Abstract
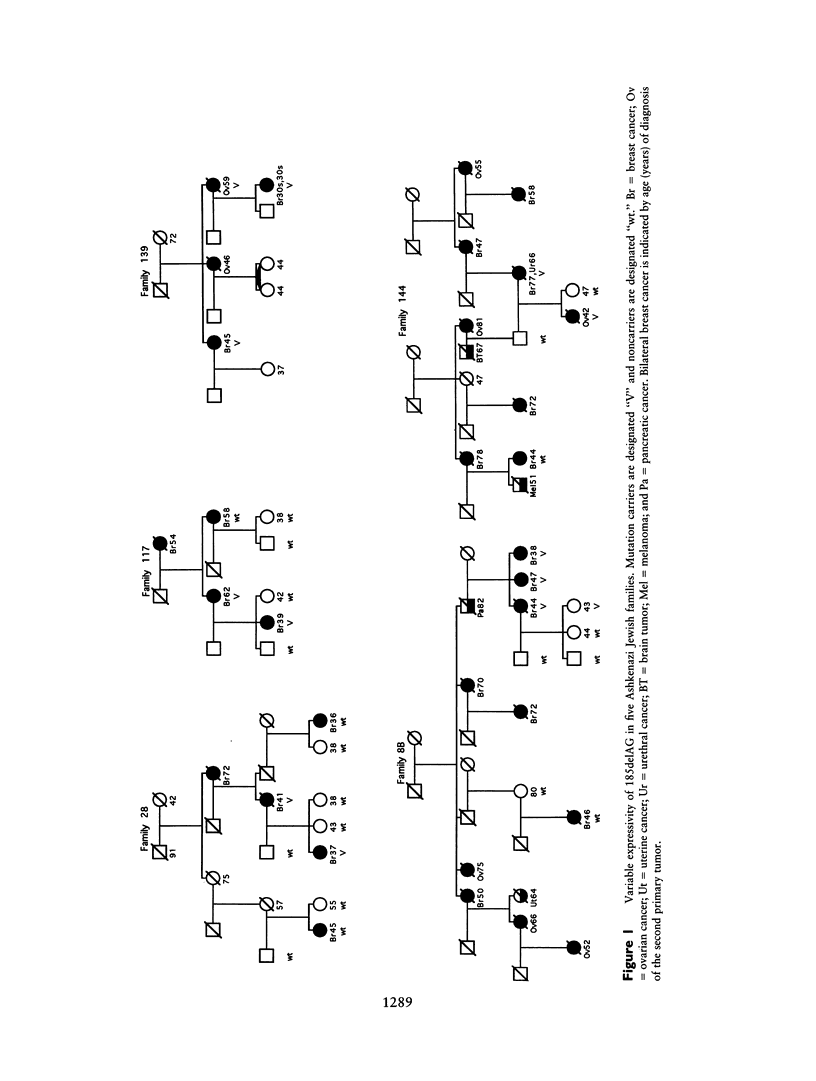
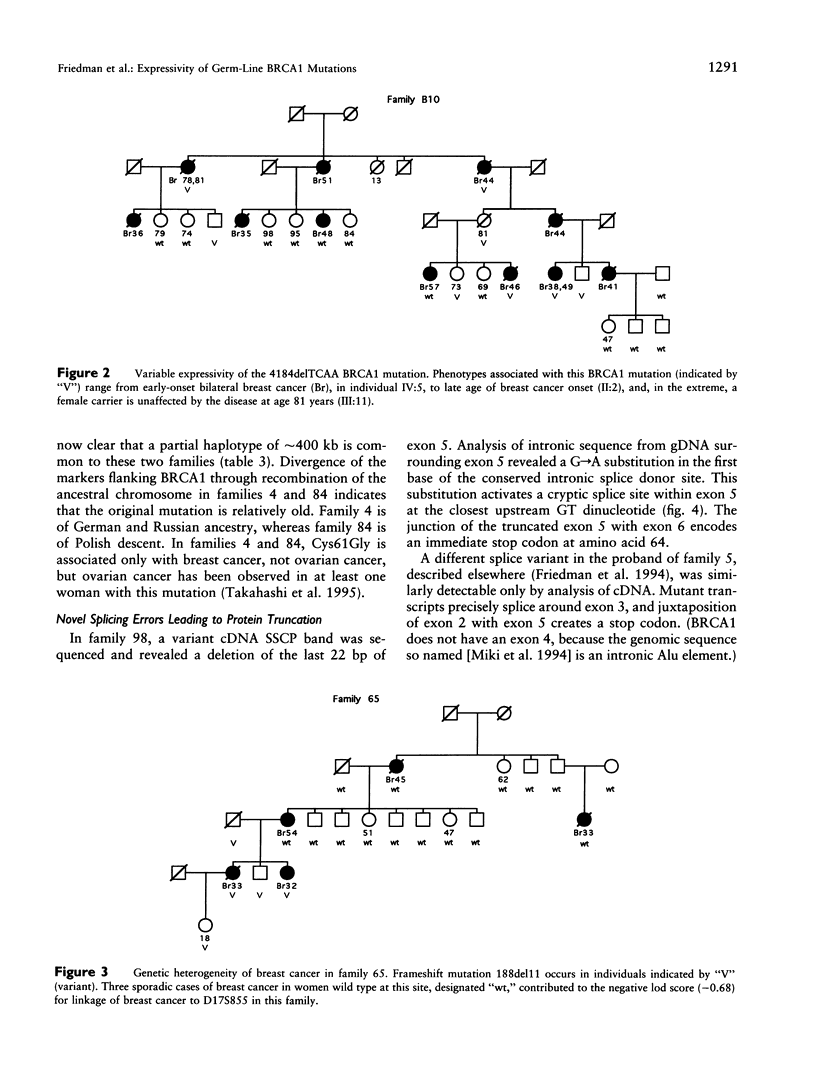
Thirty-seven families with four or more cases of breast cancer or breast and ovarian cancer were analyzed for mutations in BRCA1. Twelve different germ-line mutations, four novel and eight previously observed, were detected in 16 families. Five families of Ashkenazi Jewish descent carried the 185delAG mutation and shared the same haplotype at eight polymorphic markers spanning approximately 850 kb at BRCA1. Expressivity of 185delAG in these families varied, from early-onset breast cancer without ovarian cancer. Mutation 4184delTCAA occurred independently in two families. In one family, penetrance was complete, with females developing early-onset breast cancer or ovarian cancer and the male carrier developing prostatic cancer, whereas, in the other family, penetrance was incomplete and only breast cancer occurred, diagnosed at ages 38-81 years. Two novel nonsense mutations led to the loss of mutant BRCA1 transcript in families with 10 and 6 cases of early-onset breast cancer and ovarian cancer. A 665-nt segment of the BRCA1 3'-UTR and 1.3 kb of genomic sequence including the putative promoter region were invariant by single-strand conformation analysis in 13 families without coding-sequence mutations. Overall in our series, BRCA1 mutations have been detected in 26 families: 16 with positive BRCA1 lod scores, 7 with negative lod scores (reflecting multiple sporadic breast cancers), and 3 not tested for linkage. Three other families have positive lod scores for linkage to BRCA2, but 13 families without detected BRCA1 mutations have negative lod scores for both BRCA1 and BRCA2.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara F. M., Chen F. Y., Wright J. A. A novel transforming growth factor-beta 1 responsive cytoplasmic trans-acting factor binds selectively to the 3'-untranslated region of mammalian ribonucleotide reductase R2 mRNA: role in message stability. Nucleic Acids Res. 1993 Oct 11;21(20):4803–4809. doi: 10.1093/nar/21.20.4803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson L. A., Friedman L., Osborne-Lawrence S., Lynch E., Weissenbach J., Bowcock A., King M. C. High-density genetic map of the BRCA1 region of chromosome 17q12-q21. Genomics. 1993 Sep;17(3):618–623. doi: 10.1006/geno.1993.1381. [DOI] [PubMed] [Google Scholar]
- Blanchet-Bardon C., Nazzaro V., Chevrant-Breton J., Espie M., Kerbrat P., Le Marec B. Hereditary epidermolytic palmoplantar keratoderma associated with breast and ovarian cancer in a large kindred. Br J Dermatol. 1987 Sep;117(3):363–370. doi: 10.1111/j.1365-2133.1987.tb04144.x. [DOI] [PubMed] [Google Scholar]
