Abstract
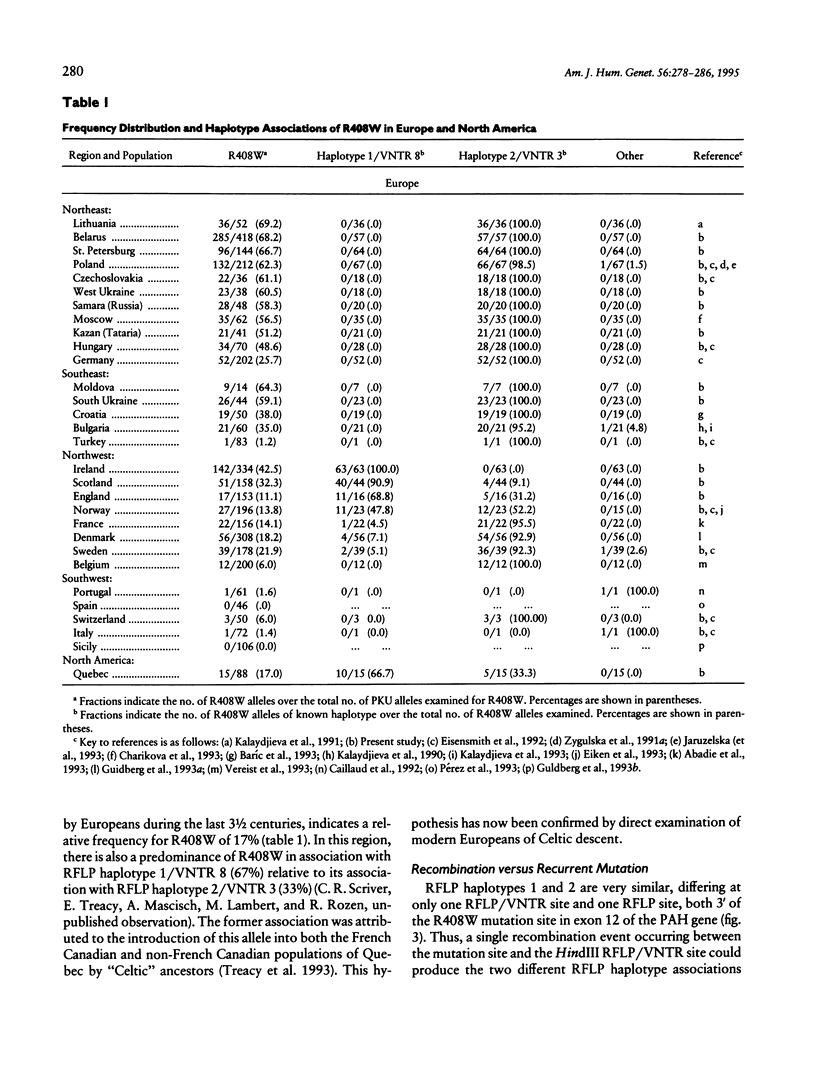
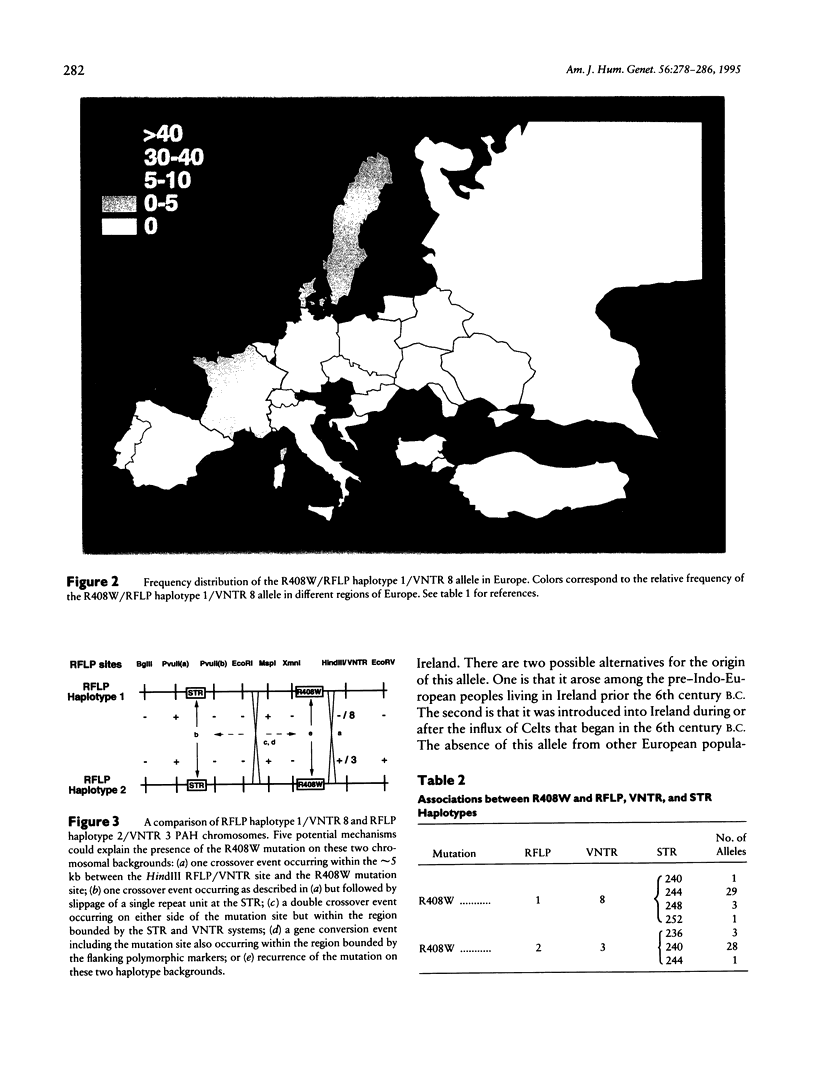
The relative frequency of the common phenylalanine hydroxylase (PAH) mutation R408W and its associations with polymorphic RFLP, VNTR, and short-tandem-repeat (STR) sites in the PAH gene were examined in many European populations and one representative North American population of defined European descent. This mutation was found to cluster in two regions: in northwest Europe among Irish and Scottish peoples, and in eastern Europe, including the Commonwealth of Independent States. This allele was significantly less frequent in intervening populations. In eastern European populations, the R408W mutation is strongly associated with RFLP haplotype 2, the three-copy VNTR allele (VNTR 3), and the 240-bp STR allele. In northwestern European populations, it is strongly associated with RFLP haplotype 1, the VNTR allele containing eight repeats (VNTR 8), and the 244-bp STR allele. An examination of the linkage between the R408W mutation and highly polymorphic RFLP, VNTR, and STR haplotypes suggests that recurrence is the most likely mechanism to account for the two different major haplotype associations of R408W in Europe.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abadie V., Lyonnet S., Maurin N., Berthelon M., Caillaud C., Giraud F., Mattei J. F., Rey J., Rey F., Munnich A. CpG dinucleotides are mutation hot spots in phenylketonuria. Genomics. 1989 Nov;5(4):936–939. doi: 10.1016/0888-7543(89)90137-7. [DOI] [PubMed] [Google Scholar]
- Avigad S., Cohen B. E., Bauer S., Schwartz G., Frydman M., Woo S. L., Niny Y., Shiloh Y. A single origin of phenylketonuria in Yemenite Jews. Nature. 1990 Mar 8;344(6262):168–170. doi: 10.1038/344168a0. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Schurr T. G., Torroni A., Gan Y. Y., Hodge J. A., Hassan K., Chen K. H., Wallace D. C. Southeast Asian mitochondrial DNA analysis reveals genetic continuity of ancient mongoloid migrations. Genetics. 1992 Jan;130(1):139–152. doi: 10.1093/genetics/130.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barić I., Mardesić D., Gjurić G., Sarnavka V., Göbel-Schreiner B., Lichter-Konecki U., Konecki D. S., Trefz F. K. Haplotype distribution and mutations at the PAH locus in Croatia. Hum Genet. 1992 Sep-Oct;90(1-2):155–157. doi: 10.1007/BF00210763. [DOI] [PubMed] [Google Scholar]
- Berthelon M., Caillaud C., Rey F., Labrune P., Melle D., Feingold J., Frézal J., Briard M. L., Farriaux J. P., Guibaud P. Spectrum of phenylketonuria mutations in western Europe and north Africa, and their relation to polymorphic DNA haplotypes at the phenylalanine hydroxylase locus. Hum Genet. 1991 Feb;86(4):355–358. doi: 10.1007/BF00201832. [DOI] [PubMed] [Google Scholar]
