Abstract
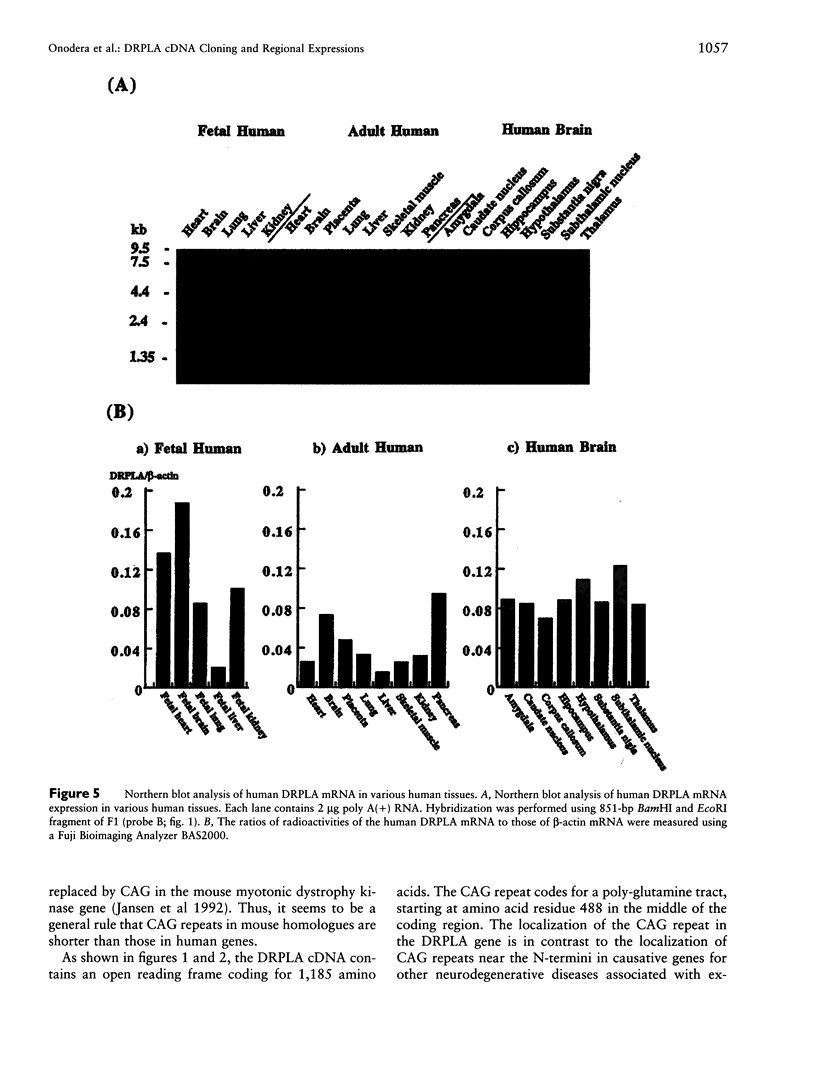
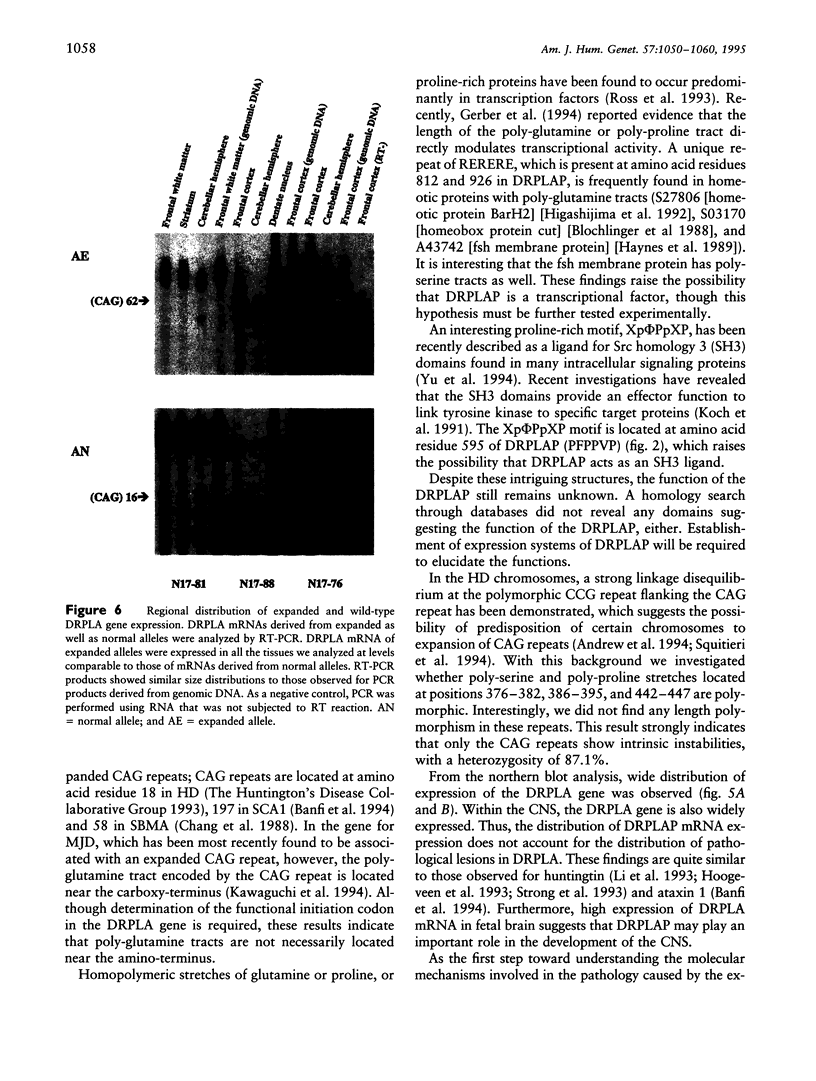
Dentatorubral-pallidoluysian atrophy (DRPLA) is an autosomal dominant neurodegenerative disorder characterized by genetic anticipation and variable combinations of symptoms including myoclonus, epilepsy, cerebellar ataxia, choreoathetosis, and dementia. Recently, we discovered that DRPLA is caused by unstable expansion of a CAG repeat of a gene on the short arm of chromosome 12. We determined the consensus DRPLA cDNA sequence containing the complete coding region for 1,185 amino acids. The CAG repeat, which is expanded in DRPLA, is located 1,462 bp downstream from the putative methionine initiation codon and encodes a poly-glutamine tract. Although poly-serine and proline tracts exist near the CAG repeats, these polyserine or proline tracts did not show any polymorphisms, which is in strong contrast to the high heterogeneity in the length of the CAG repeat. Northern blot analysis revealed a 4.7-kb transcript that is widely expressed in various tissues including heart, lung, kidney, placenta, skeletal muscle, and brain. Reverse transcription-PCR analysis revealed that the expanded alleles are transcribed to levels comparable to those of normal alleles. These results indicate that there is no difference in transcriptional efficiency between expanded and normal alleles. Furthermore, mRNA from cerebellar hemispheres of DRPLA patients showed smaller sizes of CAG repeats compared with other regions of the brain, which reflects somatic mosaicism of the expanded alleles of the DRPLA gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams M. D., Dubnick M., Kerlavage A. R., Moreno R., Kelley J. M., Utterback T. R., Nagle J. W., Fields C., Venter J. C. Sequence identification of 2,375 human brain genes. Nature. 1992 Feb 13;355(6361):632–634. doi: 10.1038/355632a0. [DOI] [PubMed] [Google Scholar]
- Amemiya Y., Miyahara J. Imaging plate illuminates many fields. Nature. 1988 Nov 3;336(6194):89–90. doi: 10.1038/336089a0. [DOI] [PubMed] [Google Scholar]
- Andrew S. E., Goldberg Y. P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M. A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington's disease. Nat Genet. 1993 Aug;4(4):398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- Andrew S. E., Goldberg Y. P., Theilmann J., Zeisler J., Hayden M. R. A CCG repeat polymorphism adjacent to the CAG repeat in the Huntington disease gene: implications for diagnostic accuracy and predictive testing. Hum Mol Genet. 1994 Jan;3(1):65–67. doi: 10.1093/hmg/3.1.65. [DOI] [PubMed] [Google Scholar]
