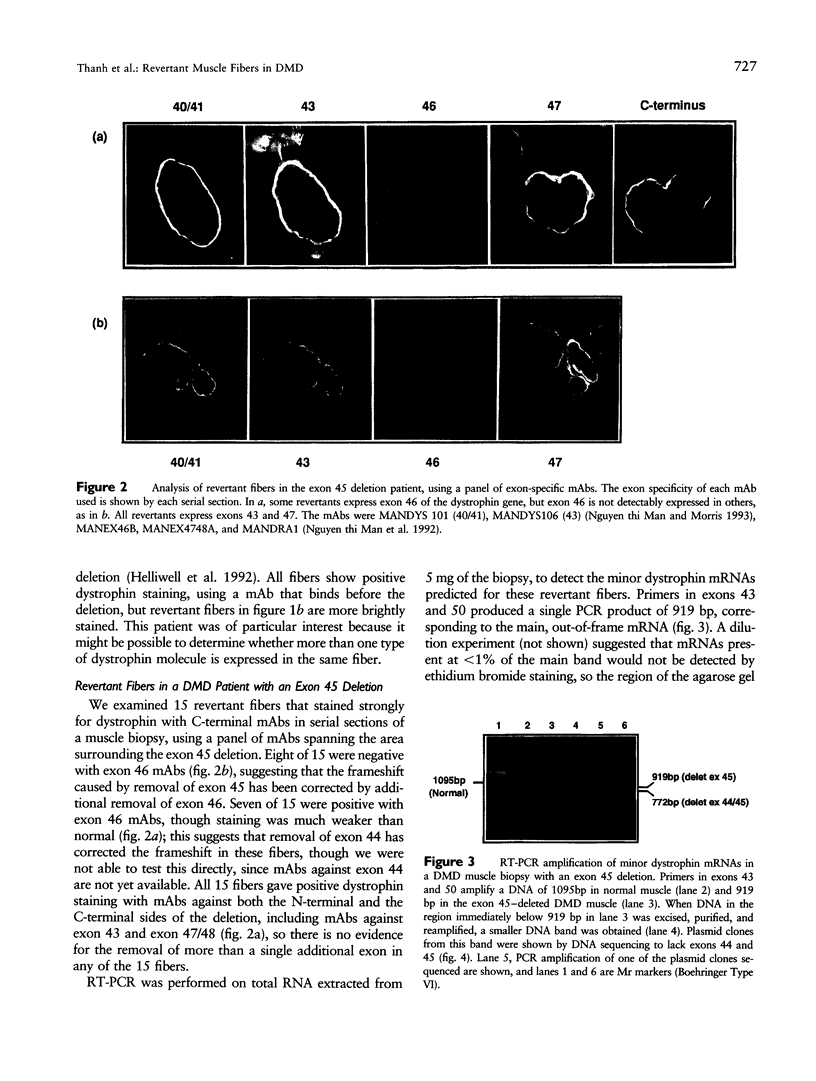
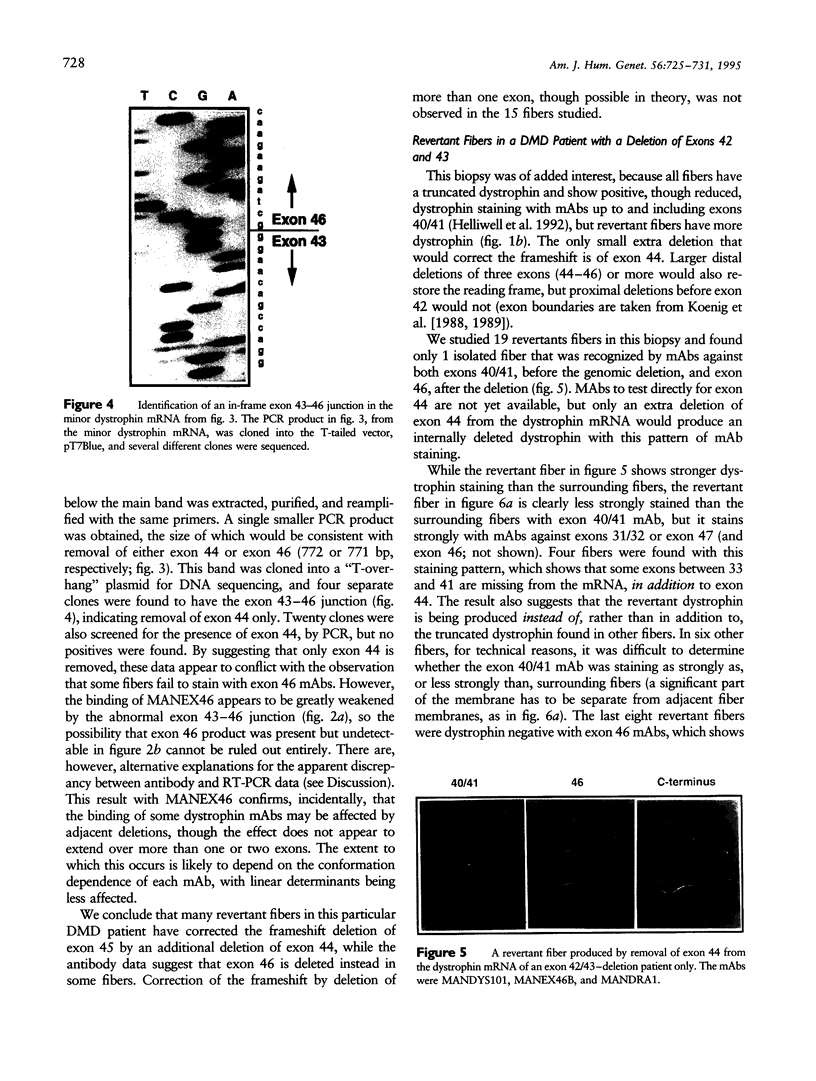
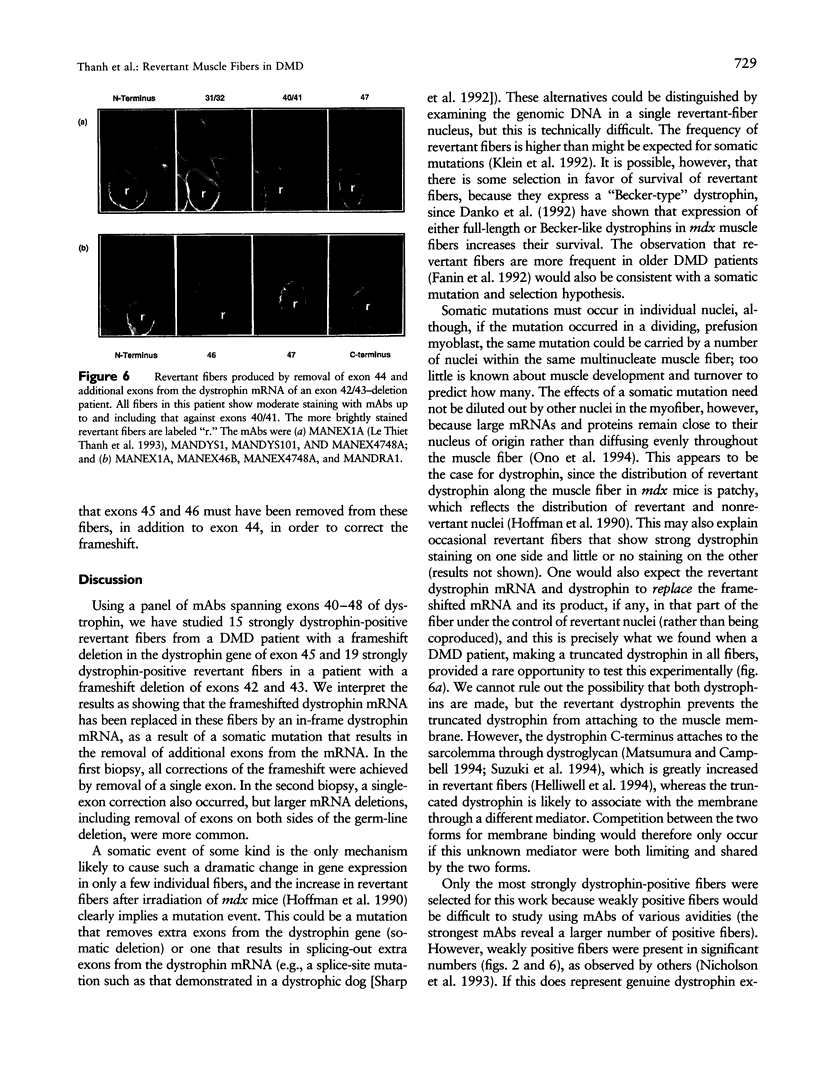
Abstract
Most Duchenne muscular dystrophy (DMD) patients have genetic deletions or point mutations in the dystrophin gene that alter the reading frame of dystrophin mRNA. This causes early termination of translation, and no dystrophin (or, less commonly, a truncated N-terminal dystrophin fragment) is produced. In many DMD patients, however, a small proportion of muscle fibers show strong dystrophin staining, and these "revertant fibers" are thought to arise by a mechanism that restores the reading frame. Exon-specific monoclonal antibodies (mAbs) have now been used to determine, for the first time, which exons are removed, in order to correct the reading frame in individual muscle fibers. Thus, 15 revertant fibers in a DMD patient with a frameshift deletion of exon 45 were shown to correct the frameshift by the additional deletion of exon 44 (or perhaps exon 46 in some fibers) from the dystrophin mRNA, but not by larger deletions. This result was consistent with reverse transcription (RT)-PCR and sequencing of a minor dystrophin mRNA with an exon 43/46 junction in this biopsy. In a DMD patient with a frameshift deletion of exons 42 and 43, however, larger deletions than the minimum necessary were used to correct the frameshift. In this patient, who produces a half-size N-terminal dystrophin fragment in all fibers, we were able to show that the revertant dystrophin replaces the truncated dystrophin in revertant-fiber sarcolemma. The results are consistent with somatic mutations in revertant-fiber nuclei, which result in removal of additional exons from dystrophin mRNA.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bies R. D., Phelps S. F., Cortez M. D., Roberts R., Caskey C. T., Chamberlain J. S. Human and murine dystrophin mRNA transcripts are differentially expressed during skeletal muscle, heart, and brain development. Nucleic Acids Res. 1992 Apr 11;20(7):1725–1731. doi: 10.1093/nar/20.7.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Gilgenkrantz H., Lambert M., Hamard G., Chafey P., Récan D., Katz P., de la Chapelle A., Koenig M., Ginjaar I. B. Effect of dystrophin gene deletions on mRNA levels and processing in Duchenne and Becker muscular dystrophies. Cell. 1990 Dec 21;63(6):1239–1248. doi: 10.1016/0092-8674(90)90419-f. [DOI] [PubMed] [Google Scholar]