- Caillaud C., Vilarinho L., Vilarinho A., Rey F., Berthelon M., Santos R., Lyonnet S., Briard M. L., Osorio R. V., Rey J. Linkage disequilibrium between phenylketonuria and RFLP haplotype 1 at the phenylalanine hydroxylase locus in Portugal. Hum Genet. 1992 Apr;89(1):69–72. doi: 10.1007/BF00207045. [DOI] [PubMed] [Google Scholar]
- Chakraborty R., Lidsky A. S., Daiger S. P., Güttler F., Sullivan S., Dilella A. G., Woo S. L. Polymorphic DNA haplotypes at the human phenylalanine hydroxylase locus and their relationship with phenylketonuria. Hum Genet. 1987 May;76(1):40–46. doi: 10.1007/BF00283048. [DOI] [PubMed] [Google Scholar]
- Charikova E. V., Khalchitskii S. E., Antoshechkin A. G., Schwartz E. I. Distribution of some point mutations in the phenylalanine hydroxylase gene of phenylketonuria patients from the Moscow region. Hum Hered. 1993 Jul-Aug;43(4):244–249. doi: 10.1159/000154138. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Youssoufian H. The CpG dinucleotide and human genetic disease. Hum Genet. 1988 Feb;78(2):151–155. doi: 10.1007/BF00278187. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Huang W. M., Woo S. L. Screening for phenylketonuria mutations by DNA amplification with the polymerase chain reaction. Lancet. 1988 Mar 5;1(8584):497–499. doi: 10.1016/s0140-6736(88)91295-0. [DOI] [PubMed] [Google Scholar]
- DiLella A. G., Marvit J., Brayton K., Woo S. L. An amino-acid substitution involved in phenylketonuria is in linkage disequilibrium with DNA haplotype 2. 1987 May 28-Jun 3Nature. 327(6120):333–336. doi: 10.1038/327333a0. [DOI] [PubMed] [Google Scholar]
- Eiken H. G., Odland E., Boman H., Skjelkvåle L., Engebretsen L. F., Apold J. Application of natural and amplification created restriction sites for the diagnosis of PKU mutations. Nucleic Acids Res. 1991 Apr 11;19(7):1427–1430. doi: 10.1093/nar/19.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiken H. G., Stangeland K., Skjelkvåle L., Knappskog P. M., Boman H., Apold J. PKU mutations R408Q and F299C in Norway: haplotype associations, geographic distributions and phenotype characteristics. Hum Genet. 1992 Mar;88(6):608–612. doi: 10.1007/BF02265283. [DOI] [PubMed] [Google Scholar]
- Eisensmith R. C., Okano Y., Dasovich M., Wang T., Güttler F., Lou H., Guldberg P., Lichter-Konecki U., Konecki D. S., Svensson E. Multiple origins for phenylketonuria in Europe. Am J Hum Genet. 1992 Dec;51(6):1355–1365. [PMC free article] [PubMed] [Google Scholar]