- Ashley C. T., Sutcliffe J. S., Kunst C. B., Leiner H. A., Eichler E. E., Nelson D. L., Warren S. T. Human and murine FMR-1: alternative splicing and translational initiation downstream of the CGG-repeat. Nat Genet. 1993 Jul;4(3):244–251. doi: 10.1038/ng0793-244. [DOI] [PubMed] [Google Scholar]
- Banfi S., Servadio A., Chung M. Y., Kwiatkowski T. J., Jr, McCall A. E., Duvick L. A., Shen Y., Roth E. J., Orr H. T., Zoghbi H. Y. Identification and characterization of the gene causing type 1 spinocerebellar ataxia. Nat Genet. 1994 Aug;7(4):513–520. doi: 10.1038/ng0894-513. [DOI] [PubMed] [Google Scholar]
- Blochlinger K., Bodmer R., Jack J., Jan L. Y., Jan Y. N. Primary structure and expression of a product from cut, a locus involved in specifying sensory organ identity in Drosophila. Nature. 1988 Jun 16;333(6174):629–635. doi: 10.1038/333629a0. [DOI] [PubMed] [Google Scholar]
- Chang C. S., Kokontis J., Liao S. T. Molecular cloning of human and rat complementary DNA encoding androgen receptors. Science. 1988 Apr 15;240(4850):324–326. doi: 10.1126/science.3353726. [DOI] [PubMed] [Google Scholar]
- Chen E. Y., Seeburg P. H. Supercoil sequencing: a fast and simple method for sequencing plasmid DNA. DNA. 1985 Apr;4(2):165–170. doi: 10.1089/dna.1985.4.165. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Duyao M., Ambrose C., Myers R., Novelletto A., Persichetti F., Frontali M., Folstein S., Ross C., Franz M., Abbott M. Trinucleotide repeat length instability and age of onset in Huntington's disease. Nat Genet. 1993 Aug;4(4):387–392. doi: 10.1038/ng0893-387. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Gerber H. P., Seipel K., Georgiev O., Höfferer M., Hug M., Rusconi S., Schaffner W. Transcriptional activation modulated by homopolymeric glutamine and proline stretches. Science. 1994 Feb 11;263(5148):808–811. doi: 10.1126/science.8303297. [DOI] [PubMed] [Google Scholar]
- Haynes S. R., Mozer B. A., Bhatia-Dey N., Dawid I. B. The Drosophila fsh locus, a maternal effect homeotic gene, encodes apparent membrane proteins. Dev Biol. 1989 Jul;134(1):246–257. doi: 10.1016/0012-1606(89)90094-8. [DOI] [PubMed] [Google Scholar]
- He W. W., Fischer L. M., Sun S., Bilhartz D. L., Zhu X. P., Young C. Y., Kelley D. B., Tindall D. J. Molecular cloning of androgen receptors from divergent species with a polymerase chain reaction technique: complete cDNA sequence of the mouse androgen receptor and isolation of androgen receptor cDNA probes from dog, guinea pig and clawed frog. Biochem Biophys Res Commun. 1990 Sep 14;171(2):697–704. doi: 10.1016/0006-291x(90)91202-4. [DOI] [PubMed] [Google Scholar]