- Danko I., Chapman V., Wolff J. A. The frequency of revertants in mdx mouse genetic models for Duchenne muscular dystrophy. Pediatr Res. 1992 Jul;32(1):128–131. doi: 10.1203/00006450-199207000-00025. [DOI] [PubMed] [Google Scholar]
- Fanin M., Danieli G. A., Vitiello L., Senter L., Angelini C. Prevalence of dystrophin-positive fibers in 85 Duchenne muscular dystrophy patients. Neuromuscul Disord. 1992;2(1):41–45. doi: 10.1016/0960-8966(92)90025-2. [DOI] [PubMed] [Google Scholar]
- Feener C. A., Koenig M., Kunkel L. M. Alternative splicing of human dystrophin mRNA generates isoforms at the carboxy terminus. Nature. 1989 Apr 6;338(6215):509–511. doi: 10.1038/338509a0. [DOI] [PubMed] [Google Scholar]
- Helliwell T. R., Ellis J. M., Mountford R. C., Appleton R. E., Morris G. E. A truncated dystrophin lacking the C-terminal domains is localized at the muscle membrane. Am J Hum Genet. 1992 Mar;50(3):508–514. [PMC free article] [PubMed] [Google Scholar]
- Helliwell T. R., Nguyen T. M., Morris G. E. Expression of the 43 kDa dystrophin-associated glycoprotein in human neuromuscular disease. Neuromuscul Disord. 1994 Mar;4(2):101–113. doi: 10.1016/0960-8966(94)90001-9. [DOI] [PubMed] [Google Scholar]
- Hoffman E. P., Morgan J. E., Watkins S. C., Partridge T. A. Somatic reversion/suppression of the mouse mdx phenotype in vivo. J Neurol Sci. 1990 Oct;99(1):9–25. doi: 10.1016/0022-510x(90)90195-s. [DOI] [PubMed] [Google Scholar]
- Klein C. J., Coovert D. D., Bulman D. E., Ray P. N., Mendell J. R., Burghes A. H. Somatic reversion/suppression in Duchenne muscular dystrophy (DMD): evidence supporting a frame-restoring mechanism in rare dystrophin-positive fibers. Am J Hum Genet. 1992 May;50(5):950–959. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Beggs A. H., Moyer M., Scherpf S., Heindrich K., Bettecken T., Meng G., Müller C. R., Lindlöf M., Kaariainen H. The molecular basis for Duchenne versus Becker muscular dystrophy: correlation of severity with type of deletion. Am J Hum Genet. 1989 Oct;45(4):498–506. [PMC free article] [PubMed] [Google Scholar]
