Abstract
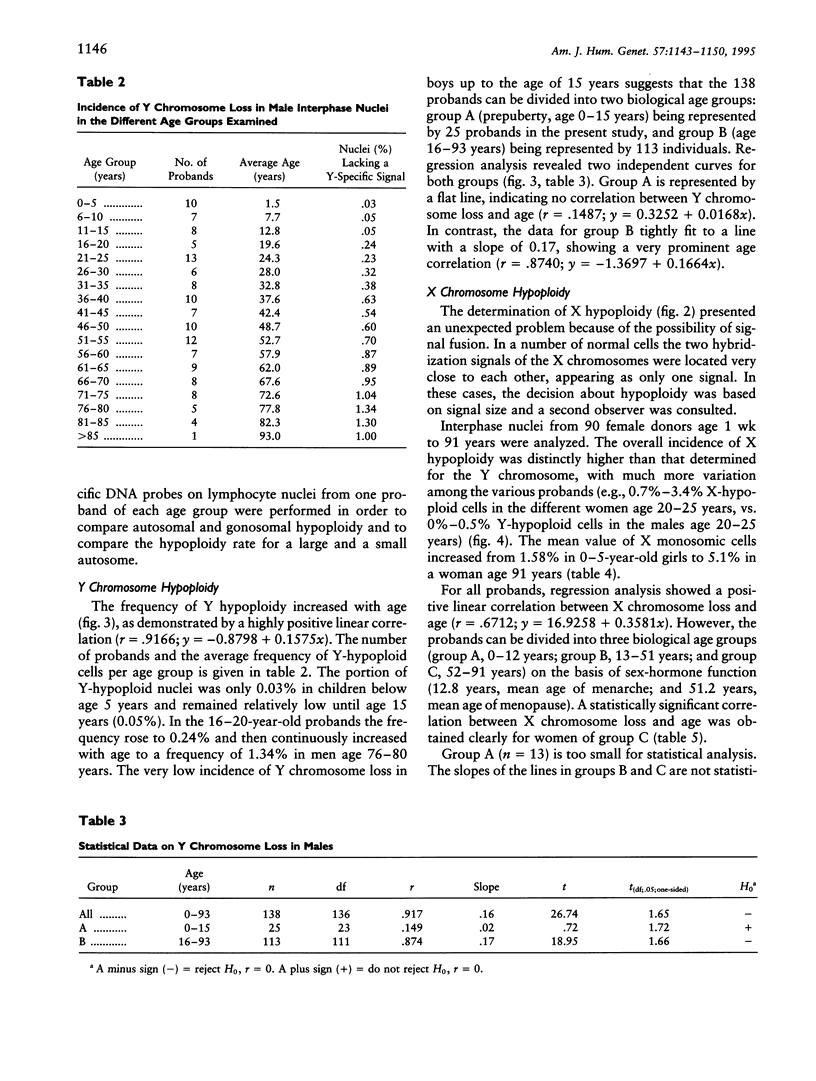
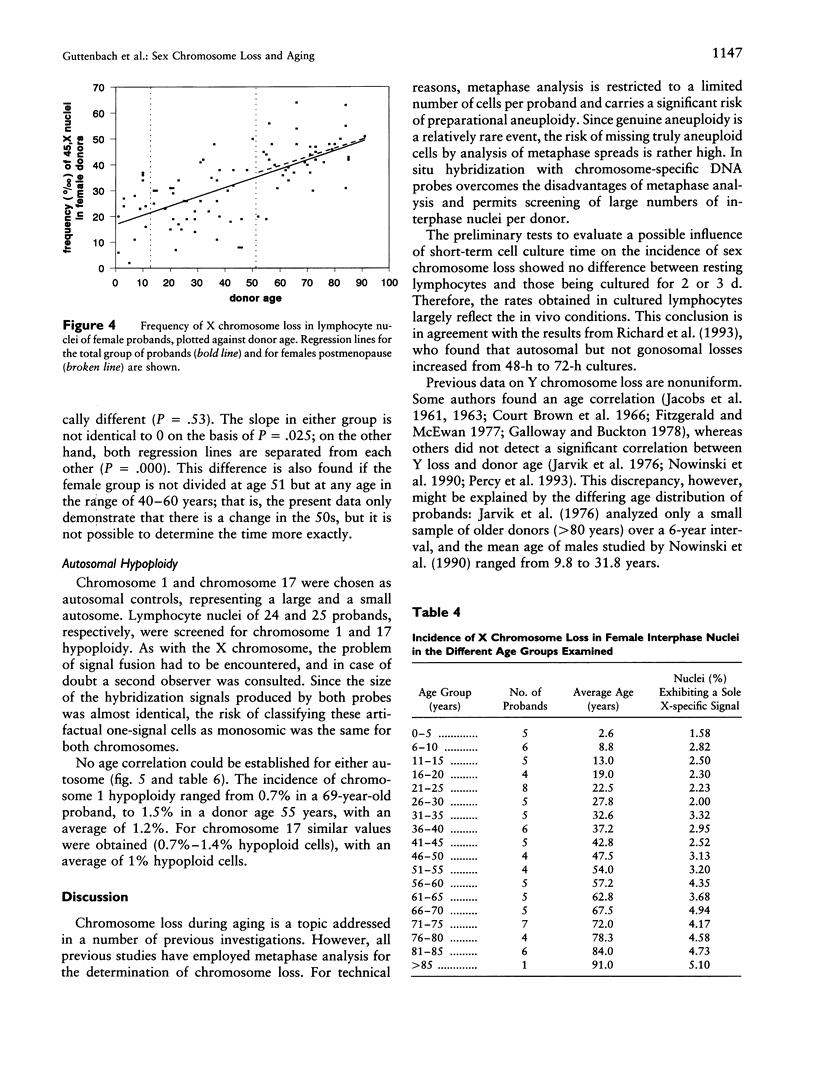
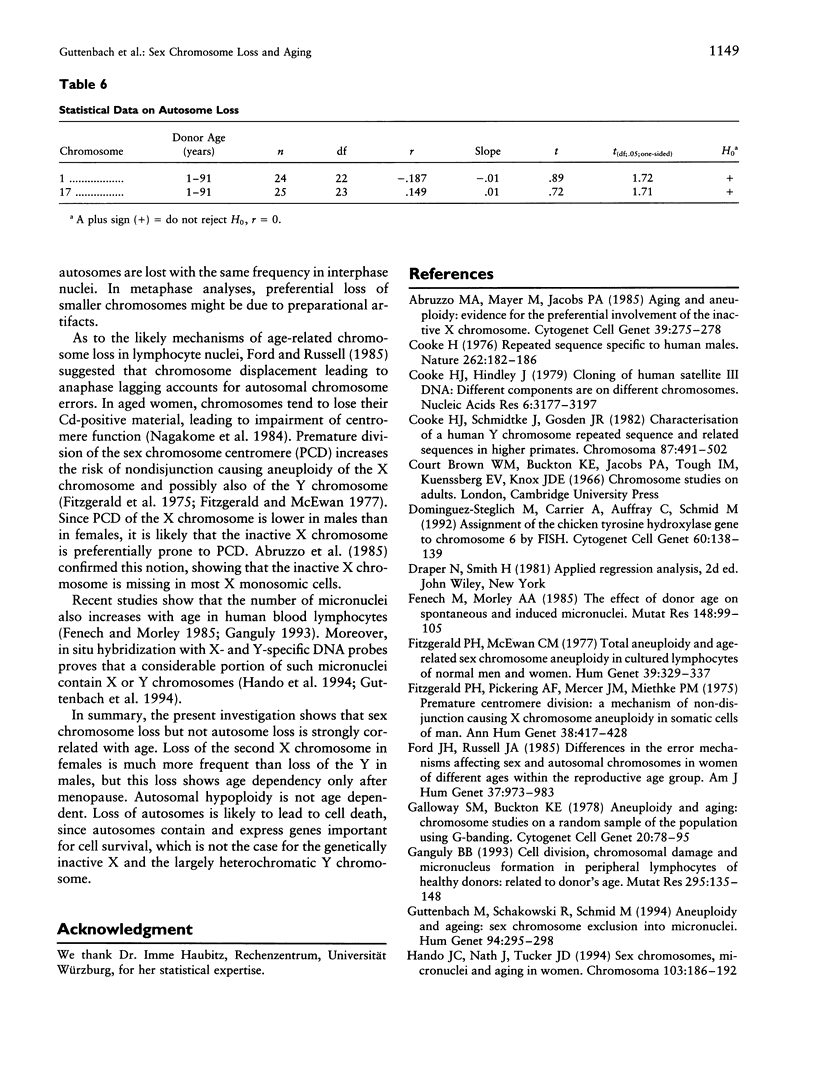
A total of 1,000 lymphocyte interphase nuclei per proband from 90 females and 138 males age 1 wk to 93 years were analyzed by in situ hybridization for loss of the X and Y chromosomes, respectively. Both sex chromosomes showed an age-dependent loss. In males, Y hypoploidy was very low up to age 15 years (0.05%) but continuously increased to a frequency of 1.34% in men age 76-80 years. In females, the baseline level for X chromosome loss is much higher than that seen for the Y chromosome in males. Even prepubertal females show a rate of X chromosome loss, on the order of 1.5%-2.5%, rising to approximately 4.5%-5% in women older than 75 years. Dividing the female probands into three biological age groups on the basis of sex hormone function (< 13 years, 13-51 years, and > 51 years), a significant correlation of X chromosome loss versus age could clearly be demonstrated in women beyond age 51 years. Females age 51-91 years showed monosomy X at a rate from 3.2% to 5.1%. In contrast to sex chromosomal loss, the frequency of autosomal monosomies does not change during the course of aging: Chromosome 1 and chromosome 17 monosomic cells were found with a constant incidence of 1.2% and 1%, respectively. These data also indicate that autosome loss in interphase nuclei is not a function of chromosome size.
Full text
PDF




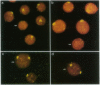
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abruzzo M. A., Mayer M., Jacobs P. A. Aging and aneuploidy: evidence for the preferential involvement of the inactive X chromosome. Cytogenet Cell Genet. 1985;39(4):275–278. doi: 10.1159/000132157. [DOI] [PubMed] [Google Scholar]