- Higashijima S., Kojima T., Michiue T., Ishimaru S., Emori Y., Saigo K. Dual Bar homeo box genes of Drosophila required in two photoreceptor cells, R1 and R6, and primary pigment cells for normal eye development. Genes Dev. 1992 Jan;6(1):50–60. doi: 10.1101/gad.6.1.50. [DOI] [PubMed] [Google Scholar]
- Hoogeveen A. T., Willemsen R., Meyer N., de Rooij K. E., Roos R. A., van Ommen G. J., Galjaard H. Characterization and localization of the Huntington disease gene product. Hum Mol Genet. 1993 Dec;2(12):2069–2073. doi: 10.1093/hmg/2.12.2069. [DOI] [PubMed] [Google Scholar]
- Igarashi S., Tanno Y., Onodera O., Yamazaki M., Sato S., Ishikawa A., Miyatani N., Nagashima M., Ishikawa Y., Sahashi K. Strong correlation between the number of CAG repeats in androgen receptor genes and the clinical onset of features of spinal and bulbar muscular atrophy. Neurology. 1992 Dec;42(12):2300–2302. doi: 10.1212/wnl.42.12.2300. [DOI] [PubMed] [Google Scholar]
- Ikeuchi T., Koide R., Tanaka H., Onodera O., Igarashi S., Takahashi H., Kondo R., Ishikawa A., Tomoda A., Miike T. Dentatorubral-pallidoluysian atrophy: clinical features are closely related to unstable expansions of trinucleotide (CAG) repeat. Ann Neurol. 1995 Jun;37(6):769–775. doi: 10.1002/ana.410370610. [DOI] [PubMed] [Google Scholar]
- Jansen G., Mahadevan M., Amemiya C., Wormskamp N., Segers B., Hendriks W., O'Hoy K., Baird S., Sabourin L., Lennon G. Characterization of the myotonic dystrophy region predicts multiple protein isoform-encoding mRNAs. Nat Genet. 1992 Jul;1(4):261–266. doi: 10.1038/ng0792-261. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y., Okamoto T., Taniwaki M., Aizawa M., Inoue M., Katayama S., Kawakami H., Nakamura S., Nishimura M., Akiguchi I. CAG expansions in a novel gene for Machado-Joseph disease at chromosome 14q32.1. Nat Genet. 1994 Nov;8(3):221–228. doi: 10.1038/ng1194-221. [DOI] [PubMed] [Google Scholar]
