Abstract
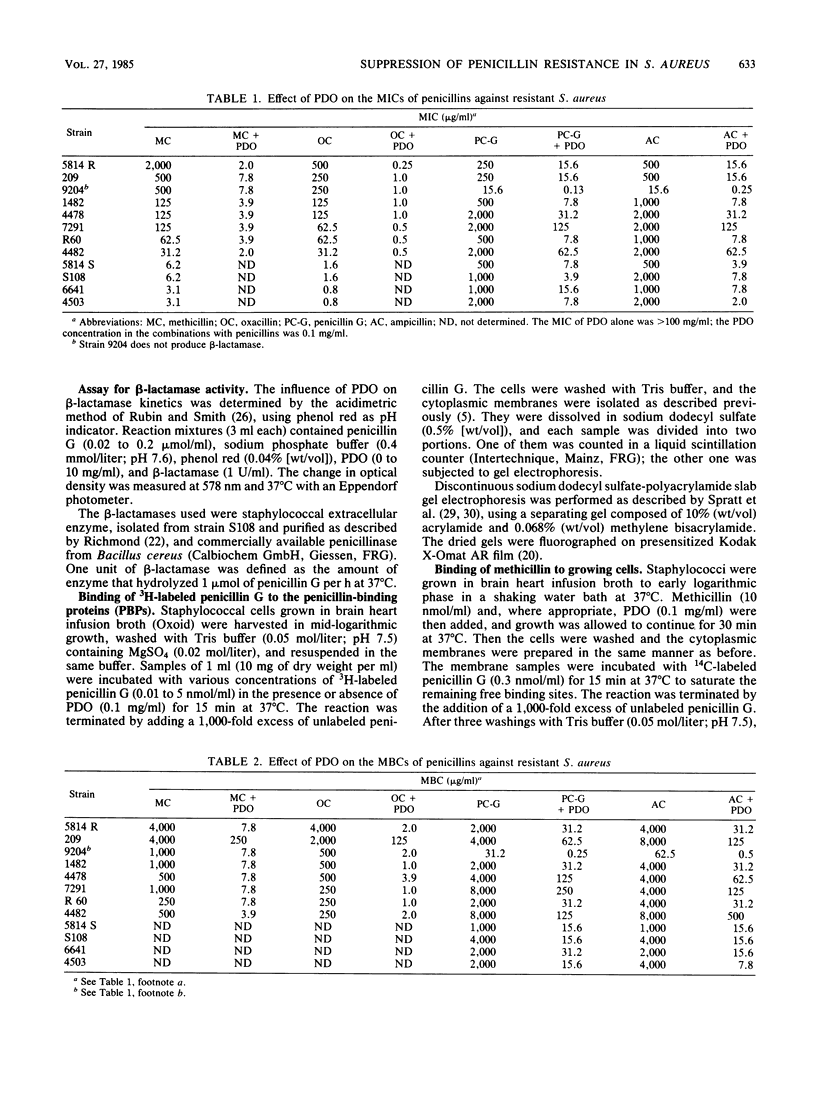
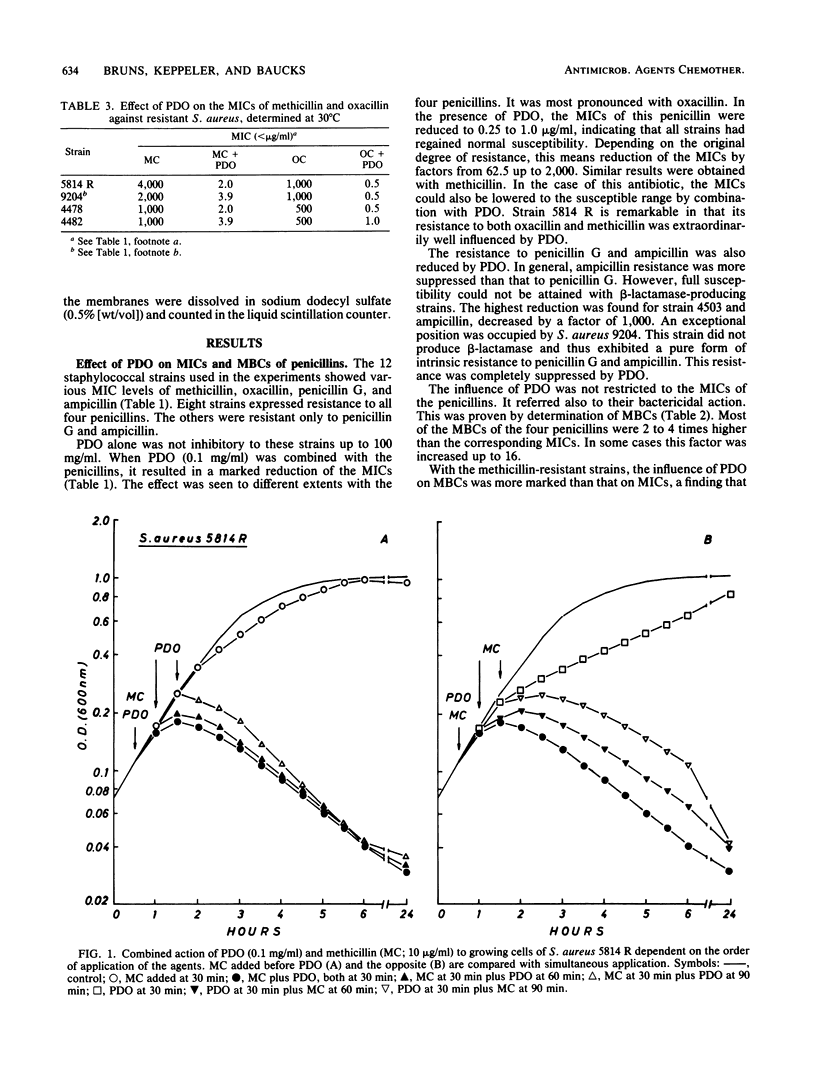
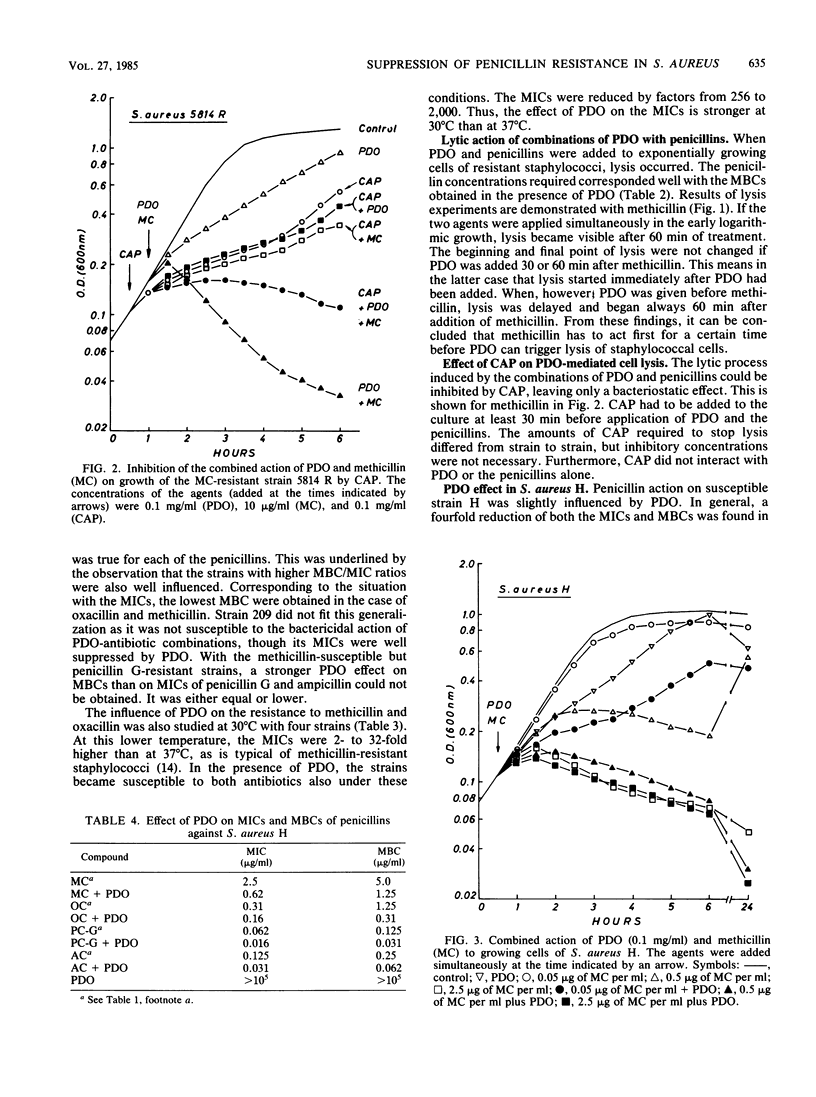
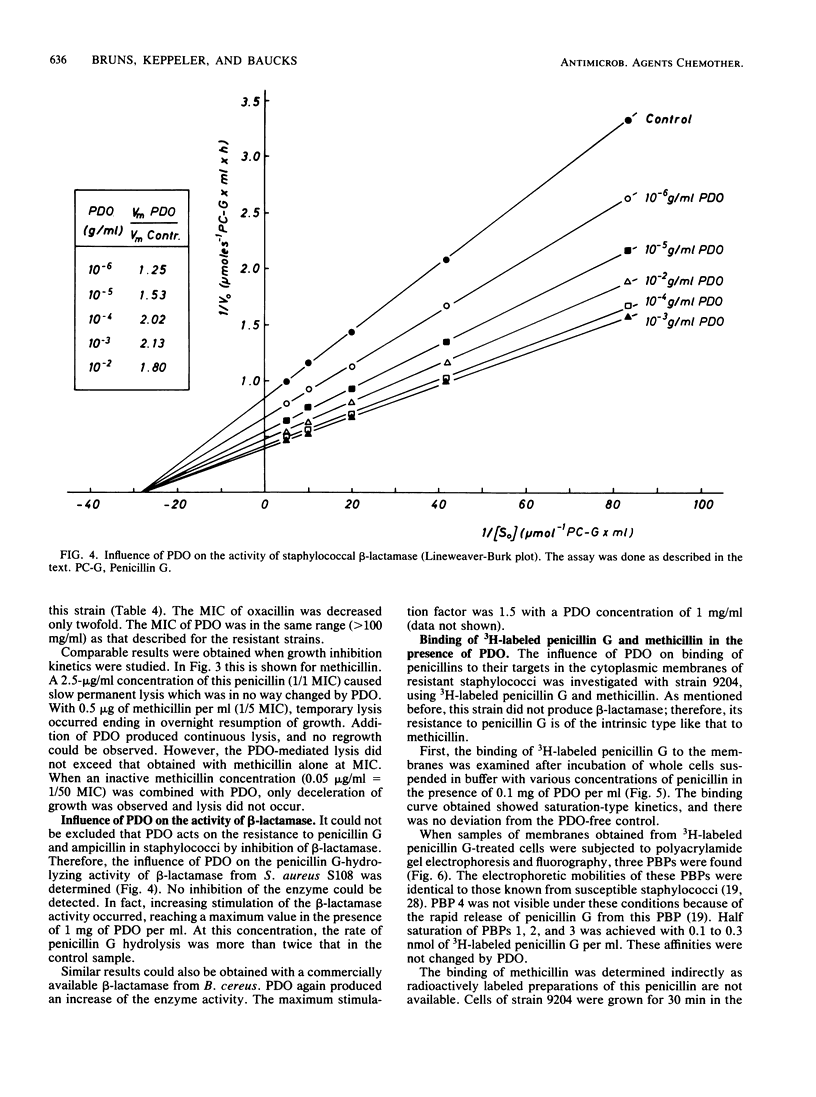
With polidocanol, it was possible to reduce the MIC as well as the MBC of methicillin, oxacillin, penicillin G, and ampicillin against resistant staphylococci. The strongest effects were obtained with methicillin and oxacillin. All strains tested could be resensitized to these penicillins independent of the original resistance levels. Polidocanol was not inhibitory by itself for Staphylococcus aureus. Furthermore, it did not inhibit the activity of staphylococcal beta-lactamase. This permits the conclusion that an intrinsic resistance mechanism is affected by this substance. Its action cannot be simply explained by an improved accessibility of the penicillin targets as uptake, and binding of methicillin and penicillin G in resistant cells was not changed by polidocanol. On the other hand, the lysis induced by combinations of this substance with small amounts of a penicillin was antagonized by chloramphenicol. This suggests that autolytic enzymes are involved in the polidocanol effect and possibly in the intrinsic resistance mechanism itself. Before polidocanol can trigger lysis, the penicillin must act first in some way. As could be seen with a susceptible strain, the resulting lysis did not exceed that obtained with penicillins alone. Thus, polidocanol does not exhibit an independent lytic mechanism but obviously is able to substitute penicillins in their lytic action.