Abstract
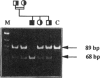
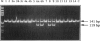
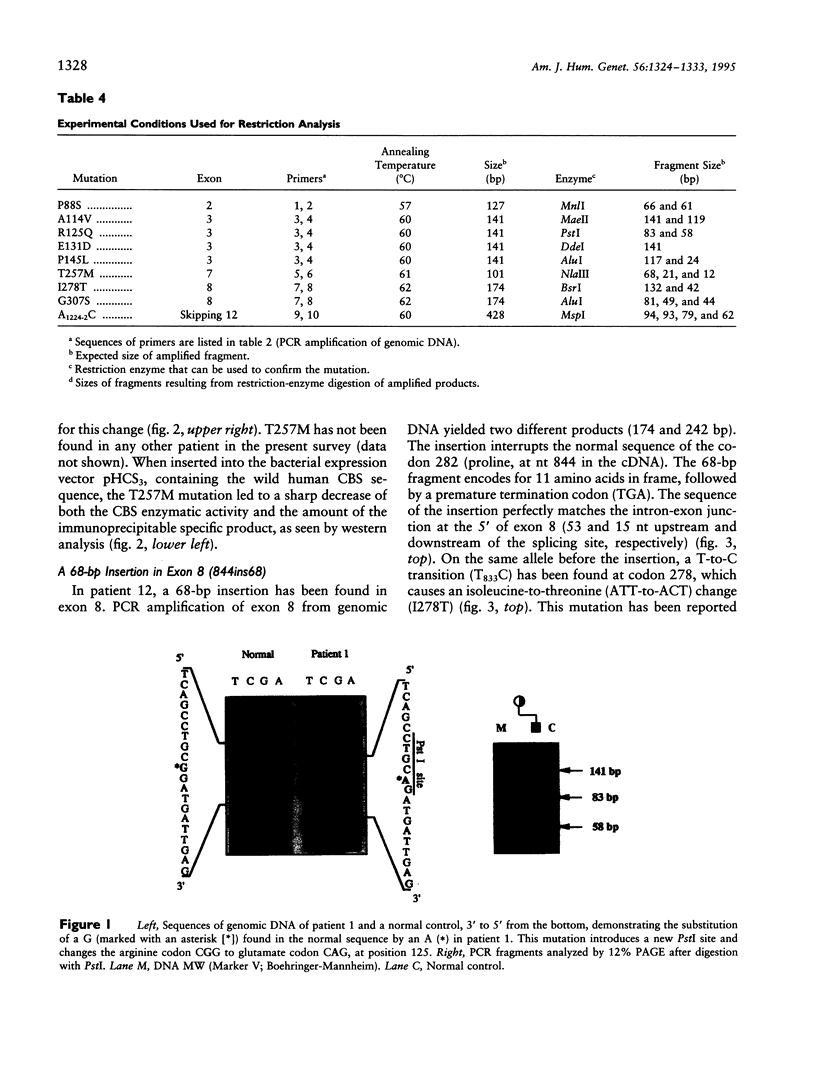
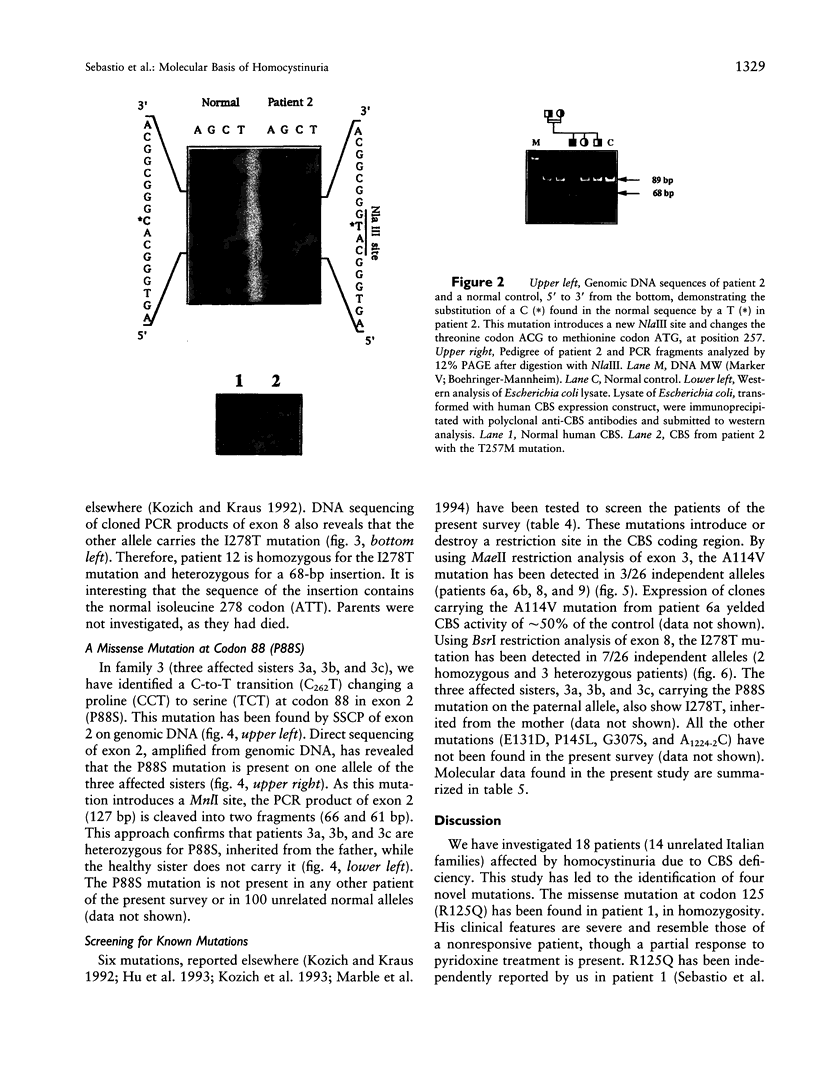
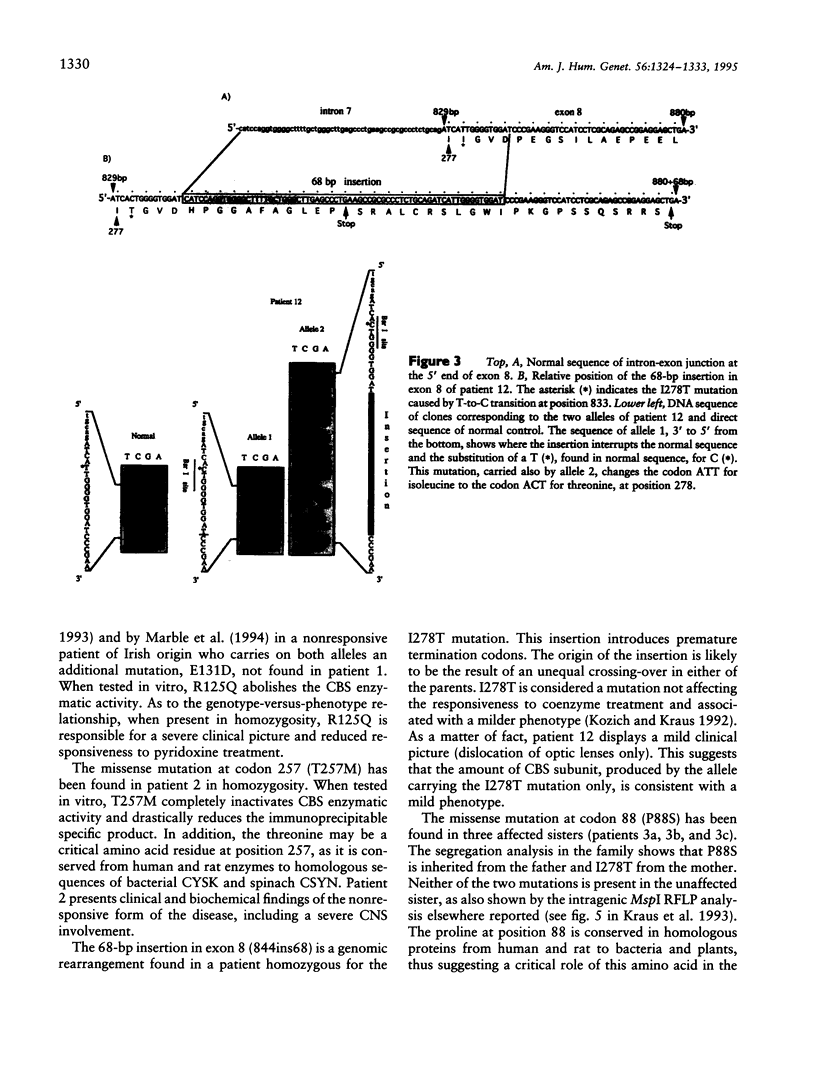
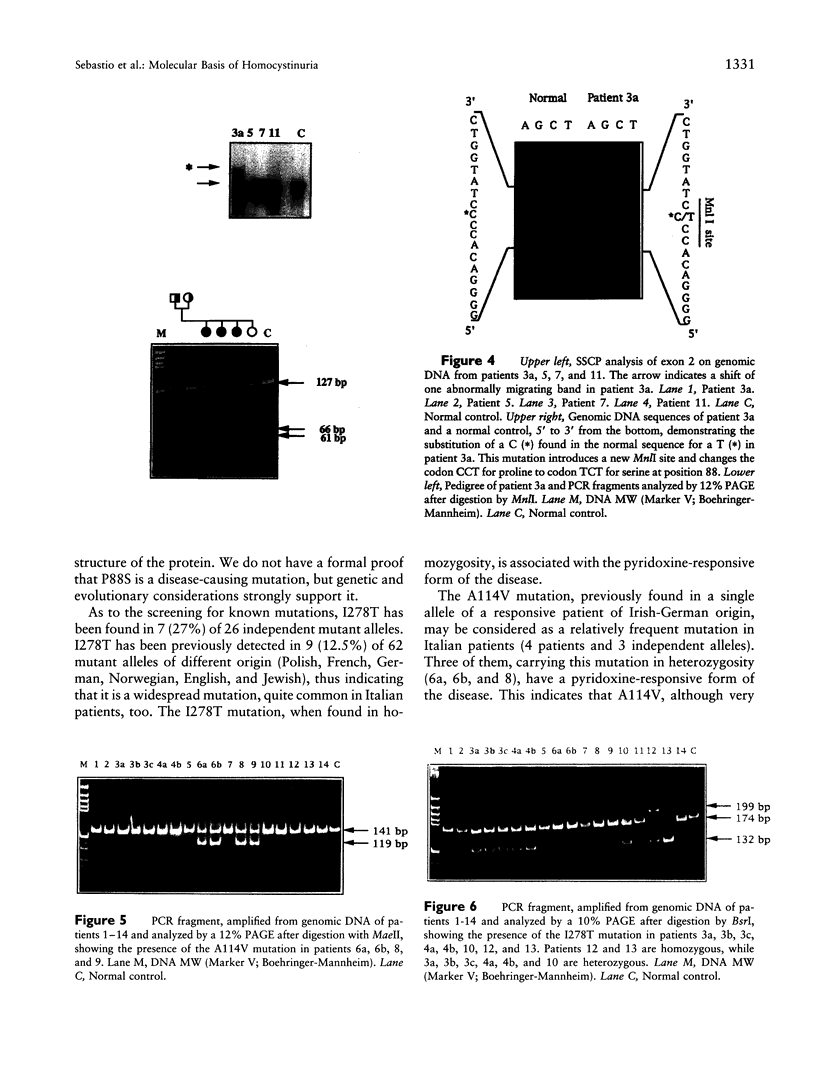
Four new mutations in the cystathionine beta-synthase (CBS) gene have been identified in Italian patients with homocystinuria. The first mutation is a G-to-A transition at base 374 in exon 3, causing an arginine-to-glutamic acid substitution at position 125 of the protein (R125Q). This mutation has been found in homozygosity in a patient partially responsive to pyridoxine treatment. The second mutation is a C-to-T transition at base 770 in exon 7, causing a threonine-to-methionine substitution at amino acid 257 of the protein (T257M). This mutation has been observed in homozygosity in a patient nonresponsive to the cofactor treatment. The third mutation, found in heterozygosity in a patient responsive to pyridoxine treatment, is an insertion of 68 bp in exon 8 at base 844, which introduces a premature termination codon. The fourth mutation is C-to-T transition in exon 2 at base 262, causing a proline-to-serine substitution at position 88 of the protein (P88S). This mutation is carried on a