- Cooke H. J., Hindley J. Cloning of human satellite III DNA: different components are on different chromosomes. Nucleic Acids Res. 1979 Jul 25;6(10):3177–3197. doi: 10.1093/nar/6.10.3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke H. J., Schmidtke J., Gosden J. R. Characterisation of a human Y chromosome repeated sequence and related sequences in higher primates. Chromosoma. 1982;87(5):491–502. doi: 10.1007/BF00333470. [DOI] [PubMed] [Google Scholar]
- Cooke H. Repeated sequence specific to human males. Nature. 1976 Jul 15;262(5565):182–186. doi: 10.1038/262182a0. [DOI] [PubMed] [Google Scholar]
- Dominguez-Steglich M., Carrier A., Auffray C., Schmid M. Assignment of the chicken tyrosine hydroxylase gene to chromosome 6 by FISH. Cytogenet Cell Genet. 1992;60(2):138–139. doi: 10.1159/000133324. [DOI] [PubMed] [Google Scholar]
- Fenech M., Morley A. A. The effect of donor age on spontaneous and induced micronuclei. Mutat Res. 1985 Jan-Feb;148(1-2):99–105. doi: 10.1016/0027-5107(85)90212-x. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., McEwan C. M. Total aneuploidy and age-related sex chromosome aneuploidy in cultured lymphocytes of normal men and women. Hum Genet. 1977 Dec 23;39(3):329–337. doi: 10.1007/BF00295428. [DOI] [PubMed] [Google Scholar]
- Fitzgerald P. H., Pickering A. F., Mercer J. M., Miethke P. M. Premature centromere division: a mechanism of non-disjunction causing X chromosome aneuploidy in somatic cells of man. Ann Hum Genet. 1975 May;38(4):417–428. doi: 10.1111/j.1469-1809.1975.tb00631.x. [DOI] [PubMed] [Google Scholar]
- Ford J. H., Russell J. A. Differences in the error mechanisms affecting sex and autosomal chromosomes in women of different ages within the reproductive age group. Am J Hum Genet. 1985 Sep;37(5):973–983. [PMC free article] [PubMed] [Google Scholar]
