Abstract
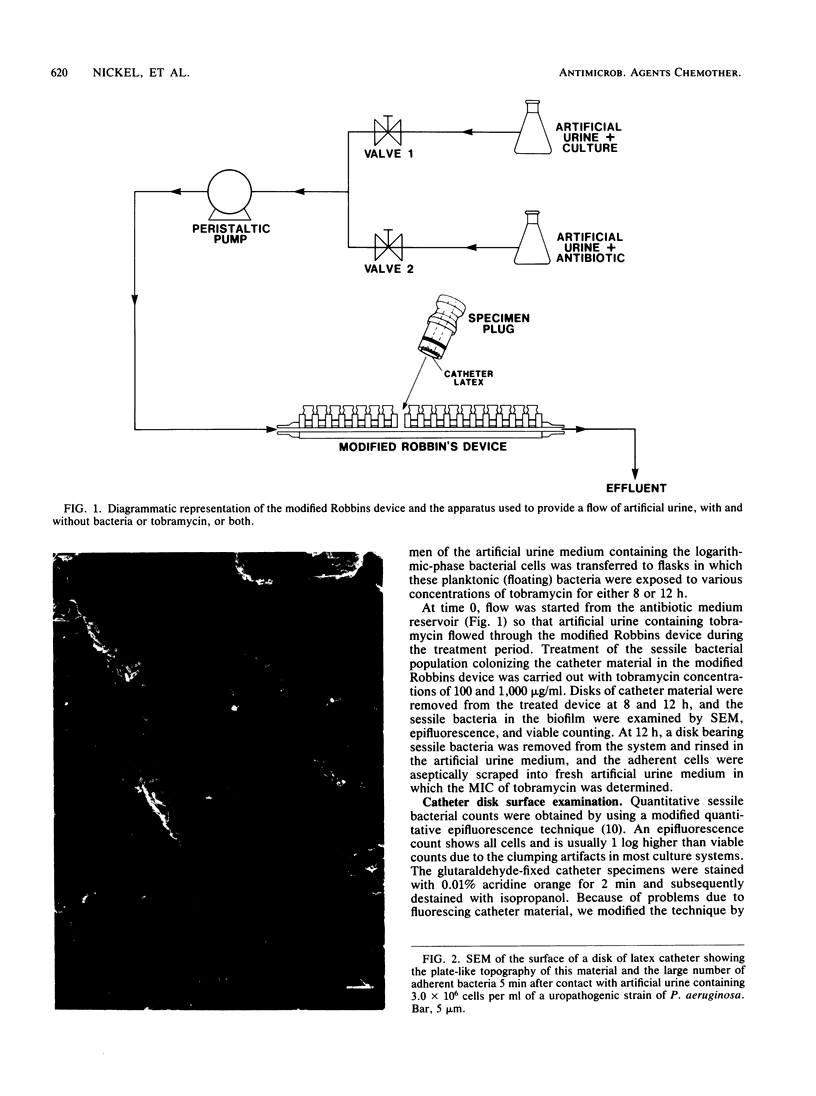
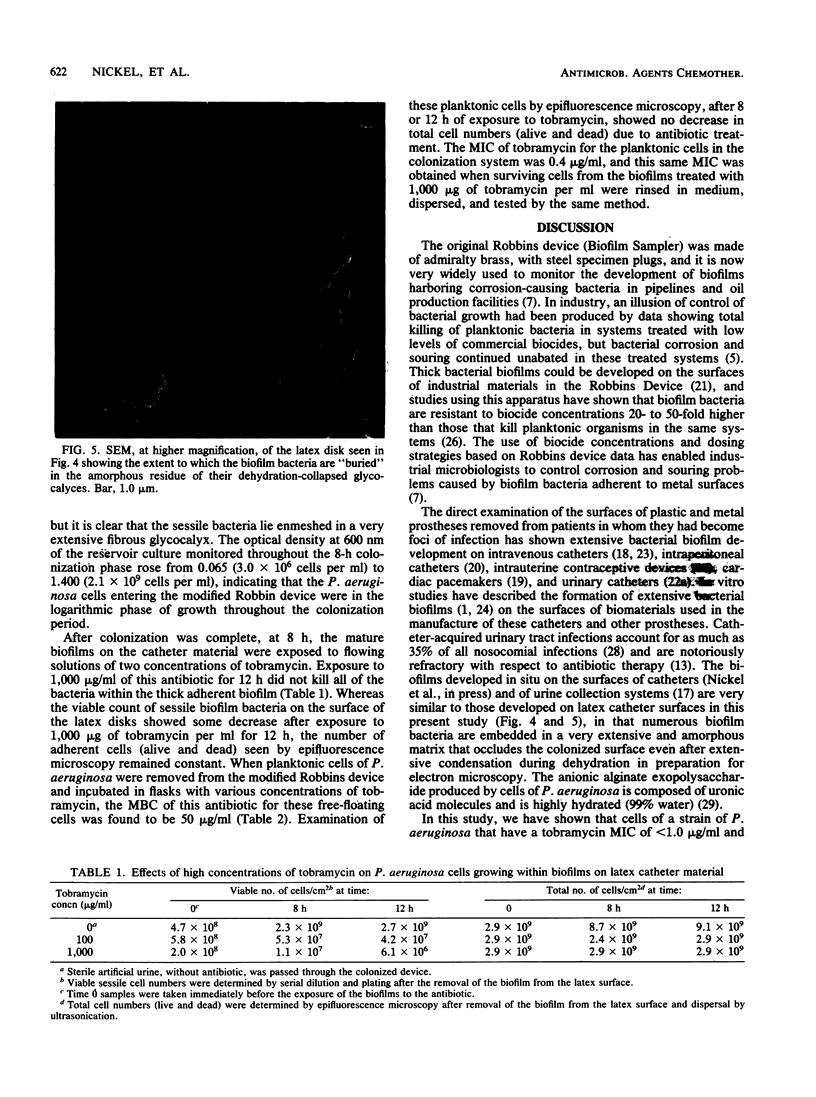
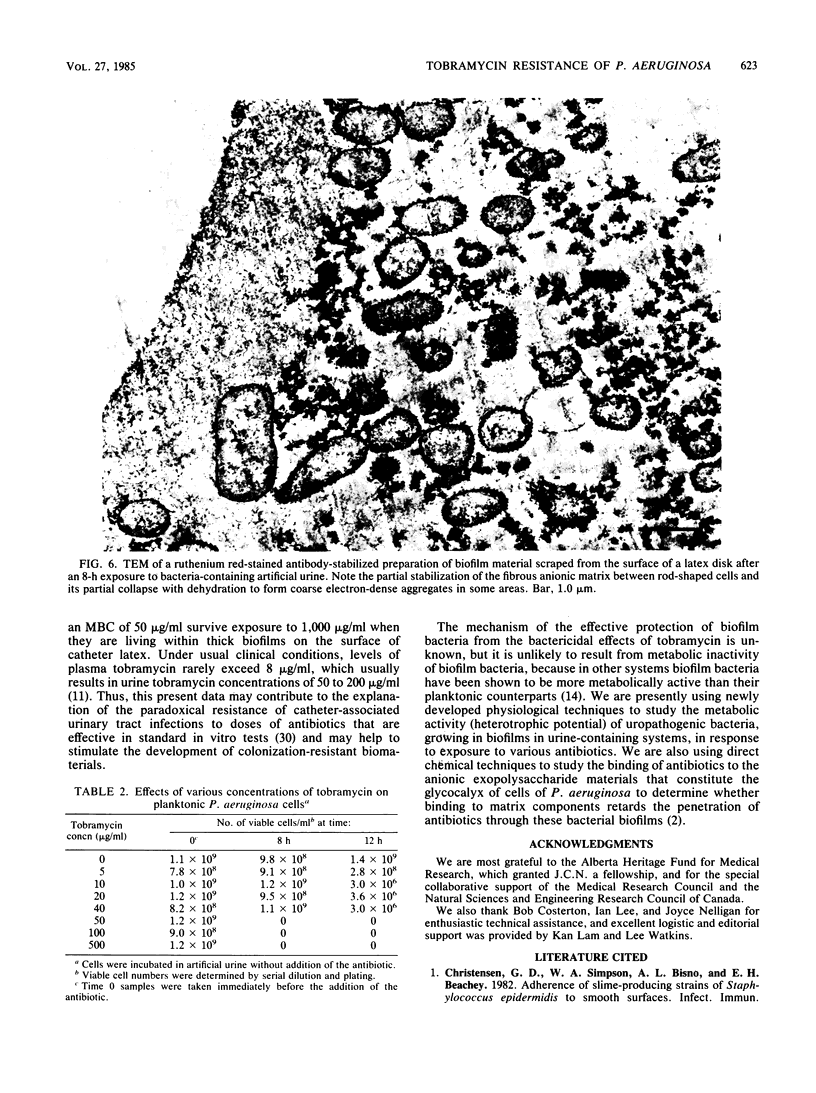
When disks of urinary catheter material were exposed to the flow of artificial urine containing cells of Pseudomonas aeruginosa, a thick adherent biofilm, composed of these bacteria and of their exopolysaccharide products, developed on the latex surface within 8 h. After this colonization, sterile artificial urine containing 1,000 micrograms of tobramycin per ml was flowed past this established biofilm, and a significant proportion of the bacterial cells within the biofilm were found to be still viable after 12 h of exposure to this very high concentration of aminoglycoside antibiotic. Planktonic (floating) cells taken from the test system just before the exposure of the biofilm to the antibiotic were completely killed by 50 micrograms of tobramycin per ml. The MIC of tobramycin for cells taken from the seeding cultures before colonization of the catheter material, and for surviving cells recovered directly from the tobramycin-treated biofilm, was found to be 0.4 micrograms/ml when dispersed cells were assayed by standard methods. These data indicate that growth within thick adherent biofilms confers a measure of tobramycin resistance on cells of P. aeruginosa.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Costerton J. W., Irvin R. T., Cheng K. J. The bacterial glycocalyx in nature and disease. Annu Rev Microbiol. 1981;35:299–324. doi: 10.1146/annurev.mi.35.100181.001503. [DOI] [PubMed] [Google Scholar]