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Best G. K., Best N. H., Koval A. V. Evidence for participation of autolysins in bactericidal action of oxacillin on Staphylococcus aureus. Antimicrob Agents Chemother. 1974 Dec;6(6):825–830. doi: 10.1128/aac.6.6.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brissette J. L., Shockman G. D., Pieringer R. A. Effects of penicillin on synthesis and excretion of lipid and lipoteichoic acid from Streptococcus mutans BHT. J Bacteriol. 1982 Aug;151(2):838–844. doi: 10.1128/jb.151.2.838-844.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. F., Reynolds P. E. Intrinsic resistance to beta-lactam antibiotics in Staphylococcus aureus. FEBS Lett. 1980 Dec 29;122(2):275–278. doi: 10.1016/0014-5793(80)80455-8. [DOI] [PubMed] [Google Scholar]
- Bruns W., Keppeler H. Mechanism of intrinsic penicillin-resistance in Staphylococcus aureus. Binding of penicillin G to the cytoplasmic membrane of resistant staphylococci. Arzneimittelforschung. 1980;30(9):1469–1475. [PubMed] [Google Scholar]
- Cleveland R. F., Daneo-Moore L., Wicken A. J., Shockman G. D. Effect of lipoteichoic acid and lipids on lysis of intact cells of Streptococcus faecalis. J Bacteriol. 1976 Sep;127(3):1582–1584. doi: 10.1128/jb.127.3.1582-1584.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland R. F., Wicken A. J., Daneo-Moore L., Shockman G. D. Inhibition of wall autolysis in Streptococcus faecalis by lipoteichoic acid and lipids. J Bacteriol. 