- Bowcock A. M., Anderson L. A., Friedman L. S., Black D. M., Osborne-Lawrence S., Rowell S. E., Hall J. M., Solomon E., King M. C. THRA1 and D17S183 flank an interval of < 4 cM for the breast-ovarian cancer gene (BRCA1) on chromosome 17q21. Am J Hum Genet. 1993 Apr;52(4):718–722. [PMC free article] [PubMed] [Google Scholar]
- Boyd M., Harris F., McFarlane R., Davidson H. R., Black D. M. A human BRCA1 gene knockout. Nature. 1995 Jun 15;375(6532):541–542. doi: 10.1038/375541b0. [DOI] [PubMed] [Google Scholar]
- Brown M. A., Nicolai H., Xu C. F., Griffiths B. L., Jones K. A., Solomon E., Hosking L., Trowsdale J., Black D. M., McFarlane R. Regulation of BRCA1. Nature. 1994 Dec 22;372(6508):733–733. doi: 10.1038/372733a0. [DOI] [PubMed] [Google Scholar]
- Castilla L. H., Couch F. J., Erdos M. R., Hoskins K. F., Calzone K., Garber J. E., Boyd J., Lubin M. B., Deshano M. L., Brody L. C. Mutations in the BRCA1 gene in families with early-onset breast and ovarian cancer. Nat Genet. 1994 Dec;8(4):387–391. doi: 10.1038/ng1294-387. [DOI] [PubMed] [Google Scholar]
- Claus E. B., Risch N., Thompson W. D. Genetic analysis of breast cancer in the cancer and steroid hormone study. Am J Hum Genet. 1991 Feb;48(2):232–242. [PMC free article] [PubMed] [Google Scholar]
- Cui Y., Hagan K. W., Zhang S., Peltz S. W. Identification and characterization of genes that are required for the accelerated degradation of mRNAs containing a premature translational termination codon. Genes Dev. 1995 Feb 15;9(4):423–436. doi: 10.1101/gad.9.4.423. [DOI] [PubMed] [Google Scholar]
- Dietrich W. F., Lander E. S., Smith J. S., Moser A. R., Gould K. A., Luongo C., Borenstein N., Dove W. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993 Nov 19;75(4):631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- Dunn J. M., Phillips R. A., Zhu X., Becker A., Gallie B. L. Mutations in the RB1 gene and their effects on transcription. Mol Cell Biol. 1989 Nov;9(11):4596–4604. doi: 10.1128/mcb.9.11.4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easton D. F., Bishop D. T., Ford D., Crockford G. P. Genetic linkage analysis in familial breast and ovarian cancer: results from 214 families. The Breast Cancer Linkage Consortium. Am J Hum Genet. 1993 Apr;52(4):678–701. [PMC free article] [PubMed] [Google Scholar]
- Easton D., Ford D., Peto J. Inherited susceptibility to breast cancer. Cancer Surv. 1993;18:95–113. [PubMed] [Google Scholar]
