Abstract
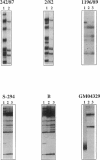
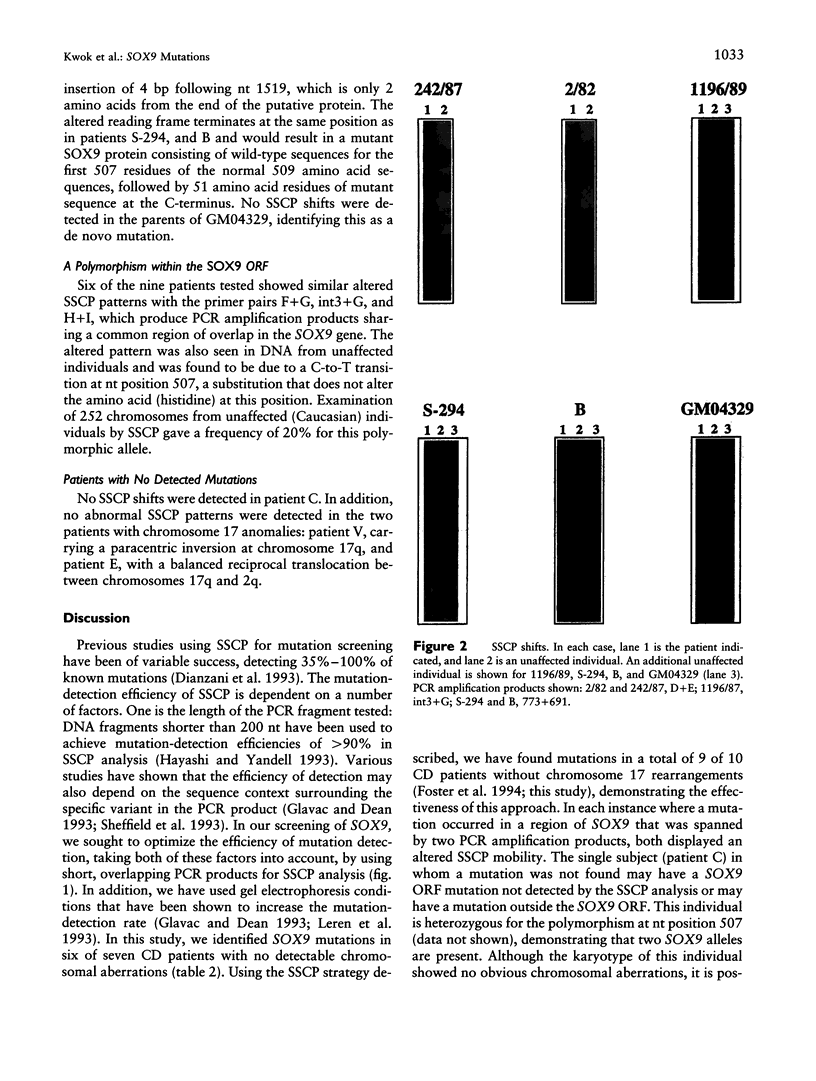
Campomelic dysplasia (CD) is a skeletal malformation syndrome frequently accompanied by 46,XY sex reversal. A mutation-screening strategy using SSCP was employed to identify mutations in SOX9, the chromosome 17q24 gene responsible for CD and autosomal sex reversal in man. We have screened seven CD patients with no cytologically detectable chromosomal aberrations and two CD patients with chromosome 17 rearrangements for mutations in the entire open reading frame of SOX9. Five different mutations have been identified in six CD patients: two missense mutations in the SOX9 putative DNA binding domain (high mobility group, or HMG, box); three frameshift mutations and a splice-acceptor mutation. An identical frameshift mutation is found in two unrelated 46,XY patients, one exhibiting a male phenotype and the other displaying a female phenotype (XY sex reversal). All mutations found affect a single allele, which is consistent with a dominant mode of inheritance. No mutations were found in the SOX9 open reading frame of two patients with chromosome 17q rearrangements, suggesting that the translocations affect SOX9 expression. These findings are consistent with the hypothesis that CD results from haploinsufficiency of SOX9.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camera G., Mastroiacovo P. Birth prevalence of skeletal dysplasias in the Italian Multicentric Monitoring System for Birth Defects. Prog Clin Biol Res. 1982;104:441–449. [PubMed] [Google Scholar]
- Cooke C. T., Mulcahy M. T., Cullity G. J., Watson M., Srague P. Campomelic dysplasia with sex reversal: morphological and cytogenetic studies of a case. Pathology. 1985 Jul;17(3):526–529. doi: 10.3109/00313028509105515. [DOI] [PubMed] [Google Scholar]
- Dianzani I., Camaschella C., Ponzone A., Cotton R. G. Dilemmas and progress in mutation detection. Trends Genet. 1993 Dec;9(12):403–405. doi: 10.1016/0168-9525(93)90100-v. [DOI] [PubMed] [Google Scholar]
- Foster J. W., Dominguez-Steglich M. A., Guioli S., Kwok C., Weller P. A., Stevanović M., Weissenbach J., Mansour S., Young I. D., Goodfellow P. N. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994 Dec 8;372(6506):525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- Glavac D., Dean M. Optimization of the single-strand conformation polymorphism (SSCP) technique for detection of point mutations. Hum Mutat. 1993;2(5):404–414. doi: 10.1002/humu.1380020513. [DOI] [PubMed] [Google Scholar]