- Koenig M., Monaco A. P., Kunkel L. M. The complete sequence of dystrophin predicts a rod-shaped cytoskeletal protein. Cell. 1988 Apr 22;53(2):219–228. doi: 10.1016/0092-8674(88)90383-2. [DOI] [PubMed] [Google Scholar]
- Le T. T., Nguyen T. M., Love D. R., Helliwell T. R., Davies K. E., Morris G. E. Monoclonal antibodies against the muscle-specific N-terminus of dystrophin: characterization of dystrophin in a muscular dystrophy patient with a frameshift deletion of exons 3-7. Am J Hum Genet. 1993 Jul;53(1):131–139. [PMC free article] [PubMed] [Google Scholar]
- Lederfein D., Levy Z., Augier N., Mornet D., Morris G., Fuchs O., Yaffe D., Nudel U. A 71-kilodalton protein is a major product of the Duchenne muscular dystrophy gene in brain and other nonmuscle tissues. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5346–5350. doi: 10.1073/pnas.89.12.5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra S. B., Hart K. A., Klamut H. J., Thomas N. S., Bodrug S. E., Burghes A. H., Bobrow M., Harper P. S., Thompson M. W., Ray P. N. Frame-shift deletions in patients with Duchenne and Becker muscular dystrophy. Science. 1988 Nov 4;242(4879):755–759. doi: 10.1126/science.3055295. [DOI] [PubMed] [Google Scholar]
- Matsumura K., Campbell K. P. Dystrophin-glycoprotein complex: its role in the molecular pathogenesis of muscular dystrophies. Muscle Nerve. 1994 Jan;17(1):2–15. doi: 10.1002/mus.880170103. [DOI] [PubMed] [Google Scholar]
- Matsuo M., Masumura T., Nishio H., Nakajima T., Kitoh Y., Takumi T., Koga J., Nakamura H. Exon skipping during splicing of dystrophin mRNA precursor due to an intraexon deletion in the dystrophin gene of Duchenne muscular dystrophy kobe. J Clin Invest. 1991 Jun;87(6):2127–2131. doi: 10.1172/JCI115244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaco A. P., Bertelson C. J., Liechti-Gallati S., Moser H., Kunkel L. M. An explanation for the phenotypic differences between patients bearing partial deletions of the DMD locus. Genomics. 1988 Jan;2(1):90–95. doi: 10.1016/0888-7543(88)90113-9. [DOI] [PubMed] [Google Scholar]