- Ford D., Easton D. F., Bishop D. T., Narod S. A., Goldgar D. E. Risks of cancer in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Lancet. 1994 Mar 19;343(8899):692–695. doi: 10.1016/s0140-6736(94)91578-4. [DOI] [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Lynch E. D., Welcsh P., Szabo C. I., Meza J. E., Anderson L. A., Dowd P., Lee M. K., Rowell S. E. 22 genes from chromosome 17q21: cloning, sequencing, and characterization of mutations in breast cancer families and tumors. Genomics. 1995 Jan 1;25(1):256–263. doi: 10.1016/0888-7543(95)80133-7. [DOI] [PubMed] [Google Scholar]
- Friedman L. S., Ostermeyer E. A., Szabo C. I., Dowd P., Lynch E. D., Rowell S. E., King M. C. Confirmation of BRCA1 by analysis of germline mutations linked to breast and ovarian cancer in ten families. Nat Genet. 1994 Dec;8(4):399–404. doi: 10.1038/ng1294-399. [DOI] [PubMed] [Google Scholar]
- Futreal P. A., Liu Q., Shattuck-Eidens D., Cochran C., Harshman K., Tavtigian S., Bennett L. M., Haugen-Strano A., Swensen J., Miki Y. BRCA1 mutations in primary breast and ovarian carcinomas. Science. 1994 Oct 7;266(5182):120–122. doi: 10.1126/science.7939630. [DOI] [PubMed] [Google Scholar]
- Hall J. M., Lee M. K., Newman B., Morrow J. E., Anderson L. A., Huey B., King M. C. Linkage of early-onset familial breast cancer to chromosome 17q21. Science. 1990 Dec 21;250(4988):1684–1689. doi: 10.1126/science.2270482. [DOI] [PubMed] [Google Scholar]
- Hartmann A., Blaszyk H., McGovern R. M., Schroeder J. J., Cunningham J., De Vries E. M., Kovach J. S., Sommer S. S. p53 gene mutations inside and outside of exons 5-8: the patterns differ in breast and other cancers. Oncogene. 1995 Feb 16;10(4):681–688. [PubMed] [Google Scholar]
- Helmrich S. P., Shapiro S., Rosenberg L., Kaufman D. W., Slone D., Bain C., Miettinen O. S., Stolley P. D., Rosenshein N. B., Knapp R. C. Risk factors for breast cancer. Am J Epidemiol. 1983 Jan;117(1):35–45. doi: 10.1093/oxfordjournals.aje.a113513. [DOI] [PubMed] [Google Scholar]
- Henics T., Sanfridson A., Hamilton B. J., Nagy E., Rigby W. F. Enhanced stability of interleukin-2 mRNA in MLA 144 cells. Possible role of cytoplasmic AU-rich sequence-binding proteins. J Biol Chem. 1994 Feb 18;269(7):5377–5383. [PubMed] [Google Scholar]
- Hogervorst F. B., Cornelis R. S., Bout M., van Vliet M., Oosterwijk J. C., Olmer R., Bakker B., Klijn J. G., Vasen H. F., Meijers-Heijboer H. Rapid detection of BRCA1 mutations by the protein truncation test. Nat Genet. 1995 Jun;10(2):208–212. doi: 10.1038/ng0695-208. [DOI] [PubMed] [Google Scholar]
- Hosking L., Trowsdale J., Nicolai H., Solomon E., Foulkes W., Stamp G., Signer E., Jeffreys A. A somatic BRCA1 mutation in an ovarian tumour. Nat Genet. 1995 Apr;9(4):343–344. doi: 10.1038/ng0495-343. [DOI] [PubMed] [Google Scholar]