single allele in three affected sisters responsive to the cofactor treatment. In addition, six previously reported mutations (A114V, E131D, P145L, I278T, G307S, and A1224-2C) have been tested in 14 independent Italian families. Mutations A114V and I278T are carried by three and by seven independent alleles, respectively. The other four mutations--including G307S and A1224-2C, common among northern European patients--have not been detected.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clarke R., Daly L., Robinson K., Naughten E., Cahalane S., Fowler B., Graham I. Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med. 1991 Apr 25;324(17):1149–1155. doi: 10.1056/NEJM199104253241701. [DOI] [PubMed] [Google Scholar]
- Hu F. L., Gu Z., Kozich V., Kraus J. P., Ramesh V., Shih V. E. Molecular basis of cystathionine beta-synthase deficiency in pyridoxine responsive and nonresponsive homocystinuria. Hum Mol Genet. 1993 Nov;2(11):1857–1860. doi: 10.1093/hmg/2.11.1857. [DOI] [PubMed] [Google Scholar]
- Kery V., Bukovska G., Kraus J. P. Transsulfuration depends on heme in addition to pyridoxal 5'-phosphate. Cystathionine beta-synthase is a heme protein. J Biol Chem. 1994 Oct 14;269(41):25283–25288. [PubMed] [Google Scholar]