- Costerton J. W. The role of electron microscopy in the elucidation of bacterial structure and function. Annu Rev Microbiol. 1979;33:459–479. doi: 10.1146/annurev.mi.33.100179.002331. [DOI] [PubMed] [Google Scholar]
- Jones H. C., Roth I. L., Sanders W. M., 3rd Electron microscopic study of a slime layer. J Bacteriol. 1969 Jul;99(1):316–325. doi: 10.1128/jb.99.1.316-325.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. F., Young P. S., Marosszeky J. E. Treatment of infection in the presence of an indwelling urethral catheter. Br J Urol. 1982 Jun;54(3):316–319. doi: 10.1111/j.1464-410x.1982.tb06987.x. [DOI] [PubMed] [Google Scholar]
- Mackie E. B., Brown K. N., Lam J., Costerton J. W. Morphological stabilization of capsules of group B streptococci, types Ia, Ib, II, and III, with specific antibody. J Bacteriol. 1979 May;138(2):609–617. doi: 10.1128/jb.138.2.609-617.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. A scanning and transmission electron microscopic study of the surfaces of intrauterine contraceptive devices. Am J Obstet Gynecol. 1983 Jun 15;146(4):384–394. doi: 10.1016/0002-9378(83)90818-9. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. A scanning electron microscopic study of urine droppers and urine collecting systems. Arch Intern Med. 1983 Jun;143(6):1135–1141. [PubMed] [Google Scholar]
- Marrie T. J., Costerton J. W. Scanning and transmission electron microscopy of in situ bacterial colonization of intravenous and intraarterial catheters. J Clin Microbiol. 1984 May;19(5):687–693. doi: 10.1128/jcm.19.5.687-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrie T. J., Nelligan J., Costerton J. W. A scanning and transmission electron microscopic study of an infected endocardial pacemaker lead. Circulation. 1982 Dec;66(6):1339–1341. doi: 10.1161/01.cir.66.6.1339. [DOI] [PubMed] [Google Scholar]