- Kelsey J. L., Gammon M. D. The epidemiology of breast cancer. CA Cancer J Clin. 1991 May-Jun;41(3):146–165. doi: 10.3322/canjclin.41.3.146. [DOI] [PubMed] [Google Scholar]
- King M. C., Rowell S., Love S. M. Inherited breast and ovarian cancer. What are the risks? What are the choices? JAMA. 1993 Apr 21;269(15):1975–1980. [PubMed] [Google Scholar]
- Kunkel T. A. Frameshift mutagenesis by eucaryotic DNA polymerases in vitro. J Biol Chem. 1986 Oct 15;261(29):13581–13587. [PubMed] [Google Scholar]
- Leeds P., Peltz S. W., Jacobson A., Culbertson M. R. The product of the yeast UPF1 gene is required for rapid turnover of mRNAs containing a premature translational termination codon. Genes Dev. 1991 Dec;5(12A):2303–2314. doi: 10.1101/gad.5.12a.2303. [DOI] [PubMed] [Google Scholar]
- Lim S. K., Sigmund C. D., Gross K. W., Maquat L. E. Nonsense codons in human beta-globin mRNA result in the production of mRNA degradation products. Mol Cell Biol. 1992 Mar;12(3):1149–1161. doi: 10.1128/mcb.12.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losson R., Lacroute F. Interference of nonsense mutations with eukaryotic messenger RNA stability. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5134–5137. doi: 10.1073/pnas.76.10.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovering R., Hanson I. M., Borden K. L., Martin S., O'Reilly N. J., Evan G. I., Rahman D., Pappin D. J., Trowsdale J., Freemont P. S. Identification and preliminary characterization of a protein motif related to the zinc finger. Proc Natl Acad Sci U S A. 1993 Mar 15;90(6):2112–2116. doi: 10.1073/pnas.90.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon K. P., Neufeld E. F. Evidence for degradation of mRNA encoding alpha-L-iduronidase in Hurler fibroblasts with premature termination alleles. Cell Mol Biol (Noisy-le-grand) 1994 Nov;40(7):999–1005. [PubMed] [Google Scholar]
- Merajver S. D., Pham T. M., Caduff R. F., Chen M., Poy E. L., Cooney K. A., Weber B. L., Collins F. S., Johnston C., Frank T. S. Somatic mutations in the BRCA1 gene in sporadic ovarian tumours. Nat Genet. 1995 Apr;9(4):439–443. doi: 10.1038/ng0495-439. [DOI] [PubMed] [Google Scholar]
- Miki Y., Swensen J., Shattuck-Eidens D., Futreal P. A., Harshman K., Tavtigian S., Liu Q., Cochran C., Bennett L. M., Ding W. A strong candidate for the breast and ovarian cancer susceptibility gene BRCA1. Science. 1994 Oct 7;266(5182):66–71. doi: 10.1126/science.7545954. [DOI] [PubMed] [Google Scholar]
- Narod S. A., Feunteun J., Lynch H. T., Watson P., Conway T., Lynch J., Lenoir G. M. Familial breast-ovarian cancer locus on chromosome 17q12-q23. Lancet. 1991 Jul 13;338(8759):82–83. doi: 10.1016/0140-6736(91)90076-2. [DOI] [PubMed] [Google Scholar]