- Galloway S. M., Buckton K. E. Aneuploidy and ageing: chromosome studies on a random sample of the population using G-banding. Cytogenet Cell Genet. 1978;20(1-6):78–95. doi: 10.1159/000130842. [DOI] [PubMed] [Google Scholar]
- Ganguly B. B. Cell division, chromosomal damage and micronucleus formation in peripheral lymphocytes of healthy donors: related to donor's age. Mutat Res. 1993 Aug;295(3):135–148. doi: 10.1016/0921-8734(93)90015-u. [DOI] [PubMed] [Google Scholar]
- Guttenbach M., Schakowski R., Schmid M. Aneuploidy and ageing: sex chromosome exclusion into micronuclei. Hum Genet. 1994 Sep;94(3):295–298. doi: 10.1007/BF00208287. [DOI] [PubMed] [Google Scholar]
- Hando J. C., Nath J., Tucker J. D. Sex chromosomes, micronuclei and aging in women. Chromosoma. 1994 Jun;103(3):186–192. doi: 10.1007/BF00368011. [DOI] [PubMed] [Google Scholar]
- Horsman D. E., Dill F. J., McGillivray B. C., Kalousek D. K. X chromosome aneuploidy in lymphocyte cultures from women with recurrent spontaneous abortions. Am J Med Genet. 1987 Dec;28(4):981–987. doi: 10.1002/ajmg.1320280425. [DOI] [PubMed] [Google Scholar]
- Hunter S., Gramlich T., Abbott K., Varma V. Y chromosome loss in esophageal carcinoma: an in situ hybridization study. Genes Chromosomes Cancer. 1993 Nov;8(3):172–177. doi: 10.1002/gcc.2870080306. [DOI] [PubMed] [Google Scholar]
- JACOBS P. A., BRUNTON M., COURT BROWN W. M., DOLL R., GOLDSTEIN H. Change of human chromosome count distribution with age: evidence for a sex differences. Nature. 1963 Mar 16;197:1080–1081. doi: 10.1038/1971080a0. [DOI] [PubMed] [Google Scholar]
- JACOBS P. A., COURT BROWN W. M., DOLL R. Distribution of human chromosome counts in relation to age. Nature. 1961 Sep 16;191:1178–1180. doi: 10.1038/1911178a0. [DOI] [PubMed] [Google Scholar]
