Abstract
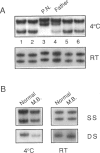
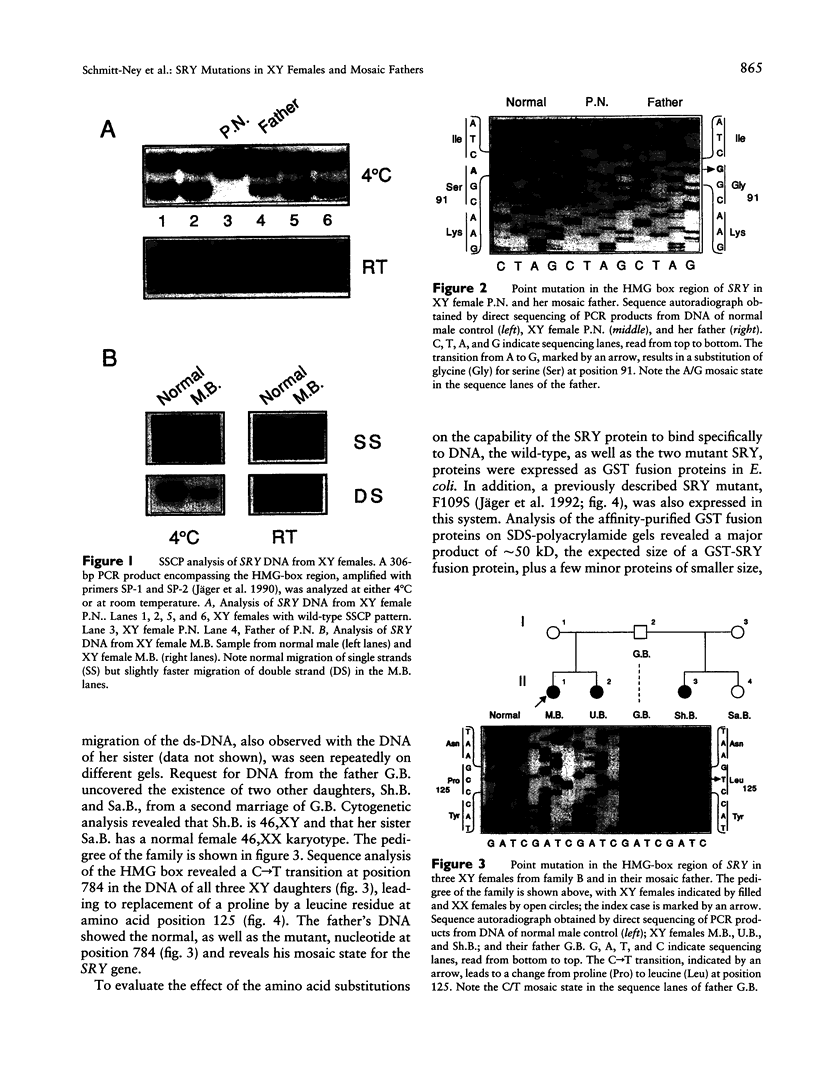
Two novel mutations in the sex-determining gene SRY were identified by screening DNA from 30 sex-reversed XY females by using the SSCP assay. Both point mutations lead to an amino acid substitution in the DNA-binding high-mobility-group domain of the SRY protein. The first mutation, changing a serine at position 91 to glycine, was found in a sporadic case. The second mutation, leading to replacement of a highly conserved proline at position 125 with leucine, is shared by three members of the same family, two sisters and a half sister having the same father. The mutant SRY proteins showed reduced DNA-binding ability in a gel-shift assay. Analysis of lymphocyte DNA from the respective fathers revealed that they carry both the wild-type and the mutant version of the SRY gene. The fact that both fathers transmitted the mutant SRY copy to their offspring implies that they are mosaic for the SRY gene in testis as well as in blood, as a result of a mutation during early embryonic development.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behlke M. A., Bogan J. S., Beer-Romero P., Page D. C. Evidence that the SRY protein is encoded by a single exon on the human Y chromosome. Genomics. 1993 Sep;17(3):736–739. doi: 10.1006/geno.1993.1395. [DOI] [PubMed] [Google Scholar]
- Berta P., Hawkins J. R., Sinclair A. H., Taylor A., Griffiths B. L., Goodfellow P. N., Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990 Nov 29;348(6300):448–450. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- Braun A., Kammerer S., Cleve H., Löhrs U., Schwarz H. P., Kuhnle U. True hermaphroditism in a 46,XY individual, caused by a postzygotic somatic point mutation in the male gonadal sex-determining locus (SRY): molecular genetics and histological findings in a sporadic case. Am J Hum Genet. 1993 Mar;52(3):578–585. [PMC free article] [PubMed] [Google Scholar]
- Ferrari S., Harley V. R., Pontiggia A., Goodfellow P. N., Lovell-Badge R., Bianchi M. E. SRY, like HMG1, recognizes sharp angles in DNA. EMBO J. 1992 Dec;11(12):4497–4506. doi: 10.1002/j.1460-2075.1992.tb05551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese K., Cox J., Grosschedl R. The HMG domain of lymphoid enhancer factor 1 bends DNA and facilitates assembly of functional nucleoprotein structures. Cell. 1992 Apr 3;69(1):185–195. doi: 10.1016/0092-8674(92)90129-z. [DOI] [PubMed] [Google Scholar]
- Giese K., Pagel J., Grosschedl R. Distinct DNA-binding properties of the high mobility group domain of murine and human SRY sex-determining factors. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3368–3372. doi: 10.1073/pnas.91.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosschedl R., Giese K., Pagel J. HMG domain proteins: architectural elements in the assembly of nucleoprotein structures. Trends Genet. 1994 Mar;10(3):94–100. doi: 10.1016/0168-9525(94)90232-1. [DOI] [PubMed] [Google Scholar]
- Gubbay J., Collignon J., Koopman P., Capel B., Economou A., Münsterberg A., Vivian N., Goodfellow P., Lovell-Badge R. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes. Nature. 1990 Jul 19;346(6281):245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Jackson D. I., Hextall P. J., Hawkins J. R., Berkovitz G. D., Sockanathan S., Lovell-Badge R., Goodfellow P. N. DNA binding activity of recombinant SRY from normal males and XY females. Science. 1992 Jan 24;255(5043):453–456. doi: 10.1126/science.1734522. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Lovell-Badge R., Goodfellow P. N. Definition of a consensus DNA binding site for SRY. Nucleic Acids Res. 1994 Apr 25;22(8):1500–1501. doi: 10.1093/nar/22.8.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Anvret M., Hall K., Scherer G. A human XY female with a frame shift mutation in the candidate testis-determining gene SRY. Nature. 1990 Nov 29;348(6300):452–454. doi: 10.1038/348452a0. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Harley V. R., Pfeiffer R. A., Goodfellow P. N., Scherer G. A familial mutation in the testis-determining gene SRY shared by both sexes. Hum Genet. 1992 Dec;90(4):350–355. doi: 10.1007/BF00220457. [DOI] [PubMed] [Google Scholar]