- Eisensmith R. C., Woo S. L. Phenylketonuria and the phenylalanine hydroxylase gene. Mol Biol Med. 1991 Feb;8(1):3–18. [PubMed] [Google Scholar]
- Goltsov A. A., Eisensmith R. C., Naughton E. R., Jin L., Chakraborty R., Woo S. L. A single polymorphic STR system in the human phenylalanine hydroxylase gene permits rapid prenatal diagnosis and carrier screening for phenylketonuria. Hum Mol Genet. 1993 May;2(5):577–581. doi: 10.1093/hmg/2.5.577. [DOI] [PubMed] [Google Scholar]
- Guldberg P., Henriksen K. F., Güttler F. Molecular analysis of phenylketonuria in Denmark: 99% of the mutations detected by denaturing gradient gel electrophoresis. Genomics. 1993 Jul;17(1):141–146. doi: 10.1006/geno.1993.1295. [DOI] [PubMed] [Google Scholar]
- Guldberg P., Romano V., Ceratto N., Bosco P., Ciuna M., Indelicato A., Mollica F., Meli C., Giovannini M., Riva E. Mutational spectrum of phenylalanine hydroxylase deficiency in Sicily: implications for diagnosis of hyperphenylalaninemia in southern Europe. Hum Mol Genet. 1993 Oct;2(10):1703–1707. doi: 10.1093/hmg/2.10.1703. [DOI] [PubMed] [Google Scholar]
- Hirst M. C., Knight S. J., Christodoulou Z., Grewal P. K., Fryns J. P., Davies K. E. Origins of the fragile X syndrome mutation. J Med Genet. 1993 Aug;30(8):647–650. doi: 10.1136/jmg.30.8.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horai S., Kondo R., Nakagawa-Hattori Y., Hayashi S., Sonoda S., Tajima K. Peopling of the Americas, founded by four major lineages of mitochondrial DNA. Mol Biol Evol. 1993 Jan;10(1):23–47. doi: 10.1093/oxfordjournals.molbev.a039987. [DOI] [PubMed] [Google Scholar]
- Ivaschenko T., Baranov V. S. Rapid and efficient PCR/StyI test for identification of common mutation R408W in phenylketonuria patients. J Med Genet. 1993 Feb;30(2):153–154. doi: 10.1136/jmg.30.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruzelska J., Matuszak R., Lyonnet S., Rey F., Rey J., Filipowicz J., Borski K., Munnich A. Genetic background of clinical homogeneity of phenylketonuria in Poland. J Med Genet. 1993 Mar;30(3):232–234. doi: 10.1136/jmg.30.3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John S. W., Rozen R., Laframboise R., Laberge C., Scriver C. R. Novel PKU mutation on haplotype 2 in French-Canadians. Am J Hum Genet. 1989 Dec;45(6):905–909. [PMC free article] [PubMed] [Google Scholar]
- John S. W., Rozen R., Scriver C. R., Laframboise R., Laberge C. Recurrent mutation, gene conversion, or recombination at the human phenylalanine hydroxylase locus: evidence in French-Canadians and a catalog of mutations. Am J Hum Genet. 1990 May;46(5):970–974. [PMC free article] [PubMed] [Google Scholar]
