Abstract
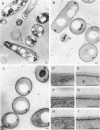
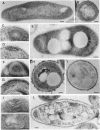
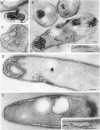
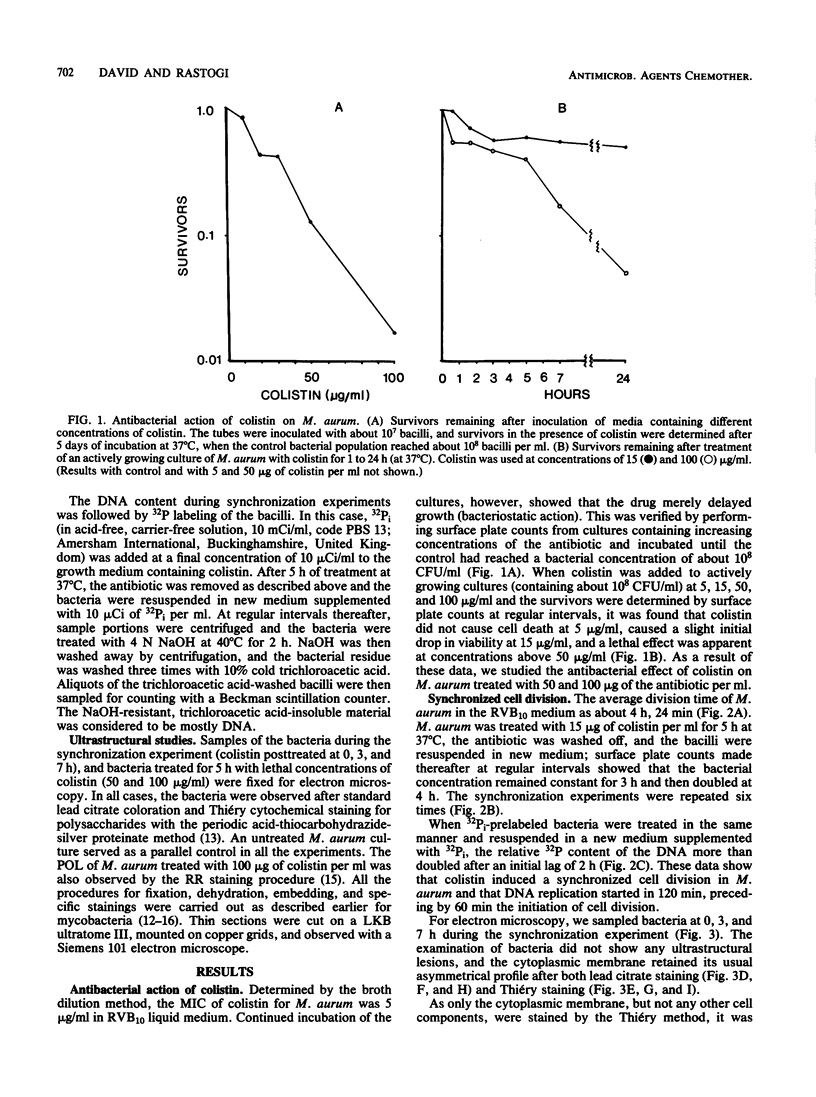
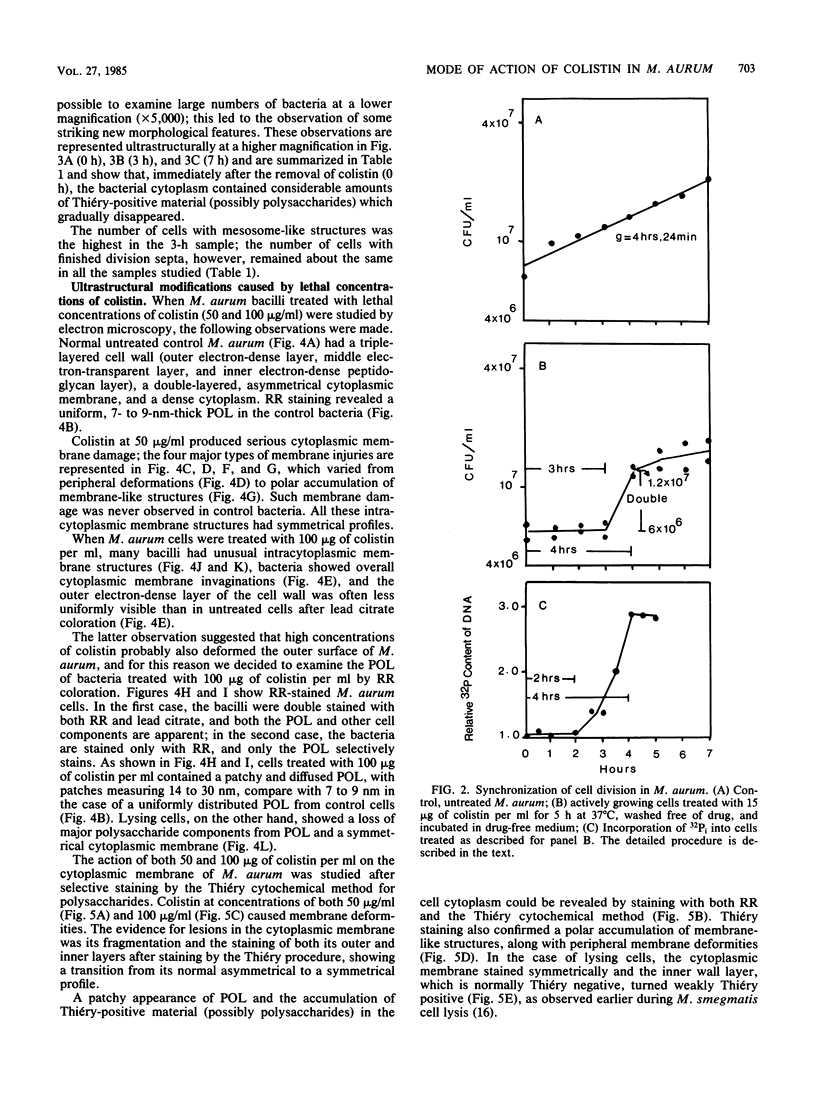
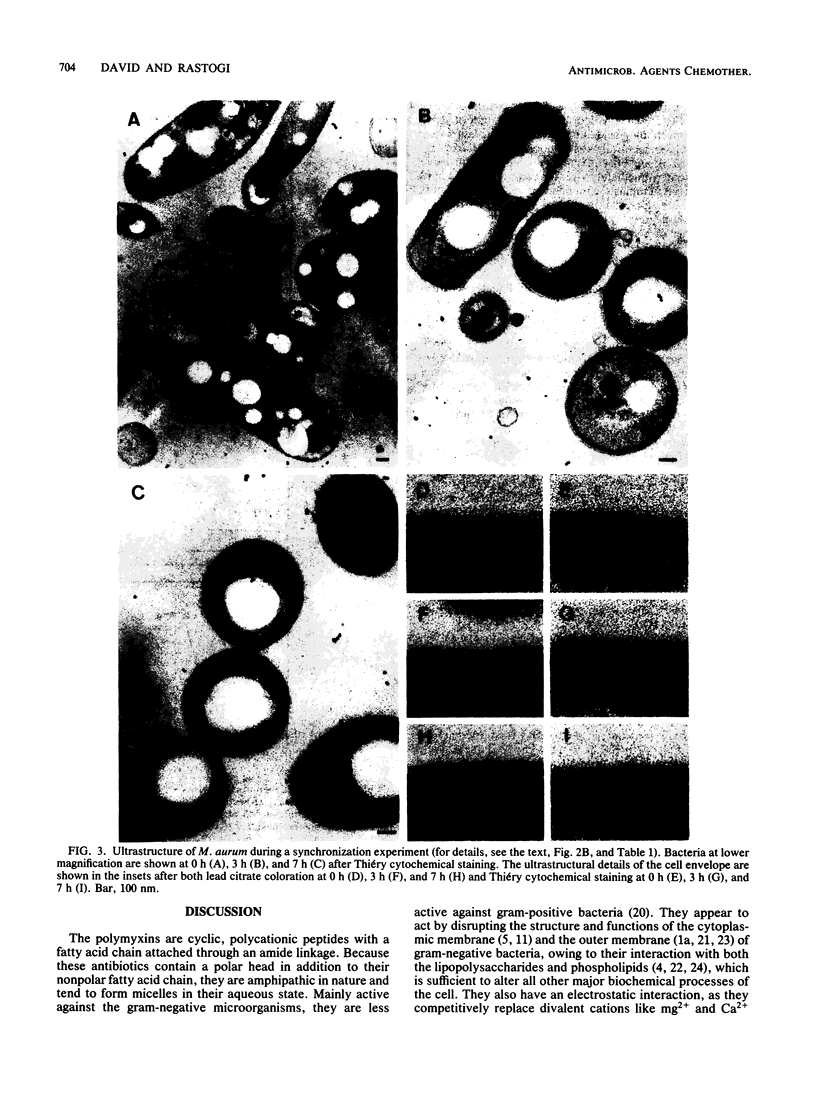
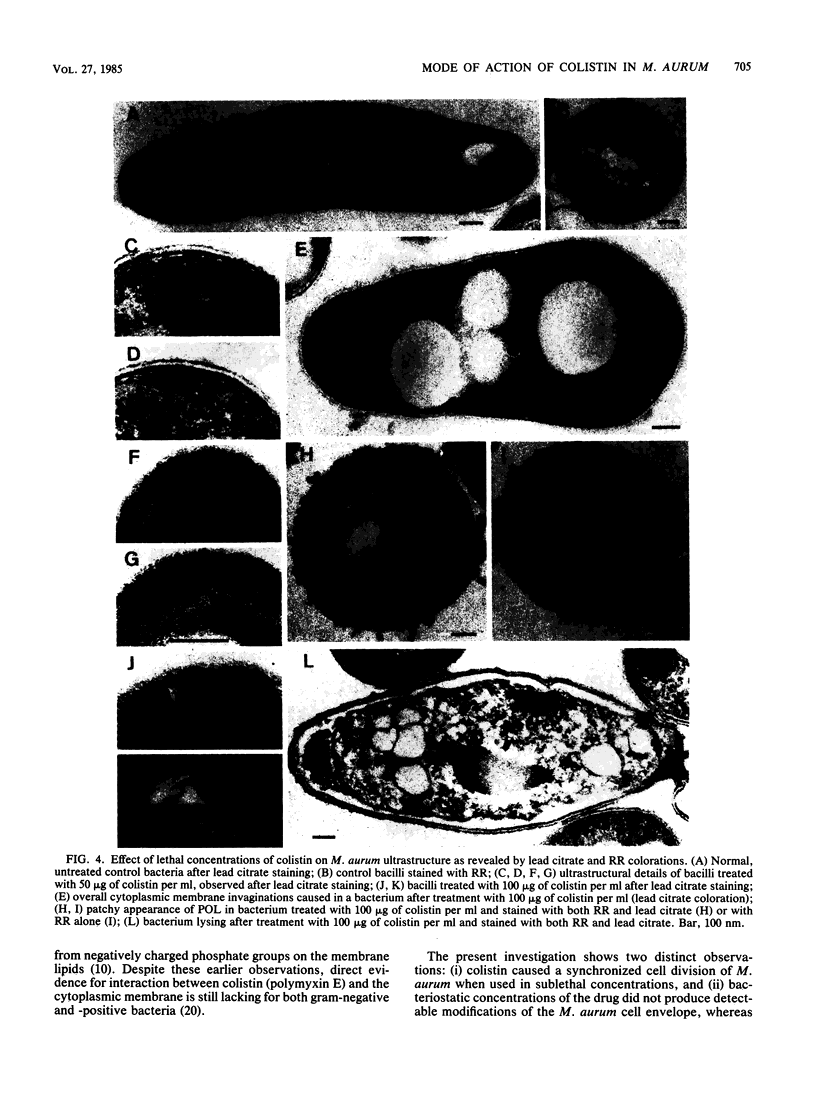
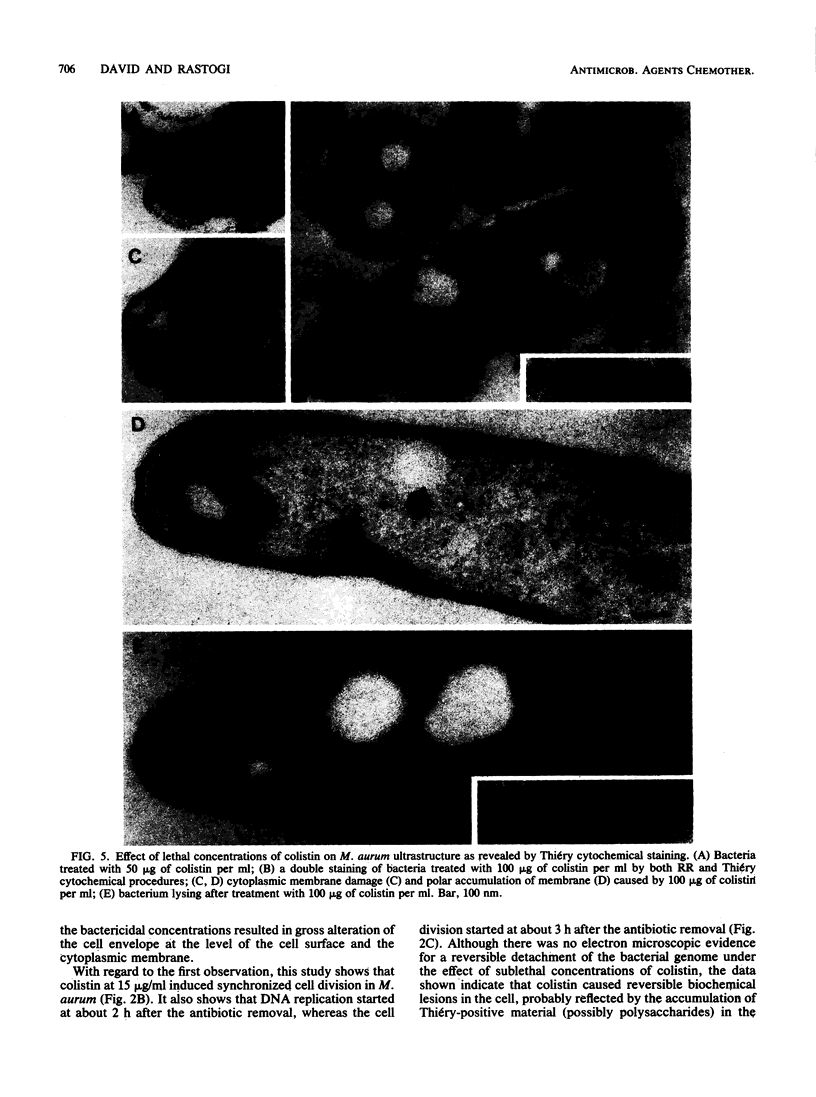
Mycobacterium aurum was susceptible to the antibiotic colistin (polymyxin E),which had an MIC of 5 micrograms/ml and an apparent bactericidal effect at concentrations above 50 micrograms/ml. Treatment of actively growing cells with sublethal concentrations of colistin (15 micrograms/ml) resulted in synchronized cell division once the antibiotic was removed. Under conditions of synchronized cell growth, one cycle of DNA replication lasted 120 min and one cycle of cell division lasted about 180 min. Although the antibiotic treatment during synchronization experiments did not produce apparent changes in the bacterial envelope, it was accompanied by the accumulation of a polysaccharide-like substance in the bacterial cytoplasm which gradually decreased after the removal of antibiotic and by an increase in the number