- Newman B., Austin M. A., Lee M., King M. C. Inheritance of human breast cancer: evidence for autosomal dominant transmission in high-risk families. Proc Natl Acad Sci U S A. 1988 May;85(9):3044–3048. doi: 10.1073/pnas.85.9.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulak R., Anderson P. mRNA surveillance by the Caenorhabditis elegans smg genes. Genes Dev. 1993 Oct;7(10):1885–1897. doi: 10.1101/gad.7.10.1885. [DOI] [PubMed] [Google Scholar]
- Roest P. A., Roberts R. G., van der Tuijn A. C., Heikoop J. C., van Ommen G. J., den Dunnen J. T. Protein truncation test (PTT) to rapidly screen the DMD gene for translation terminating mutations. Neuromuscul Disord. 1993 Sep-Nov;3(5-6):391–394. doi: 10.1016/0960-8966(93)90083-v. [DOI] [PubMed] [Google Scholar]
- Roy N., Laflamme G., Raymond V. 5' untranslated sequences modulate rapid mRNA degradation mediated by 3' AU-rich element in v-/c-fos recombinants. Nucleic Acids Res. 1992 Nov 11;20(21):5753–5762. doi: 10.1093/nar/20.21.5753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlötterer C., Tautz D. Slippage synthesis of simple sequence DNA. Nucleic Acids Res. 1992 Jan 25;20(2):211–215. doi: 10.1093/nar/20.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck-Eidens D., McClure M., Simard J., Labrie F., Narod S., Couch F., Hoskins K., Weber B., Castilla L., Erdos M. A collaborative survey of 80 mutations in the BRCA1 breast and ovarian cancer susceptibility gene. Implications for presymptomatic testing and screening. JAMA. 1995 Feb 15;273(7):535–541. [PubMed] [Google Scholar]
- Simard J., Tonin P., Durocher F., Morgan K., Rommens J., Gingras S., Samson C., Leblanc J. F., Bélanger C., Dion F. Common origins of BRCA1 mutations in Canadian breast and ovarian cancer families. Nat Genet. 1994 Dec;8(4):392–398. doi: 10.1038/ng1294-392. [DOI] [PubMed] [Google Scholar]
- Smith S. A., Easton D. F., Ford D., Peto J., Anderson K., Averill D., Stratton M., Ponder M., Pye C., Ponder B. A. Genetic heterogeneity and localization of a familial breast-ovarian cancer gene on chromosome 17q12-q21. Am J Hum Genet. 1993 Apr;52(4):767–776. [PMC free article] [PubMed] [Google Scholar]
- Streisinger G., Okada Y., Emrich J., Newton J., Tsugita A., Terzaghi E., Inouye M. Frameshift mutations and the genetic code. This paper is dedicated to Professor Theodosius Dobzhansky on the occasion of his 66th birthday. Cold Spring Harb Symp Quant Biol. 1966;31:77–84. doi: 10.1101/sqb.1966.031.01.014. [DOI] [PubMed] [Google Scholar]
