Abstract
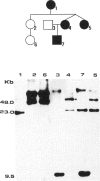
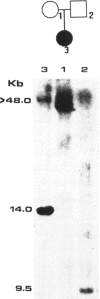
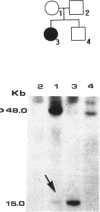
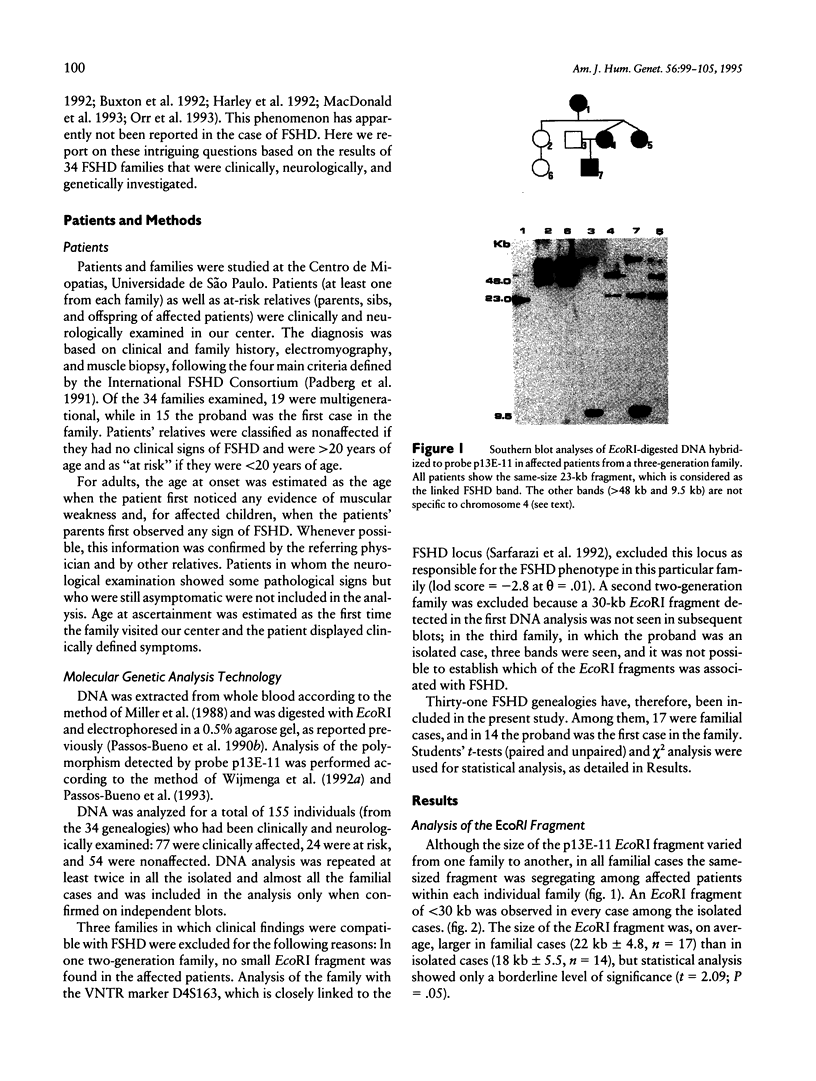
A gene responsible for facioscapulohumeral muscular dystrophy (FSHD) has been localized at 4q35. Subsequently, it was found that probe p13E-11 detects a polymorphic EcoRI fragment, usually > 28 kb, in normal individuals, whereas in sporadic and familial FSHD cases, an EcoRI fragment, usually < 28 kb, was found. Although these findings have been amply confirmed, several aspects are as yet either controversial or unsolved. In the present investigation, 34 Brazilian FSHD families were studied at the clinical and the molecular level for the following purposes: to assess the frequency of new mutations and their effect on estimates of biological fitness, to characterize FSHD-associated EcoRI fragments detected with probe p13E-11 in familial--as compared with isolated--FSHD cases, and to assess whether anticipation occurs in multigenerational families. Results from our study suggest that new mutations are apparently frequent for FSHD and may account for at least one-third of the cases, that somatic mosaicism may not be rare, and that biological fitness appeared to be reduced in FSHD, ranging from 0.6 to 0.82 by different estimates, with no difference in sexes. Interestingly, the size of the new EcoRI fragment is apparently smaller in more severely affected isolated patients. Moreover, the age at onset of clinical signs, as well as the age at ascertainment, in patients from multigenerational families suggests that anticipation occurs for FSHD in the majority of the families.