- Marrie T. J., Noble M. A., Costerton J. W. Examination of the morphology of bacteria adhering to peritoneal dialysis catheters by scanning and transmission electron microscopy. J Clin Microbiol. 1983 Dec;18(6):1388–1398. doi: 10.1128/jcm.18.6.1388-1398.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuth J. N., Musher D. M., Thorsteinsson S. B. Inhibition of the antibacterial activity of gentamicin by urine. J Infect Dis. 1976 Jan;133(1):14–21. doi: 10.1093/infdis/133.1.14. [DOI] [PubMed] [Google Scholar]
- Nickel J. C., Gristina A. G., Costerton J. W. Electron microscopic study of an infected Foley catheter. Can J Surg. 1985 Jan;28(1):50-1, 54. [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982 Oct;146(4):479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- Peters G., Locci R., Pulverer G. Microbial colonization of prosthetic devices. II. Scanning electron microscopy of naturally infected intravenous catheters. Zentralbl Bakteriol Mikrobiol Hyg B. 1981;173(5):293–299. [PubMed] [Google Scholar]
- RANTZ L. A., RANDALL E. Use of autoclaved extracts of hemolytic streptococci for serological grouping. Stanford Med Bull. 1955 May;13(2):290–291. [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Warren J. W., Muncie H. L., Jr, Bergquist E. J., Hoopes J. M. Sequelae and management of urinary infection in the patient requiring chronic catheterization. J Urol. 1981 Jan;125(1):1–8. doi: 10.1016/s0022-5347(17)54874-0. [DOI] [PubMed] [Google Scholar]