- Kalaydjieva L., Dworniczak B., Aulehla-Scholz C., Kremensky I., Bronzova J., Eigel A., Horst J. Classical phenylketonuria in Bulgaria: RFLP haplotypes and frequency of the major mutations. J Med Genet. 1990 Dec;27(12):742–745. doi: 10.1136/jmg.27.12.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaydjieva L., Dworniczak B., Kucinskas V., Yurgeliavicius V., Kunert E., Horst J. Geographical distribution gradients of the major PKU mutations and the linked haplotypes. Hum Genet. 1991 Feb;86(4):411–413. doi: 10.1007/BF00201847. [DOI] [PubMed] [Google Scholar]
- Lin C. H., Hsiao K. J., Tsai T. F., Chao H. K., Su T. S. Identification of a missense phenylketonuria mutation at codon 408 in Chinese. Hum Genet. 1992 Aug;89(6):593–596. doi: 10.1007/BF00221944. [DOI] [PubMed] [Google Scholar]
- Lyonnet S., Melle D., de Braekeleer M., Laframboise R., Rey F., John S. W., Berthelon M., Berthelot J., Journel H., Le Marec B. Time and space clusters of the French-Canadian M1V phenylketonuria mutation in France. Am J Hum Genet. 1992 Jul;51(1):191–196. [PMC free article] [PubMed] [Google Scholar]
- Matsumoto H. Characteristics of Mongoloid and neighboring populations based on the genetic markers of human immunoglobulins. Hum Genet. 1988 Nov;80(3):207–218. doi: 10.1007/BF01790088. [DOI] [PubMed] [Google Scholar]
- Menozzi P., Piazza A., Cavalli-Sforza L. Synthetic maps of human gene frequencies in Europeans. Science. 1978 Sep 1;201(4358):786–792. doi: 10.1126/science.356262. [DOI] [PubMed] [Google Scholar]
- Morral N., Bertranpetit J., Estivill X., Nunes V., Casals T., Giménez J., Reis A., Varon-Mateeva R., Macek M., Jr, Kalaydjieva L. The origin of the major cystic fibrosis mutation (delta F508) in European populations. Nat Genet. 1994 Jun;7(2):169–175. doi: 10.1038/ng0694-169. [DOI] [PubMed] [Google Scholar]
- Okano Y., Wang T., Eisensmith R. C., Güttler F., Woo S. L. Recurrent mutation in the human phenylalanine hydroxylase gene. Am J Hum Genet. 1990 May;46(5):919–924. [PMC free article] [PubMed] [Google Scholar]
- Piazza A. Who are the Europeans? Science. 1993 Jun 18;260(5115):1767–1769. doi: 10.1126/science.8511584. [DOI] [PubMed] [Google Scholar]
- Pérez B., Desviat L. R., Die M., Ugarte M. Mutation analysis of phenylketonuria in Spain: prevalence of two Mediterranean mutations. Hum Genet. 1992 May;89(3):341–342. doi: 10.1007/BF00220555. [DOI] [PubMed] [Google Scholar]