Full text
PDF
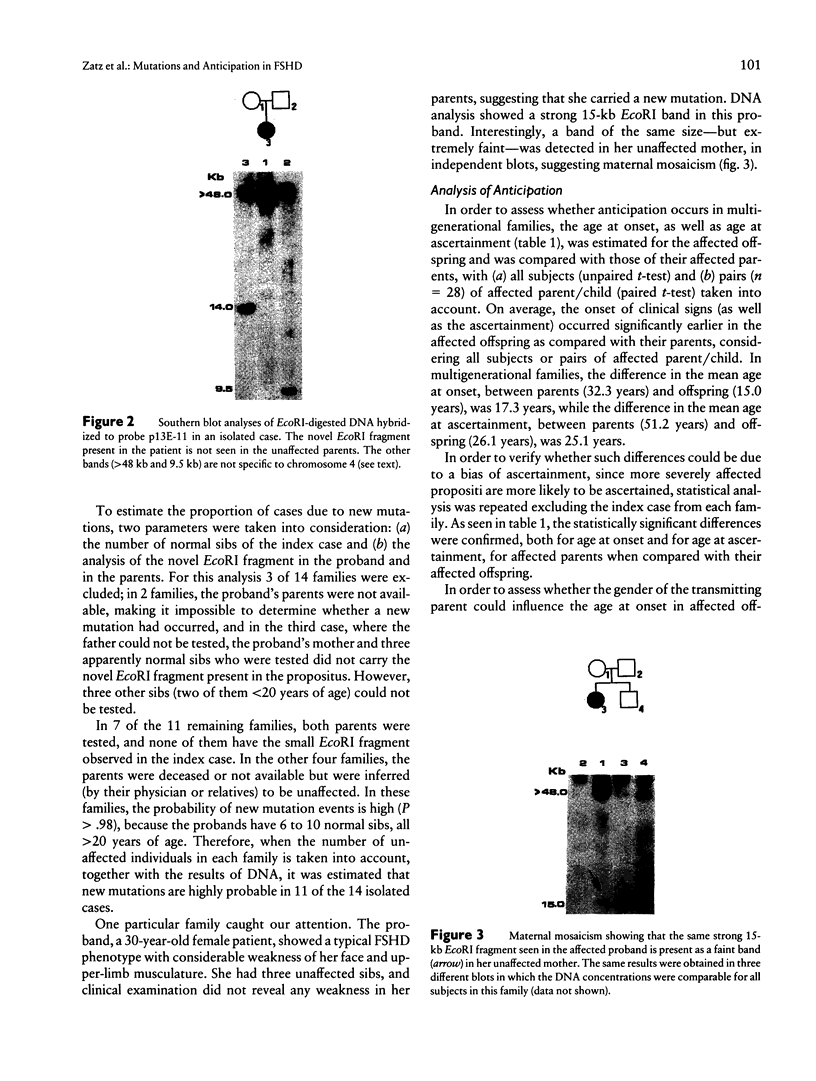




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aslanidis C., Jansen G., Amemiya C., Shutler G., Mahadevan M., Tsilfidis C., Chen C., Alleman J., Wormskamp N. G., Vooijs M. Cloning of the essential myotonic dystrophy region and mapping of the putative defect. Nature. 1992 Feb 6;355(6360):548–551. doi: 10.1038/355548a0. [DOI] [PubMed] [Google Scholar]
- Bakker E., Veenema H., Den Dunnen J. T., van Broeckhoven C., Grootscholten P. M., Bonten E. J., van Ommen G. J., Pearson P. L. Germinal mosaicism increases the recurrence risk for 'new' Duchenne muscular dystrophy mutations. J Med Genet. 1989 Sep;26(9):553–559. doi: 10.1136/jmg.26.9.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bröcker-Vriends A. H., Briët E., Dreesen J. C., Bakker B., Reitsma P., Pannekoek H., van de Kamp J. J., Pearson P. L. Somatic origin of inherited haemophilia A. Hum Genet. 1990 Aug;85(3):288–292. doi: 10.1007/BF00206748. [DOI] [PubMed] [Google Scholar]
- Buxton J., Shelbourne P., Davies J., Jones C., Van Tongeren T., Aslanidis C., de Jong P., Jansen G., Anvret M., Riley B. Detection of an unstable fragment of DNA specific to individuals with myotonic dystrophy. Nature. 1992 Feb 6;355(6360):547–548. doi: 10.1038/355547a0. [DOI] [PubMed] [Google Scholar]
- Eggers S., Passos-Bueno M. R., Zatz M. Facioscapulohumeral muscular dystrophy: aspects of genetic counselling, acceptance of preclinical diagnosis, and fitness. J Med Genet. 1993 Jul;30(7):589–592. doi: 10.1136/jmg.30.7.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griggs R. C., Tawil R., Storvick D., Mendell J. R., Altherr M. R. Genetics of facioscapulohumeral muscular dystrophy: new mutations in sporadic cases. Neurology. 1993 Nov;43(11):2369–2372. doi: 10.1212/wnl.43.11.2369. [DOI] [PubMed] [Google Scholar]
- Harley H. G., Brook J. D., Rundle S. A., Crow S., Reardon W., Buckler A. J., Harper P. S., Housman D. E., Shaw D. J. Expansion of an unstable DNA region and phenotypic variation in myotonic dystrophy. Nature. 1992 Feb 6;355(6360):545–546. doi: 10.1038/355545a0. [DOI] [PubMed] [Google Scholar]
- Hewitt J. E., Lyle R., Clark L. N., Valleley E. M., Wright T. J., Wijmenga C., van Deutekom J. C., Francis F., Sharpe P. T., Hofker M. Analysis of the tandem repeat locus D4Z4 associated with facioscapulohumeral muscular dystrophy. Hum Mol Genet. 1994 Aug;3(8):1287–1295. doi: 10.1093/hmg/3.8.1287. [DOI] [PubMed] [Google Scholar]