- Goodfellow P. N., Lovell-Badge R. SRY and sex determination in mammals. Annu Rev Genet. 1993;27:71–92. doi: 10.1146/annurev.ge.27.120193.000443. [DOI] [PubMed] [Google Scholar]
- Harley V. R., Goodfellow P. N. The biochemical role of SRY in sex determination. Mol Reprod Dev. 1994 Oct;39(2):184–193. doi: 10.1002/mrd.1080390211. [DOI] [PubMed] [Google Scholar]
- Hawkins J. R. Sex determination. Hum Mol Genet. 1994;3(Spec No):1463–1467. doi: 10.1093/hmg/3.suppl_1.1463. [DOI] [PubMed] [Google Scholar]
- Hayashi K., Yandell D. W. How sensitive is PCR-SSCP? Hum Mutat. 1993;2(5):338–346. doi: 10.1002/humu.1380020503. [DOI] [PubMed] [Google Scholar]
- Hoefnagel D., Wuster-Hill D. H., Dupree W. B., Benirschke K., Fuld G. L. Camptomelic dwarfism associated with XY-gonadal dysgenesis and chromosome anomalies. Clin Genet. 1978 Jun;13(6):489–499. doi: 10.1111/j.1399-0004.1978.tb01204.x. [DOI] [PubMed] [Google Scholar]
- Houston C. S., Opitz J. M., Spranger J. W., Macpherson R. I., Reed M. H., Gilbert E. F., Herrmann J., Schinzel A. The campomelic syndrome: review, report of 17 cases, and follow-up on the currently 17-year-old boy first reported by Maroteaux et al in 1971. Am J Med Genet. 1983 May;15(1):3–28. doi: 10.1002/ajmg.1320150103. [DOI] [PubMed] [Google Scholar]
- Hovmöller M. L., Osuna A., Eklöf O., Fredga K., Hjerpe A., Linsten J., Ritzen M., Stanescu V., Svenningsen N. Camptomelic dwarfism. A genetically determined mesenchymal disorder combined with sex reversal. Hereditas. 1977;86(1):51–62. doi: 10.1111/j.1601-5223.1977.tb01212.x. [DOI] [PubMed] [Google Scholar]
- Jäger R. J., Harley V. R., Pfeiffer R. A., Goodfellow P. N., Scherer G. A familial mutation in the testis-determining gene SRY shared by both sexes. Hum Genet. 1992 Dec;90(4):350–355. doi: 10.1007/BF00220457. [DOI] [PubMed] [Google Scholar]
- Laudet V., Stehelin D., Clevers H. Ancestry and diversity of the HMG box superfamily. Nucleic Acids Res. 1993 May 25;21(10):2493–2501. doi: 10.1093/nar/21.10.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leren T. P., Solberg K., Rødningen O. K., Ose L., Tonstad S., Berg K. Evaluation of running conditions for SSCP analysis: application of SSCP for detection of point mutations in the LDL receptor gene. PCR Methods Appl. 1993 Dec;3(3):159–162. doi: 10.1101/gr.3.3.159. [DOI] [PubMed] [Google Scholar]