- Koch C. A., Anderson D., Moran M. F., Ellis C., Pawson T. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991 May 3;252(5006):668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- Koide R., Ikeuchi T., Onodera O., Tanaka H., Igarashi S., Endo K., Takahashi H., Kondo R., Ishikawa A., Hayashi T. Unstable expansion of CAG repeat in hereditary dentatorubral-pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Jan;6(1):9–13. doi: 10.1038/ng0194-9. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Roling D. B., Harding A. E., Warner C. L., Spiegel R., Hausmanowa-Petrusewicz I., Yee W. C., Fischbeck K. H. Meiotic stability and genotype-phenotype correlation of the trinucleotide repeat in X-linked spinal and bulbar muscular atrophy. Nat Genet. 1992 Dec;2(4):301–304. doi: 10.1038/ng1292-301. [DOI] [PubMed] [Google Scholar]
- La Spada A. R., Wilson E. M., Lubahn D. B., Harding A. E., Fischbeck K. H. Androgen receptor gene mutations in X-linked spinal and bulbar muscular atrophy. Nature. 1991 Jul 4;352(6330):77–79. doi: 10.1038/352077a0. [DOI] [PubMed] [Google Scholar]
- Li S. H., McInnis M. G., Margolis R. L., Antonarakis S. E., Ross C. A. Novel triplet repeat containing genes in human brain: cloning, expression, and length polymorphisms. Genomics. 1993 Jun;16(3):572–579. doi: 10.1006/geno.1993.1232. [DOI] [PubMed] [Google Scholar]
- Li S. H., Schilling G., Young W. S., 3rd, Li X. J., Margolis R. L., Stine O. C., Wagster M. V., Abbott M. H., Franz M. L., Ranen N. G. Huntington's disease gene (IT15) is widely expressed in human and rat tissues. Neuron. 1993 Nov;11(5):985–993. doi: 10.1016/0896-6273(93)90127-d. [DOI] [PubMed] [Google Scholar]
- Lin B., Nasir J., MacDonald H., Hutchinson G., Graham R. K., Rommens J. M., Hayden M. R. Sequence of the murine Huntington disease gene: evidence for conservation, alternate splicing and polymorphism in a triplet (CCG) repeat [corrected]. Hum Mol Genet. 1994 Jan;3(1):85–92. doi: 10.1093/hmg/3.1.85. [DOI] [PubMed] [Google Scholar]
- Murray V. Improved double-stranded DNA sequencing using the linear polymerase chain reaction. Nucleic Acids Res. 1989 Nov 11;17(21):8889–8889. doi: 10.1093/nar/17.21.8889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi S., Yanagisawa H., Ohsaki E., Shirayama T., Tadokoro K., Inoue T., Yamada M. Structure and expression of the gene responsible for the triplet repeat disorder, dentatorubral and pallidoluysian atrophy (DRPLA). Nat Genet. 1994 Oct;8(2):177–182. doi: 10.1038/ng1094-177. [DOI] [PubMed] [Google Scholar]
- Nagafuchi S., Yanagisawa H., Sato K., Shirayama T., Ohsaki E., Bundo M., Takeda T., Tadokoro K., Kondo I., Murayama N. Dentatorubral and pallidoluysian atrophy expansion of an unstable CAG trinucleotide on chromosome 12p. Nat Genet. 1994 Jan;6(1):14–18. doi: 10.1038/ng0194-14. [DOI] [PubMed] [Google Scholar]