- Koopman P., Gubbay J., Vivian N., Goodfellow P., Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991 May 9;351(6322):117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- McElreavey K., Vilain E., Cotinot C., Payen E., Fellous M. Control of sex determination in animals. Eur J Biochem. 1993 Dec 15;218(3):769–783. doi: 10.1111/j.1432-1033.1993.tb18432.x. [DOI] [PubMed] [Google Scholar]
- Nasrin N., Buggs C., Kong X. F., Carnazza J., Goebl M., Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. doi: 10.1038/354317a0. [DOI] [PubMed] [Google Scholar]
- Ner S. S. HMGs everywhere. Curr Biol. 1992 Apr;2(4):208–210. doi: 10.1016/0960-9822(92)90541-h. [DOI] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Poulat F., Soullier S., Gozé C., Heitz F., Calas B., Berta P. Description and functional implications of a novel mutation in the sex-determining gene SRY. Hum Mutat. 1994;3(3):200–204. doi: 10.1002/humu.1380030305. [DOI] [PubMed] [Google Scholar]
- Read C. M., Cary P. D., Crane-Robinson C., Driscoll P. C., Norman D. G. Solution structure of a DNA-binding domain from HMG1. Nucleic Acids Res. 1993 Jul 25;21(15):3427–3436. doi: 10.1093/nar/21.15.3427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saad F. A., Halliger B., Müller C. R., Roberts R. G., Danieli G. A. Single base substitutions are detected by double strand conformation analysis. Nucleic Acids Res. 1994 Oct 11;22(20):4352–4353. doi: 10.1093/nar/22.20.4352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair A. H., Berta P., Palmer M. S., Hawkins J. R., Griffiths B. L., Smith M. J., Foster J. W., Frischauf A. M., Lovell-Badge R., Goodfellow P. N. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature. 1990 Jul 19;346(6281):240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Tajima T., Nakae J., Shinohara N., Fujieda K. A novel mutation localized in the 3' non-HMG box region of the SRY gene in 46,XY gonadal dysgenesis. Hum Mol Genet. 1994 Jul;3(7):1187–1189. doi: 10.1093/hmg/3.7.1187. [DOI] [PubMed] [Google Scholar]
- Vilain E., McElreavey K., Jaubert F., Raymond J. P., Richaud F., Fellous M. Familial case with sequence variant in the testis-determining region associated with two sex phenotypes. Am J Hum Genet. 1992 May;50(5):1008–1011. [PMC free article] [PubMed] [Google Scholar]
- Weir H. M., Kraulis P. J., Hill C. S., Raine A. R., Laue E. D., Thomas J. O. Structure of the HMG box motif in the B-domain of HMG1. EMBO J. 1993 Apr;12(4):1311–1319. doi: 10.1002/j.1460-2075.1993.tb05776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf U., Schempp W., Scherer G. Molecular biology of the human Y chromosome. Rev Physiol Biochem Pharmacol. 1992;121:147–213. doi: 10.1007/BFb0033195. [DOI] [PubMed] [Google Scholar]
- Wright E. M., Snopek B., Koopman P. Seven new members of the Sox gene family expressed during mouse development. Nucleic Acids Res. 1993 Feb 11;21(3):744–744. doi: 10.1093/nar/21.3.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y. T., Ren Z. R., Zhang M. L., Huang Y., Zeng F. Y., Huang S. Z. A new de novo mutation (A113T) in HMG box of the SRY gene leads to XY gonadal dysgenesis. J Med Genet. 1993 Aug;30(8):655–657. doi: 10.1136/jmg.30.8.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Wetering M., Clevers H. Sequence-specific interaction of the HMG box proteins TCF-1 and SRY occurs within the minor groove of a Watson-Crick double helix. EMBO J. 1992 Aug;11(8):3039–3044. doi: 10.1002/j.1460-2075.1992.tb05374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]