- Kozich V., Kraus J. P. Screening for mutations by expressing patient cDNA segments in E. coli: homocystinuria due to cystathionine beta-synthase deficiency. Hum Mutat. 1992;1(2):113–123. doi: 10.1002/humu.1380010206. [DOI] [PubMed] [Google Scholar]
- Kozich V., de Franchis R., Kraus J. P. Molecular defect in a patient with pyridoxine-responsive homocystinuria. Hum Mol Genet. 1993 Jun;2(6):815–816. doi: 10.1093/hmg/2.6.815. [DOI] [PubMed] [Google Scholar]
- Kraus J. P. Komrower Lecture. Molecular basis of phenotype expression in homocystinuria. J Inherit Metab Dis. 1994;17(4):383–390. doi: 10.1007/BF00711354. [DOI] [PubMed] [Google Scholar]
- Kraus J. P., Le K., Swaroop M., Ohura T., Tahara T., Rosenberg L. E., Roper M. D., Kozich V. Human cystathionine beta-synthase cDNA: sequence, alternative splicing and expression in cultured cells. Hum Mol Genet. 1993 Oct;2(10):1633–1638. doi: 10.1093/hmg/2.10.1633. [DOI] [PubMed] [Google Scholar]
- Marble M., Geraghty M. T., de Franchis R., Kraus J. P., Valle D. Characterization of a cystathionine beta-synthase allele with three mutations in cis in a patient with B6 nonresponsive homocystinuria. Hum Mol Genet. 1994 Oct;3(10):1883–1886. doi: 10.1093/hmg/3.10.1883. [DOI] [PubMed] [Google Scholar]