- Naito H., Oyanagi S. Familial myoclonus epilepsy and choreoathetosis: hereditary dentatorubral-pallidoluysian atrophy. Neurology. 1982 Aug;32(8):798–807. doi: 10.1212/wnl.32.8.798. [DOI] [PubMed] [Google Scholar]
- Orr H. T., Chung M. Y., Banfi S., Kwiatkowski T. J., Jr, Servadio A., Beaudet A. L., McCall A. E., Duvick L. A., Ranum L. P., Zoghbi H. Y. Expansion of an unstable trinucleotide CAG repeat in spinocerebellar ataxia type 1. Nat Genet. 1993 Jul;4(3):221–226. doi: 10.1038/ng0793-221. [DOI] [PubMed] [Google Scholar]
- Ross C. A., McInnis M. G., Margolis R. L., Li S. H. Genes with triplet repeats: candidate mediators of neuropsychiatric disorders. Trends Neurosci. 1993 Jul;16(7):254–260. doi: 10.1016/0166-2236(93)90175-l. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short J. M., Fernandez J. M., Sorge J. A., Huse W. D. Lambda ZAP: a bacteriophage lambda expression vector with in vivo excision properties. Nucleic Acids Res. 1988 Aug 11;16(15):7583–7600. doi: 10.1093/nar/16.15.7583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squitieri F., Andrew S. E., Goldberg Y. P., Kremer B., Spence N., Zeisler J., Nichol K., Theilmann J., Greenberg J., Goto J. DNA haplotype analysis of Huntington disease reveals clues to the origins and mechanisms of CAG expansion and reasons for geographic variations of prevalence. Hum Mol Genet. 1994 Dec;3(12):2103–2114. doi: 10.1093/hmg/3.12.2103. [DOI] [PubMed] [Google Scholar]
- Strong T. V., Tagle D. A., Valdes J. M., Elmer L. W., Boehm K., Swaroop M., Kaatz K. W., Collins F. S., Albin R. L. Widespread expression of the human and rat Huntington's disease gene in brain and nonneural tissues. Nat Genet. 1993 Nov;5(3):259–265. doi: 10.1038/ng1193-259. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens M., Stephenson P. Role of the conserved AAUAAA sequence: four AAUAAA point mutants prevent messenger RNA 3' end formation. Science. 1984 Nov 30;226(4678):1045–1051. doi: 10.1126/science.6208611. [DOI] [PubMed] [Google Scholar]
- Yazawa I., Nukina N., Hashida H., Goto J., Yamada M., Kanazawa I. Abnormal gene product identified in hereditary dentatorubral-pallidoluysian atrophy (DRPLA) brain. Nat Genet. 1995 May;10(1):99–103. doi: 10.1038/ng0595-99. [DOI] [PubMed] [Google Scholar]
- Yu H., Chen J. K., Feng S., Dalgarno D. C., Brauer A. W., Schreiber S. L. Structural basis for the binding of proline-rich peptides to SH3 domains. Cell. 1994 Mar 11;76(5):933–945. doi: 10.1016/0092-8674(94)90367-0. [DOI] [PubMed] [Google Scholar]