- Jarvik L. F., Yen F. S., Fu T. K., Matsuyama S. S. Chromosomes in old age: a six year longitudinal study. Hum Genet. 1976 Jul 7;33(1):17–22. doi: 10.1007/BF00447282. [DOI] [PubMed] [Google Scholar]
- Kormann-Bortolotto M. H., de Arruda Cardoso Smith M., Toniolo Neto J. Alzheimer's disease and ageing: a chromosomal approach. Gerontology. 1993;39(1):1–6. doi: 10.1159/000213508. [DOI] [PubMed] [Google Scholar]
- Nakagome Y., Abe T., Misawa S., Takeshita T., Iinuma K. The "loss" of centromeres from chromosomes of aged women. Am J Hum Genet. 1984 Mar;36(2):398–404. [PMC free article] [PubMed] [Google Scholar]
- Neurath P., DeRemer K., Bell B., Jarvik, Kato T. Chromosome loss compared with chromosome size, age and sex of subjects. Nature. 1970 Jan 17;225(5229):281–282. doi: 10.1038/225281a0. [DOI] [PubMed] [Google Scholar]
- Nowinski G. P., Van Dyke D. L., Tilley B. C., Jacobsen G., Babu V. R., Worsham M. J., Wilson G. N., Weiss L. The frequency of aneuploidy in cultured lymphocytes is correlated with age and gender but not with reproductive history. Am J Hum Genet. 1990 Jun;46(6):1101–1111. [PMC free article] [PubMed] [Google Scholar]
- Percy M. E., Markovic V. D., Dalton A. J., McLachlan D. R., Berg J. M., Rusk A. C., Somerville M. J., Chodakowski B., Andrews D. F. Age-associated chromosome 21 loss in Down syndrome: possible relevance to mosaicism and Alzheimer disease. Am J Med Genet. 1993 Mar 1;45(5):584–588. doi: 10.1002/ajmg.1320450513. [DOI] [PubMed] [Google Scholar]
- Richard F., Aurias A., Couturier J., Dutrillaux A. M., Flüry-Hérard A., Gerbault-Seureau M., Hoffschir F., Lamoliatte E., Lefrançois D., Lombard M. Aneuploidy in human lymphocytes: an extensive study of eight individuals of various ages. Mutat Res. 1993 Mar;295(2):71–80. doi: 10.1016/0921-8734(93)90003-l. [DOI] [PubMed] [Google Scholar]
