T. J., 3rd, Hsieh C. Y., Yang Y. S., Lee T. Y. The prevalence of recurrent spontaneous abortions, cancer, and congenital anomalies in the families of couples with recurrent spontaneous abortions or gestational trophoblastic tumors. Am J Obstet Gynecol. 1991 Aug;165(2):461–466. doi: 10.1016/0002-9378(91)90117-a. [DOI] [PubMed] [Google Scholar]
- Ho H. N., Gill T. J., 3rd, Nsieh R. P., Hsieh H. J., Lee T. Y. Sharing of human leukocyte antigens in primary and secondary recurrent spontaneous abortions. Am J Obstet Gynecol. 1990 Jul;163(1 Pt 1):178–188. doi: 10.1016/s0002-9378(11)90696-6. [DOI] [PubMed] [Google Scholar]
- Ho H. N., Yang Y. S., Hsieh R. P., Lin H. R., Chen S. U., Chen H. F., Huang S. C., Lee T. Y., Gill T. J., 3rd Sharing of human leukocyte antigens in couples with unexplained infertility affects the success of in vitro fertilization and tubal embryo transfer. Am J Obstet Gynecol. 1994 Jan;170(1 Pt 1):63–71. doi: 10.1016/s0002-9378(94)70385-x. [DOI] [PubMed] [Google Scholar]
- Jackson D. G., Capra J. D. TAP1 alleles in insulin-dependent diabetes mellitus: a newly defined centromeric boundary of disease susceptibility. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11079–11083. doi: 10.1073/pnas.90.23.11079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanbour A., Ho H. N., Misra D. N., MacPherson T. A., Kunz H. W., Gill T. J., 3rd Differential expression of MHC class I antigens on the placenta of the rat. A mechanism for the survival of the fetal allograft. J Exp Med. 1987 Dec 1;166(6):1861–1882. doi: 10.1084/jem.166.6.1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klitz W., Aldrich C. L., Fildes N., Horning S. J., Begovich A. B. Localization of predisposition to Hodgkin disease in the HLA class II region. Am J Hum Genet. 1994 Mar;54(3):497–505. [PMC free article] [PubMed] [Google Scholar]
- Kockum I., Wassmuth R., Holmberg E., Michelsen B., Lernmark A. HLA-DQ primarily confers protection and HLA-DR susceptibility in type I (insulin-dependent) diabetes studied in population-based affected families and controls. Am J Hum Genet. 1993 Jul;53(1):150–167. [PMC free article] [PubMed] [Google Scholar]
- Kostyu D. D., Dawson D. V., Elias S., Ober C. Deficit of HLA homozygotes in a Caucasian isolate. Hum Immunol. 1993 Jul;37(3):135–142. doi: 10.1016/0198-8859(93)90178-4. [DOI] [PubMed] [Google Scholar]
- Lepage V., Lamm L. U., Charron D. Molecular aspects of HLA class II and some autoimmune diseases. Eur J Immunogenet. 1993 Jun;20(3):153–164. doi: 10.1111/j.1744-313x.1993.tb00106.x. [DOI] [PubMed] [Google Scholar]
- Lu D., Kunz H. W., Melhem M. F., Gill T. J., 3rd Cell lines from grc congenic strains of rats having different susceptibilities to chemical carcinogens. Cancer Res. 1993 Sep 1;53(17):4089–4095. [PubMed] [Google Scholar]
- Marshall B., Leelayuwat C., Abraham L. J., Pinelli M., Dawkins R. L. Large transcripts and sequence from a polymorphic 170 kb MHC region implicated in susceptibility to autoimmune disease. Immunogenetics. 1994;39(1):15–20. doi: 10.1007/BF00171792. [DOI] [PubMed] [Google Scholar]
- Melhem M. F., Kunz H. W., Gill T. J., 3rd A major histocompatibility complex-linked locus in the rat critically influences resistance to diethylnitrosamine carcinogenesis. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1967–1971. doi: 10.1073/pnas.90.5.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mowbray J. F., Gibbings C., Liddell H., Reginald P. W., Underwood J. L., Beard R. W. Controlled trial of treatment of recurrent spontaneous abortion by immunisation with paternal cells. Lancet. 1985 Apr 27;1(8435):941–943. doi: 10.1016/s0140-6736(85)91723-4. [DOI] [PubMed] [Google Scholar]
- Nelson J. L., Hansen J. A. Autoimmune diseases and HLA. Crit Rev Immunol. 1990;10(4):307–328. [PubMed] [Google Scholar]
- Nelson J. L., Koepsell T. D., Dugowson C. E., Voigt L. F., Daling J. R., Hansen J. A. Fecundity before disease onset in women with rheumatoid arthritis. Arthritis Rheum. 1993 Jan;36(1):7–14. doi: 10.1002/art.1780360103. [DOI] [PubMed] [Google Scholar]
- Nepom G. T., Erlich H. MHC class-II molecules and autoimmunity. Annu Rev Immunol. 1991;9:493–525. doi: 10.1146/annurev.iy.09.040191.002425. [DOI] [PubMed] [Google Scholar]
- Partanen J., Koskimies S., Sipilä I., Lipsanen V. Major-histocompatibility-complex gene markers and restriction-fragment analysis of steroid 21-hydroxylase (CYP21) and complement C4 genes in classical congenital adrenal hyperplasia patients in a single population. Am J Hum Genet. 1989 May;44(5):660–670. [PMC free article] [PubMed] [Google Scholar]
- Schacter B., Muir A., Gyves M., Tasin M. HLA-A,B compatibility in parents of offspring with neural-tube defects or couples experiencing involuntary fetal wastage. Lancet. 1979 Apr 14;1(8120):796–799. doi: 10.1016/s0140-6736(79)91317-5. [DOI] [PubMed] [Google Scholar]
- Shelton A. J., Harger J. H., Dorman J. S., Kuller L. H., LaPorte R. E., Gill T. J., 3rd Association between familial autoimmune diseases and recurrent spontaneous abortions. Am J Reprod Immunol. 1994 Sep;32(2):82–87. doi: 10.1111/j.1600-0897.1994.tb01097.x. [DOI] [PubMed] [Google Scholar]
- Sinha A. A., Lopez M. T., McDevitt H. O. Autoimmune diseases: the failure of self tolerance. Science. 1990 Jun 15;248(4961):1380–1388. doi: 10.1126/science.1972595. [DOI] [PubMed] [Google Scholar]
- Vardimon D., Locker J., Kunz H. W., Gill T. J., 3rd Physical mapping of the MHC and grc by pulse field electrophoresis. Immunogenetics. 1992;35(3):166–175. doi: 10.1007/BF00185110. [DOI] [PubMed] [Google Scholar]
- Weitkamp L. R., Schacter B. Z. Transferrin and HLA: spontaneous abortion, neural tube defects, and natural selection. N Engl J Med. 1985 Oct 10;313(15):925–932. doi: 10.1056/NEJM198510103131505. [DOI] [PubMed] [Google Scholar]
- White P. C., New M. I., Dupont B. HLA-linked congenital adrenal hyperplasia results from a defective gene encoding a cytochrome P-450 specific for steroid 21-hydroxylation. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7505–7509. doi: 10.1073/pnas.81.23.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winchester R., Dwyer E., Rose S. The genetic basis of rheumatoid arthritis. The shared epitope hypothesis. Rheum Dis Clin North Am. 1992 Nov;18(4):761–783. [PubMed] [Google Scholar]