- Narita N., Nishio H., Kitoh Y., Ishikawa Y., Ishikawa Y., Minami R., Nakamura H., Matsuo M. Insertion of a 5' truncated L1 element into the 3' end of exon 44 of the dystrophin gene resulted in skipping of the exon during splicing in a case of Duchenne muscular dystrophy. J Clin Invest. 1993 May;91(5):1862–1867. doi: 10.1172/JCI116402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. M., Ginjaar I. B., van Ommen G. J., Morris G. E. Monoclonal antibodies for dystrophin analysis. Epitope mapping and improved binding to SDS-treated muscle sections. Biochem J. 1992 Dec 1;288(Pt 2):663–668. doi: 10.1042/bj2880663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. M., Morris G. E. Use of epitope libraries to identify exon-specific monoclonal antibodies for characterization of altered dystrophins in muscular dystrophy. Am J Hum Genet. 1993 Jun;52(6):1057–1066. [PMC free article] [PubMed] [Google Scholar]
- Nicholson L. V., Bushby K. M., Johnson M. A., den Dunnen J. T., Ginjaar I. B., van Ommen G. J. Predicted and observed sizes of dystrophin in some patients with gene deletions that disrupt the open reading frame. J Med Genet. 1992 Dec;29(12):892–896. doi: 10.1136/jmg.29.12.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson L. V., Davison K., Johnson M. A., Slater C. R., Young C., Bhattacharya S., Gardner-Medwin D., Harris J. B. Dystrophin in skeletal muscle. II. Immunoreactivity in patients with Xp21 muscular dystrophy. J Neurol Sci. 1989 Dec;94(1-3):137–146. doi: 10.1016/0022-510x(89)90224-4. [DOI] [PubMed] [Google Scholar]
- Nicholson L. V., Johnson M. A., Bushby K. M., Gardner-Medwin D. Functional significance of dystrophin positive fibres in Duchenne muscular dystrophy. Arch Dis Child. 1993 May;68(5):632–636. doi: 10.1136/adc.68.5.632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T., Ono K., Mizukawa K., Ohta T., Tsuchiya T., Tsuda M. Limited diffusibility of gene products directed by a single nucleus in the cytoplasm of multinucleated myofibres. FEBS Lett. 1994 Jan 3;337(1):18–22. doi: 10.1016/0014-5793(94)80621-7. [DOI] [PubMed] [Google Scholar]
- Roberts R. G., Barby T. F., Manners E., Bobrow M., Bentley D. R. Direct detection of dystrophin gene rearrangements by analysis of dystrophin mRNA in peripheral blood lymphocytes. Am J Hum Genet. 1991 Aug;49(2):298–310. [PMC free article] [PubMed] [Google Scholar]
- Roberts R. G., Coffey A. J., Bobrow M., Bentley D. R. Exon structure of the human dystrophin gene. Genomics. 1993 May;16(2):536–538. doi: 10.1006/geno.1993.1225. [DOI] [PubMed] [Google Scholar]