- Riske C. B., Morgan R., Ondreyco S., Sandberg A. A. X and Y chromosome loss as sole abnormality in acute non-lymphocytic leukemia (ANLL) Cancer Genet Cytogenet. 1994 Jan;72(1):44–47. doi: 10.1016/0165-4608(94)90108-2. [DOI] [PubMed] [Google Scholar]
- Schmid M., Guttenbach M., Nanda I., Studer R., Epplen J. T. Organization of DYZ2 repetitive DNA on the human Y chromosome. Genomics. 1990 Feb;6(2):212–218. doi: 10.1016/0888-7543(90)90559-d. [DOI] [PubMed] [Google Scholar]
- Vaziri H., Schächter F., Uchida I., Wei L., Zhu X., Effros R., Cohen D., Harley C. B. Loss of telomeric DNA during aging of normal and trisomy 21 human lymphocytes. Am J Hum Genet. 1993 Apr;52(4):661–667. [PMC free article] [PubMed] [Google Scholar]
- Wenger S. L., Golden W. L., Dennis S. P., Steele M. W. Are the occasional aneuploid cells in peripheral blood cultures significant? Am J Med Genet. 1984 Dec;19(4):715–719. doi: 10.1002/ajmg.1320190411. [DOI] [PubMed] [Google Scholar]
- Wheeler W. J., Cherry L. M., Downs T., Hsu T. C. Mitotic inhibition and aneuploidy induction by naturally occurring and synthetic estrogens in Chinese hamster cells in vitro. Mutat Res. 1986 Jul;171(1):31–41. doi: 10.1016/0165-1218(86)90006-6. [DOI] [PubMed] [Google Scholar]
- Willard H. F., Smith K. D., Sutherland J. Isolation and characterization of a major tandem repeat family from the human X chromosome. Nucleic Acids Res. 1983 Apr 11;11(7):2017–2033. doi: 10.1093/nar/11.7.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang T. P., Hansen S. K., Oishi K. K., Ryder O. A., Hamkalo B. A. Characterization of a cloned repetitive DNA sequence concentrated on the human X chromosome. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6593–6597. doi: 10.1073/pnas.79.21.6593. [DOI] [PMC free article] [PubMed] [Google Scholar]