1976 Apr;126(1):192–197. doi: 10.1128/jb.126.1.192-197.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornett J. B., Shockman G. D. Cellular lysis of Streptococcus faecalis induced with triton X-100. J Bacteriol. 1978 Jul;135(1):153–160. doi: 10.1128/jb.135.1.153-160.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnick J. K., O'Leary W. M. Correlation of bacteria lipid composition with antibiotic resistance. J Bacteriol. 1970 Mar;101(3):892–900. doi: 10.1128/jb.101.3.892-900.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyke K. G., Jevons M. P., Parker M. T. Penicillinase production and intrinsic resistance to penicillins in Staphylococcus aures. Lancet. 1966 Apr 16;1(7442):835–838. doi: 10.1016/s0140-6736(66)90182-6. [DOI] [PubMed] [Google Scholar]
- Hartman B., Tomasz A. Altered penicillin-binding proteins in methicillin-resistant strains of Staphylococcus aureus. Antimicrob Agents Chemother. 1981 May;19(5):726–735. doi: 10.1128/aac.19.5.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt J. H., Coe A. W., Parker M. T. The detection of methicillin resistance in Staphylococcus aureus. J Med Microbiol. 1969 Nov 4;2(4):443–456. doi: 10.1099/00222615-2-4-443. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugo W. B., Stretton R. J. The role of cellular lipid in the resistance of gram-positive bacteria to penicillins. J Gen Microbiol. 1966 Jan;42(1):133–138. doi: 10.1099/00221287-42-1-133. [DOI] [PubMed] [Google Scholar]