- Lunt P. W. A workshop on facioscapulohumeral (Landouzy-Déjérine) disease, Manchester, 16 to 17 November 1988. J Med Genet. 1989 Aug;26(8):535–537. doi: 10.1136/jmg.26.8.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt P. W., Compston D. A., Harper P. S. Estimation of age dependent penetrance in facioscapulohumeral muscular dystrophy by minimising ascertainment bias. J Med Genet. 1989 Dec;26(12):755–760. doi: 10.1136/jmg.26.12.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORTON N. E., CHUNG C. S. Formal genetics of muscular dystrophy. Am J Hum Genet. 1959 Dec;11:360–379. [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. E., Barnes G., Srinidhi J., Duyao M. P., Ambrose C. M., Myers R. H., Gray J., Conneally P. M., Young A., Penney J. Gametic but not somatic instability of CAG repeat length in Huntington's disease. J Med Genet. 1993 Dec;30(12):982–986. doi: 10.1136/jmg.30.12.982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Padberg G. W., Lunt P. W., Koch M., Fardeau M. Diagnostic criteria for facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 1991;1(4):231–234. doi: 10.1016/0960-8966(91)90094-9. [DOI] [PubMed] [Google Scholar]
- Passos-Bueno M. R., Bakker E., Kneppers A. L., Takata R. I., Rapaport D., den Dunnen J. T., Zatz M., van Ommen G. J. Different mosaicism frequencies for proximal and distal Duchenne muscular dystrophy (DMD) mutations indicate difference in etiology and recurrence risk. Am J Hum Genet. 1992 Nov;51(5):1150–1155. [PMC free article] [PubMed] [Google Scholar]
- Passos-Bueno M. R., Lima M. A., Zatz M. Estimate of germinal mosaicism in Duchenne muscular dystrophy. J Med Genet. 1990 Nov;27(11):727–728. doi: 10.1136/jmg.27.11.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos-Bueno M. R., Rapaport D., Love D., Flint T., Bortolini E. R., Zatz M., Davies K. E. Screening of deletions in the dystrophin gene with the cDNA probes Cf23a, Cf56a, and Cf115. J Med Genet. 1990 Mar;27(3):145–150. doi: 10.1136/jmg.27.3.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passos-Bueno M. R., Wijmenga C., Takata R. E., Marie S. K., Vainzof M., Pavanello R. C., Hewitt J. E., Bakker E., Carvalho A., Akiyama J. No evidence of genetic heterogeneity in Brazilian facioscapulohumeral muscular dystrophy families (FSHD) with 4q markers. Hum Mol Genet. 1993 May;2(5):557–562. doi: 10.1093/hmg/2.5.557. [DOI] [PubMed] [Google Scholar]
- Sarfarazi M., Wijmenga C., Upadhyaya M., Weiffenbach B., Hyser C., Mathews K., Murray J., Gilbert J., Pericak-Vance M., Lunt P. Regional mapping of facioscapulohumeral muscular dystrophy gene on 4q35: combined analysis of an international consortium. Am J Hum Genet. 1992 Aug;51(2):396–403. [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. A new simplified method for estimating relative fitness in man. Jinrui Idengaku Zasshi. 1974 Dec;19(3):195–202. [PubMed] [Google Scholar]
- Upadhyaya M., Jardine P., Maynard J., Farnham J., Sarfarazi M., Wijmenga C., Hewitt J. E., Frants R., Harper P. S., Lunt P. W. Molecular analysis of British facioscapulohumeral dystrophy families for 4q DNA rearrangements. Hum Mol Genet. 1993 Jul;2(7):981–987. doi: 10.1093/hmg/2.7.981. [DOI] [PubMed] [Google Scholar]