- Mansour S., Hall C. M., Pembrey M. E., Young I. D. A clinical and genetic study of campomelic dysplasia. J Med Genet. 1995 Jun;32(6):415–420. doi: 10.1136/jmg.32.6.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maraia R., Saal H. M., Wangsa D. A chromosome 17q de novo paracentric inversion in a patient with campomelic dysplasia; case report and etiologic hypothesis. Clin Genet. 1991 Jun;39(6):401–408. doi: 10.1111/j.1399-0004.1991.tb03050.x. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Tjian R. Transcriptional regulation in mammalian cells by sequence-specific DNA binding proteins. Science. 1989 Jul 28;245(4916):371–378. doi: 10.1126/science.2667136. [DOI] [PubMed] [Google Scholar]
- Nasrin N., Buggs C., Kong X. F., Carnazza J., Goebl M., Alexander-Bridges M. DNA-binding properties of the product of the testis-determining gene and a related protein. Nature. 1991 Nov 28;354(6351):317–320. doi: 10.1038/354317a0. [DOI] [PubMed] [Google Scholar]
- Normann E. K., Pedersen J. C., Stiris G., van der Hagen C. B. Campomelic dysplasia--an underdiagnosed condition? Eur J Pediatr. 1993 Apr;152(4):331–333. doi: 10.1007/BF01956747. [DOI] [PubMed] [Google Scholar]
- Poulat F., Girard F., Chevron M. P., Gozé C., Rebillard X., Calas B., Lamb N., Berta P. Nuclear localization of the testis determining gene product SRY. J Cell Biol. 1995 Mar;128(5):737–748. doi: 10.1083/jcb.128.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Beck J. S., Kwitek A. E., Sandstrom D. W., Stone E. M. The sensitivity of single-strand conformation polymorphism analysis for the detection of single base substitutions. Genomics. 1993 May;16(2):325–332. doi: 10.1006/geno.1993.1193. [DOI] [PubMed] [Google Scholar]
- Tommerup N., Schempp W., Meinecke P., Pedersen S., Bolund L., Brandt C., Goodpasture C., Guldberg P., Held K. R., Reinwein H. Assignment of an autosomal sex reversal locus (SRA1) and campomelic dysplasia (CMPD1) to 17q24.3-q25.1. Nat Genet. 1993 Jun;4(2):170–174. doi: 10.1038/ng0693-170. [DOI] [PubMed] [Google Scholar]
- Wagner T., Wirth J., Meyer J., Zabel B., Held M., Zimmer J., Pasantes J., Bricarelli F. D., Keutel J., Hustert E. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell. 1994 Dec 16;79(6):1111–1120. doi: 10.1016/0092-8674(94)90041-8. [DOI] [PubMed] [Google Scholar]
- Wright E., Hargrave M. R., Christiansen J., Cooper L., Kun J., Evans T., Gangadharan U., Greenfield A., Koopman P. The Sry-related gene Sox9 is expressed during chondrogenesis in mouse embryos. Nat Genet. 1995 Jan;9(1):15–20. doi: 10.1038/ng0195-15. [DOI] [PubMed] [Google Scholar]
- Young I. D., Zuccollo J. M., Maltby E. L., Broderick N. J. Campomelic dysplasia associated with a de novo 2q;17q reciprocal translocation. J Med Genet. 1992 Apr;29(4):251–252. doi: 10.1136/jmg.29.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]