- Ramus S. J., Forrest S. M., Saleeba J. A., Cotton R. G. CpG hotspot causes second mutation in codon 408 of the phenylalanine hydroxylase gene. Hum Genet. 1992 Sep-Oct;90(1-2):147–148. doi: 10.1007/BF00210760. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Friend K., Kremer E., Hillen D., Staples A., Brown W. T., Goonewardena P., Tarleton J., Schwartz C. Evidence of founder chromosomes in fragile X syndrome. Nat Genet. 1992 Jul;1(4):257–260. doi: 10.1038/ng0792-257. [DOI] [PubMed] [Google Scholar]
- Saugstad L. F. Anthropological significance of phenylketonuria. Clin Genet. 1975 Jan;7(1):52–61. [PubMed] [Google Scholar]
- Schanfield M. S. Immunoglobulin allotypes (GM and KM) indicate multiple founding populations of Native Americans: evidence of at least four migrations to the New World. Hum Biol. 1992 Jun;64(3):381–402. [PubMed] [Google Scholar]
- Sokal R. R., Harding R. M., Oden N. L. Spatial patterns of human gene frequencies in Europe. Am J Phys Anthropol. 1989 Nov;80(3):267–294. doi: 10.1002/ajpa.1330800302. [DOI] [PubMed] [Google Scholar]
- Sokal R. R., Oden N. L., Wilson C. Genetic evidence for the spread of agriculture in Europe by demic diffusion. Nature. 1991 May 9;351(6322):143–145. doi: 10.1038/351143a0. [DOI] [PubMed] [Google Scholar]
- Svensson E., Eisensmith R. C., Dworniczak B., von Döbeln U., Hagenfeldt L., Horst J., Woo S. L. Two missense mutations causing mild hyperphenylalaninemia associated with DNA haplotype 12. Hum Mutat. 1992;1(2):129–137. doi: 10.1002/humu.1380010208. [DOI] [PubMed] [Google Scholar]
- Svensson E., von Döbeln U., Eisensmith R. C., Hagenfeldt L., Woo S. L. Relation between genotype and phenotype in Swedish phenylketonuria and hyperphenylalaninemia patients. Eur J Pediatr. 1993 Feb;152(2):132–139. doi: 10.1007/BF02072490. [DOI] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Cabell M. F., Brown M. D., Neel J. V., Larsen M., Smith D. G., Vullo C. M., Wallace D. C. Asian affinities and continental radiation of the four founding Native American mtDNAs. Am J Hum Genet. 1993 Sep;53(3):563–590. [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Schurr T. G., Yang C. C., Szathmary E. J., Williams R. C., Schanfield M. S., Troup G. A., Knowler W. C., Lawrence D. N., Weiss K. M. Native American mitochondrial DNA analysis indicates that the Amerind and the Nadene populations were founded by two independent migrations. Genetics. 1992 Jan;130(1):153–162. doi: 10.1093/genetics/130.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torroni A., Sukernik R. I., Schurr T. G., Starikorskaya Y. B., Cabell M. F., Crawford M. H., Comuzzie A. G., Wallace D. C. mtDNA variation of aboriginal Siberians reveals distinct genetic affinities with Native Americans. Am J Hum Genet. 1993 Sep;53(3):591–608. [PMC free article] [PubMed] [Google Scholar]