of mesosomes at 3 h after antibiotic removal. This step was closely linked to the doubling time of bacteria. Lethal concentrations of colistin of 50 and 100 micrograms/ml, which caused about 90 and 99% cell death, respectively, produced significant cytoplasmic membrane injuries, patchy appearance of the cell wall outer polysaccharide layer, and little cell lysis. These data indicate that the cytoplasmic membrane is a site of action of colistin and raise a question as to whether an outer bilayer exists in mycobacteria, at least functionally.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti E. L., Dunia I., Ludosky M. A., Nguyen V. M., Dang D. T., Rastogi N., David H. L. Freeze-etching and freeze-fracture structural features of cell envelopes in mycobacteria and leprosy derived corynebacteria. Acta Leprol. 1984 Oct-Dec;2(2-4):237–248. [PubMed] [Google Scholar]
- Cerny G., Teuber M. Differential release of periplasmic versus cytoplasmic enzymes from Escherichia coli B by polymixin B. Arch Mikrobiol. 1971;78(2):166–179. doi: 10.1007/BF00424873. [DOI] [PubMed] [Google Scholar]
- David H. L., Clavel S., Clement F., Moniz-Pereira J. Effects of antituberculosis and antileprosy drugs on mycobacteriophage D29 growth. Antimicrob Agents Chemother. 1980 Aug;18(2):357–359. doi: 10.1128/aac.18.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delius H., Worcel A. Letter: Electron microscopic visualization of the folded chromosome of Escherichia coli. J Mol Biol. 1974 Jan 5;82(1):107–109. doi: 10.1016/0022-2836(74)90577-4. [DOI] [PubMed] [Google Scholar]
- HsuChen C. C., Feingold D. S. The mechanism of polymyxin B action and selectivity toward biologic membranes. Biochemistry. 1973 May 22;12(11):2105–2111. doi: 10.1021/bi00735a014. [DOI] [PubMed] [Google Scholar]
- Klemperer R. M., Gilbert P., Meier A. M., Cozens R. M., Brown M. R. Influence of suspending media upon the susceptibility of Pseudomonas aeruginosa NCTC 6750 and its spheroplasts to polymyxin B. Antimicrob Agents Chemother. 1979 Feb;15(2):147–151. doi: 10.1128/aac.15.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike M., Iida K. Effect of polymyxin on the bacteriophage receptors of the cell walls of gram-negative bacteria. J Bacteriol. 1971 Dec;108(3):1402–1411. doi: 10.1128/jb.108.3.1402-1411.