- Mudd S. H., Skovby F., Levy H. L., Pettigrew K. D., Wilcken B., Pyeritz R. E., Andria G., Boers G. H., Bromberg I. L., Cerone R. The natural history of homocystinuria due to cystathionine beta-synthase deficiency. Am J Hum Genet. 1985 Jan;37(1):1–31. [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Rubba P., Faccenda F., Pauciullo P., Carbone L., Mancini M., Strisciuglio P., Carrozzo R., Sartorio R., del Giudice E., Andria G. Early signs of vascular disease in homocystinuria: a noninvasive study by ultrasound methods in eight families with cystathionine-beta-synthase deficiency. Metabolism. 1990 Nov;39(11):1191–1195. doi: 10.1016/0026-0495(90)90093-r. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swaroop M., Bradley K., Ohura T., Tahara T., Roper M. D., Rosenberg L. E., Kraus J. P. Rat cystathionine beta-synthase. Gene organization and alternative splicing. J Biol Chem. 1992 Jun 5;267(16):11455–11461. [PubMed] [Google Scholar]
- de Franchis R., Kozich V., McInnes R. R., Kraus J. P. Identical genotypes in siblings with different homocystinuric phenotypes: identification of three mutations in cystathionine beta-synthase using an improved bacterial expression system. Hum Mol Genet. 1994 Jul;3(7):1103–1108. doi: 10.1093/hmg/3.7.1103. [DOI] [PubMed] [Google Scholar]