- Sharp N. J., Kornegay J. N., Van Camp S. D., Herbstreith M. H., Secore S. L., Kettle S., Hung W. Y., Constantinou C. D., Dykstra M. J., Roses A. D. An error in dystrophin mRNA processing in golden retriever muscular dystrophy, an animal homologue of Duchenne muscular dystrophy. Genomics. 1992 May;13(1):115–121. doi: 10.1016/0888-7543(92)90210-j. [DOI] [PubMed] [Google Scholar]
- Sherratt T. G., Vulliamy T., Dubowitz V., Sewry C. A., Strong P. N. Exon skipping and translation in patients with frameshift deletions in the dystrophin gene. Am J Hum Genet. 1993 Nov;53(5):1007–1015. [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Yoshida M., Hayashi K., Mizuno Y., Hagiwara Y., Ozawa E. Molecular organization at the glycoprotein-complex-binding site of dystrophin. Three dystrophin-associated proteins bind directly to the carboxy-terminal portion of dystrophin. Eur J Biochem. 1994 Mar 1;220(2):283–292. doi: 10.1111/j.1432-1033.1994.tb18624.x. [DOI] [PubMed] [Google Scholar]
- Wallgren-Pettersson C., Jasani B., Rosser L. G., Lazarou L. P., Nicholson L. V., Clarke A. Immunohistological evidence for second or somatic mutations as the underlying cause of dystrophin expression by isolated fibres in Xp21 muscular dystrophy of Duchenne-type severity. J Neurol Sci. 1993 Aug;118(1):56–63. doi: 10.1016/0022-510x(93)90246-u. [DOI] [PubMed] [Google Scholar]
- Wilton S. D., Johnsen R. D., Pedretti J. R., Laing N. G. Two distinct mutations in a single dystrophin gene: identification of an altered splice-site as the primary Becker muscular dystrophy mutation. Am J Med Genet. 1993 Jun 15;46(5):563–569. doi: 10.1002/ajmg.1320460521. [DOI] [PubMed] [Google Scholar]
- Winnard A. V., Klein C. J., Coovert D. D., Prior T., Papp A., Snyder P., Bulman D. E., Ray P. N., McAndrew P., King W. Characterization of translational frame exception patients in Duchenne/Becker muscular dystrophy. Hum Mol Genet. 1993 Jun;2(6):737–744. doi: 10.1093/hmg/2.6.737. [DOI] [PubMed] [Google Scholar]
- Worton R. G., Thompson M. W. Genetics of Duchenne muscular dystrophy. Annu Rev Genet. 1988;22:601–629. doi: 10.1146/annurev.ge.22.120188.003125. [DOI] [PubMed] [Google Scholar]