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes J., Inniss W. E. Electron microscopy of effect of polymyxin on Escherichia coli lipopolysaccharide. J Bacteriol. 1969 Nov;100(2):1128–1129. doi: 10.1128/jb.100.2.1128-1130.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEWTON B. A. Reversal of the antibacterial activity of polymyxin by divalent cations. Nature. 1953 Jul 25;172(4369):160–161. [PubMed] [Google Scholar]
- NEWTON B. A. The properties and mode of action of the polymyxins. Bacteriol Rev. 1956 Mar;20(1):14–27. doi: 10.1128/br.20.1.14-27.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard B., Frehel C., Rastogi N. Cytochemical characterization of mycobacterial outer surfaces. Acta Leprol. 1984 Oct-Dec;2(2-4):227–235. [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., David H. L. Comparative ultrastructure of Mycobacterium leprae and M. avium grown in experimental hosts. Ann Microbiol (Paris) 1982 Jul-Aug;133(1):109–128. [PubMed] [Google Scholar]
- Rastogi N., Frehel C., Ryter A., Ohayon H., Lesourd M., David H. L. Multiple drug resistance in Mycobacterium avium: is the wall architecture responsible for exclusion of antimicrobial agents? Antimicrob Agents Chemother. 1981 Nov;20(5):666–677. doi: 10.1128/aac.20.5.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond W. B., Ward D. M. Media and methods for phage-typing mycobacteria. Bull World Health Organ. 1966;35(4):563–568. [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. T., Macedo P. M. The interpretation of the ultrastructure of mycobacterial cells in transmission electron microscopy of ultrathin sections. Int J Lepr Other Mycobact Dis. 1983 Jun;51(2):225–234. [PubMed] [Google Scholar]
- Storm D. R., Rosenthal K. S., Swanson P. E. Polymyxin and related peptide antibiotics. Annu Rev Biochem. 1977;46:723–763. doi: 10.1146/annurev.bi.46.070177.003451. [DOI] [PubMed] [Google Scholar]
- Teuber M. Lysozyme-dependent production of spheroplast-like bodies from polymyxin B-treated Salmonella typhimurium. Arch Mikrobiol. 1970;70(2):139–146. doi: 10.1007/BF00412204. [DOI] [PubMed] [Google Scholar]
- WARREN G. H., GRAY J., YURCHENCO J. A. Effect of polymyxin on the lysis of Neisseria catarrhalis by lysozyme. J Bacteriol. 1957 Dec;74(6):788–793. doi: 10.1128/jb.74.6.788-793.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]