- Höltje J. V., Tomasz A. Lipoteichoic acid: a specific inhibitor of autolysin activity in Pneumococcus. Proc Natl Acad Sci U S A. 1975 May;72(5):1690–1694. doi: 10.1073/pnas.72.5.1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozarich J. W., Strominger J. L. A membrane enzyme from Staphylococcus aureus which catalyzes transpeptidase, carboxypeptidase, and penicillinase activities. J Biol Chem. 1978 Feb 25;253(4):1272–1278. [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Pearson R. D., Steigbigel R. T., Davis H. T., Chapman S. W. Method of reliable determination of minimal lethal antibiotic concentrations. Antimicrob Agents Chemother. 1980 Nov;18(5):699–708. doi: 10.1128/aac.18.5.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H. Beta-lactamase (Staphylococcus aureus). Methods Enzymol. 1975;43:664–672. doi: 10.1016/0076-6879(75)43131-7. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rozgonyi F. Genotypic stability of methicillin resistance in Staphylococcus aureus at supraoptimal temperature. Antimicrob Agents Chemother. 1976 Aug;10(2):377–379. doi: 10.1128/aac.10.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozgonyi F., Váczi L., Sebessy-Gönczy P., Rédai I. Phospholipid content of staphylococci sensitive and resistant to methicillin. Contrib Microbiol Immunol. 1973;1:172–179. [PubMed] [Google Scholar]
- Rubin F. A., Smith D. H. Characterization of R factor beta-lactamases by the acidimetric method. Antimicrob Agents Chemother. 1973 Jan;3(1):68–73. doi: 10.1128/aac.3.