- Weiffenbach B., Dubois J., Storvick D., Tawil R., Jacobsen S. J., Gilbert J., Wijmenga C., Mendell J. R., Winokur S., Altherr M. R. Mapping the facioscapulohumeral muscular dystrophy gene is complicated by chromsome 4q35 recombination events. Nat Genet. 1993 Jun;4(2):165–169. doi: 10.1038/ng0693-165. [DOI] [PubMed] [Google Scholar]
- Wijmenga C., Frants R. R., Brouwer O. F., Moerer P., Weber J. L., Padberg G. W. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet. 1990 Sep 15;336(8716):651–653. doi: 10.1016/0140-6736(90)92148-b. [DOI] [PubMed] [Google Scholar]
- Wijmenga C., Frants R. R., Hewitt J. E., van Deutekom J. C., van Geel M., Wright T. J., Padberg G. W., Hofker M. H., van Ommen G. J. Molecular genetics of facioscapulohumeral muscular dystrophy. Neuromuscul Disord. 1993 Sep-Nov;3(5-6):487–491. doi: 10.1016/0960-8966(93)90102-p. [DOI] [PubMed] [Google Scholar]
- Wijmenga C., Hewitt J. E., Sandkuijl L. A., Clark L. N., Wright T. J., Dauwerse H. G., Gruter A. M., Hofker M. H., Moerer P., Williamson R. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat Genet. 1992 Sep;2(1):26–30. doi: 10.1038/ng0992-26. [DOI] [PubMed] [Google Scholar]
- Wijmenga C., Padberg G. W., Moerer P., Wiegant J., Liem L., Brouwer O. F., Milner E. C., Weber J. L., van Ommen G. B., Sandkuyl L. A. Mapping of facioscapulohumeral muscular dystrophy gene to chromosome 4q35-qter by multipoint linkage analysis and in situ hybridization. Genomics. 1991 Apr;9(4):570–575. doi: 10.1016/0888-7543(91)90348-i. [DOI] [PubMed] [Google Scholar]
- Wijmenga C., Sandkuijl L. A., Moerer P., van der Boorn N., Bodrug S. E., Ray P. N., Brouwer O. F., Murray J. C., van Ommen G. J., Padberg G. W. Genetic linkage map of facioscapulohumeral muscular dystrophy and five polymorphic loci on chromosome 4q35-qter. Am J Hum Genet. 1992 Aug;51(2):411–415. [PMC free article] [PubMed] [Google Scholar]
- Wijmenga C., van Deutekom J. C., Hewitt J. E., Padberg G. W., van Ommen G. J., Hofker M. H., Frants R. R. Pulsed-field gel electrophoresis of the D4F104S1 locus reveals the size and the parental origin of the facioscapulohumeral muscular dystrophy (FSHD)-associated deletions. Genomics. 1994 Jan 1;19(1):21–26. doi: 10.1006/geno.1994.1006. [DOI] [PubMed] [Google Scholar]
- Winokur S. T., Bengtsson U., Feddersen J., Mathews K. D., Weiffenbach B., Bailey H., Markovich R. P., Murray J. C., Wasmuth J. J., Altherr M. R. The DNA rearrangement associated with facioscapulohumeral muscular dystrophy involves a heterochromatin-associated repetitive element: implications for a role of chromatin structure in the pathogenesis of the disease. Chromosome Res. 1994 May;2(3):225–234. doi: 10.1007/BF01553323. [DOI] [PubMed] [Google Scholar]