- Treacy E., Byck S., Clow C., Scriver C. R. 'Celtic' phenylketonuria chromosomes found? Evidence in two regions of Quebec Province. Eur J Hum Genet. 1993;1(3):220–228. doi: 10.1159/000472415. [DOI] [PubMed] [Google Scholar]
- Tsai T. F., Hsiao K. J., Su T. S. Phenylketonuria mutation in Chinese haplotype 44 identical with haplotype 2 mutation in northern-European Caucasians. Hum Genet. 1990 Apr;84(5):409–411. doi: 10.1007/BF00195810. [DOI] [PubMed] [Google Scholar]
- Tyfield L. A., Osborn M. J., Holton J. B. Molecular heterogeneity at the phenylalanine hydroxylase locus in the population of the south-west of England. J Med Genet. 1991 Apr;28(4):244–247. doi: 10.1136/jmg.28.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Okano Y., Eisensmith R. C., Harvey M. L., Lo W. H., Huang S. Z., Zeng Y. T., Yuan L. F., Furuyama J. I., Oura T. Founder effect of a prevalent phenylketonuria mutation in the Oriental population. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2146–2150. doi: 10.1073/pnas.88.6.2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T., Okano Y., Eisensmith R. C., Lo W. H., Huang S. Z., Zeng Y. T., Woo S. L. Identification of a novel phenylketonuria (PKU) mutation in the Chinese: further evidence for multiple origins of PKU in Asia. Am J Hum Genet. 1991 Mar;48(3):628–630. [PMC free article] [PubMed] [Google Scholar]
- Wood N., Tyfield L., Bidwell J. Rapid classification of phenylketonuria genotypes by analysis of heteroduplexes generated by PCR-amplifiable synthetic DNA. Hum Mutat. 1993;2(2):131–137. doi: 10.1002/humu.1380020213. [DOI] [PubMed] [Google Scholar]
- Wood S., Schertzer M., Hayden M., Ma Y. Support for founder effect for two lipoprotein lipase (LPL) gene mutations in French Canadians by analysis of GT microsatellites flanking the LPL gene. Hum Genet. 1993 May;91(4):312–316. doi: 10.1007/BF00217348. [DOI] [PubMed] [Google Scholar]
- Zygulska M., Eigel A., Aulehla-Scholz C., Pietrzyk J. J., Horst J. Molecular analysis of PKU haplotypes in the population of southern Poland. Hum Genet. 1991 Jan;86(3):292–294. doi: 10.1007/BF00202412. [DOI] [PubMed] [Google Scholar]
- Zygulska M., Eigel A., Dworniczak B., Sutkowska A., Pietrzyk J. J., Horst J. Phenylketonuria in Poland: 66% of PKU alleles are caused by three mutations. Hum Genet. 1991 Nov;88(1):91–94. doi: 10.1007/BF00204935. [DOI] [PubMed] [Google Scholar]