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabath L. D., Wheeler N., Laverdiere M., Blazevic D., Wilkinson B. J. A new type of penicillin resistance of Staphylococcus aureus. Lancet. 1977 Feb 26;1(8009):443–447. doi: 10.1016/s0140-6736(77)91941-9. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Biochemical and genetical approaches to the mechanism of action of penicillin. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):273–283. doi: 10.1098/rstb.1980.0045. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Jobanputra V. Mutants of Escherichia coli which lack a component of penicillin-binding protein 1 are viable. FEBS Lett. 1977 Jul 15;79(2):374–378. doi: 10.1016/0014-5793(77)80824-7. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Pardee A. B. Penicillin-binding proteins and cell shape in E. coli. Nature. 1975 Apr 10;254(5500):516–517. doi: 10.1038/254516a0. [DOI] [PubMed] [Google Scholar]
- Suginaka H., Shimatani M., Ogawa M., Kotani S. Prevention of penicillin-induced lysis of Staphylococcus aureus by cellular lipoteichoic acid. J Antibiot (Tokyo) 1979 Jan;32(1):73–77. doi: 10.7164/antibiotics.32.73. [DOI] [PubMed] [Google Scholar]
- Suling W. J., O'Leary W. M. Effect of surfactants on antibiotic resistance. Antimicrob Agents Chemother. 1975 Sep;8(3):334–343. doi: 10.1128/aac.8.3.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyke A. W., Ward J. B., Hayes M. V. Synthesis of peptidoglycan in vivo in methicillin-resistant Staphylococcus aureus. Eur J Biochem. 1982 Oct;127(3):553–558. doi: 10.1111/j.1432-1033.1982.tb06907.x. [DOI] [PubMed] [Google Scholar]



