Abstract
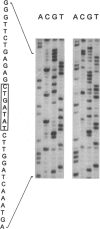
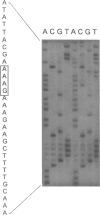
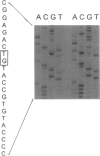
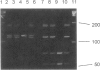
Canavan disease is an infantile neurodegenerative disease that is due to aspartoacylase deficiency. The disease has been reported mainly in Ashkenazi Jews but also occurs in other ethnic groups. Determination of enzymatic activity for carrier detection and prenatal diagnosis is considered unreliable. In the present study, nine mutations were found in the aspartoacylase gene of 19 non-Jewish patients. These included four point mutations (A305E [39.5% of the mutated alleles], C218X [15.8%], F295S [2.6%], and G274R [5.3%]); four deletion mutations (827delGT [5.3%], 870del4 [2.6%], 566del7 [2.6%], and 527del6 [2.6%]); and one exon skip (527del108 [5.3%]). The A305E mutation is pan-European and probably the most ancient mutation, identified in patients of Greek, Polish, Danish, French, Spanish, Italian, and British origin. In contrast, the G274R and 527del108 mutations were found only in patients of Turkish origin, and the C218X mutation was identified only in patients of Gypsy origin. Homozygosity for the A305E mutation was identified in patients with both the severe and the mild forms of Canavan disease. Mutations were identified in 31 of the 38 alleles, resulting in an overall detection rate of 81.6%. All nine mutations identified in non-Jewish patients reside in exons 4–6 of the aspartoacylase gene. The results would enable accurate genetic counseling in the families of 13 (68.4%) of 19 patients, in whom two mutations were identified in the aspartoacylase cDNA.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartalini G., Margollicci M., Balestri P., Farnetani M. A., Cioni M., Fois A. Biochemical diagnosis of Canavan disease. Childs Nerv Syst. 1992 Dec;8(8):468–470. doi: 10.1007/BF00274411. [DOI] [PubMed] [Google Scholar]
- Bennett M. J., Gibson K. M., Sherwood W. G., Divry P., Rolland M. O., Elpeleg O. N., Rinaldo P., Jakobs C. Reliable prenatal diagnosis of Canavan disease (aspartoacylase deficiency): comparison of enzymatic and metabolite analysis. J Inherit Metab Dis. 1993;16(5):831–836. doi: 10.1007/BF00714274. [DOI] [PubMed] [Google Scholar]
- Divry P., Vianey-Liaud C., Gay C., Macabeo V., Rapin F., Echenne B. N-acetylaspartic aciduria: report of three new cases in children with a neurological syndrome associating macrocephaly and leukodystrophy. J Inherit Metab Dis. 1988;11(3):307–308. doi: 10.1007/BF01800378. [DOI] [PubMed] [Google Scholar]
- Elpeleg O. N., Anikster Y., Barash V., Branski D., Shaag A. The frequency of the C854 mutation in the aspartoacylase gene in Ashkenazi Jews in Israel. Am J Hum Genet. 1994 Aug;55(2):287–288. [PMC free article] [PubMed] [Google Scholar]
- Elpeleg O. N., Shaag A., Anikster Y., Jakobs C. Prenatal detection of Canavan disease (aspartoacylase deficiency) by DNA analysis. J Inherit Metab Dis. 1994;17(6):664–666. doi: 10.1007/BF00712008. [DOI] [PubMed] [Google Scholar]
- Goodhue W. W., Jr, Couch R. D., Namiki H. Spongy degeneration of the CNS: an instance of the rare juvenile form. Arch Neurol. 1979 Aug;36(8):481–484. doi: 10.1001/archneur.1979.00500440051009. [DOI] [PubMed] [Google Scholar]
- Jellinger K., Seitelberger F. Juvenile form of spongy degeneration of the CNS. Acta Neuropathol. 1969;13(3):276–281. doi: 10.1007/BF00690647. [DOI] [PubMed] [Google Scholar]
- Kaul R., Balamurugan K., Gao G. P., Matalon R. Canavan disease: genomic organization and localization of human ASPA to 17p13-ter and conservation of the ASPA gene during evolution. Genomics. 1994 May 15;21(2):364–370. doi: 10.1006/geno.1994.1278. [DOI] [PubMed] [Google Scholar]
- Kaul R., Gao G. P., Aloya M., Balamurugan K., Petrosky A., Michals K., Matalon R. Canavan disease: mutations among Jewish and non-Jewish patients. Am J Hum Genet. 1994 Jul;55(1):34–41. [PMC free article] [PubMed] [Google Scholar]
- Kaul R., Gao G. P., Balamurugan K., Matalon R. Cloning of the human aspartoacylase cDNA and a common missense mutation in Canavan disease. Nat Genet. 1993 Oct;5(2):118–123. doi: 10.1038/ng1093-118. [DOI] [PubMed] [Google Scholar]
- Krawczak M., Reiss J., Cooper D. N. The mutational spectrum of single base-pair substitutions in mRNA splice junctions of human genes: causes and consequences. Hum Genet. 1992 Sep-Oct;90(1-2):41–54. doi: 10.1007/BF00210743. [DOI] [PubMed] [Google Scholar]
- Matalon R., Michals K., Sebesta D., Deanching M., Gashkoff P., Casanova J. Aspartoacylase deficiency and N-acetylaspartic aciduria in patients with Canavan disease. Am J Med Genet. 1988 Feb;29(2):463–471. doi: 10.1002/ajmg.1320290234. [DOI] [PubMed] [Google Scholar]
- Michelakakis H., Giouroukos S., Divry P., Katsarou E., Rolland M. O., Skardoutsou A. Canavan disease: findings in four new cases. J Inherit Metab Dis. 1991;14(2):267–268. doi: 10.1007/BF01800603. [DOI] [PubMed] [Google Scholar]
- Rolland M. O., Mandon G., Bernard A., Zabot M. T., Mathieu M. Unreliable verification of prenatal diagnosis of Canavan disease: aspartoacylase activity in deficient and normal fetal skin fibroblasts. J Inherit Metab Dis. 1994;17(6):748–748. doi: 10.1007/BF00712018. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toft P. B., Geiss-Holtorff R., Rolland M. O., Pryds O., Müller-Forell W., Christensen E., Lehnert W., Lou H. C., Ott D., Hennig J. Magnetic resonance imaging in juvenile Canavan disease. Eur J Pediatr. 1993 Sep;152(9):750–753. doi: 10.1007/BF01953994. [DOI] [PubMed] [Google Scholar]