- Struewing J. P., Brody L. C., Erdos M. R., Kase R. G., Giambarresi T. R., Smith S. A., Collins F. S., Tucker M. A. Detection of eight BRCA1 mutations in 10 breast/ovarian cancer families, including 1 family with male breast cancer. Am J Hum Genet. 1995 Jul;57(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Takahashi H., Behbakht K., McGovern P. E., Chiu H. C., Couch F. J., Weber B. L., Friedman L. S., King M. C., Furusato M., LiVolsi V. A. Mutation analysis of the BRCA1 gene in ovarian cancers. Cancer Res. 1995 Jul 15;55(14):2998–3002. [PubMed] [Google Scholar]
- Tautz D. Hypervariability of simple sequences as a general source for polymorphic DNA markers. Nucleic Acids Res. 1989 Aug 25;17(16):6463–6471. doi: 10.1093/nar/17.16.6463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson M. E., Jensen R. A., Obermiller P. S., Page D. L., Holt J. T. Decreased expression of BRCA1 accelerates growth and is often present during sporadic breast cancer progression. Nat Genet. 1995 Apr;9(4):444–450. doi: 10.1038/ng0495-444. [DOI] [PubMed] [Google Scholar]
- Tonin P., Serova O., Lenoir G., Lynch H., Durocher F., Simard J., Morgan K., Narod S. BRCA1 mutations in Ashkenazi Jewish women. Am J Hum Genet. 1995 Jul;57(1):189–189. [PMC free article] [PubMed] [Google Scholar]
- Torchard D., Blanchet-Bardon C., Serova O., Langbein L., Narod S., Janin N., Goguel A. F., Bernheim A., Franke W. W., Lenoir G. M. Epidermolytic palmoplantar keratoderma cosegregates with a keratin 9 mutation in a pedigree with breast and ovarian cancer. Nat Genet. 1994 Jan;6(1):106–110. doi: 10.1038/ng0194-106. [DOI] [PubMed] [Google Scholar]
- Vidal-Puig A., Moller D. E. Comparative sensitivity of alternative single-strand conformation polymorphism (SSCP) methods. Biotechniques. 1994 Sep;17(3):490-2, 494, 496. [PubMed] [Google Scholar]
- Wang X., Kiledjian M., Weiss I. M., Liebhaber S. A. Detection and characterization of a 3' untranslated region ribonucleoprotein complex associated with human alpha-globin mRNA stability. Mol Cell Biol. 1995 Mar;15(3):1769–1777. doi: 10.1128/mcb.15.3.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood W. I., Gitschier J., Lasky L. A., Lawn R. M. Base composition-independent hybridization in tetramethylammonium chloride: a method for oligonucleotide screening of highly complex gene libraries. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1585–1588. doi: 10.1073/pnas.82.6.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooster R., Neuhausen S. L., Mangion J., Quirk Y., Ford D., Collins N., Nguyen K., Seal S., Tran T., Averill D. Localization of a breast cancer susceptibility gene, BRCA2, to chromosome 13q12-13. Science. 1994 Sep 30;265(5181):2088–2090. doi: 10.1126/science.8091231. [DOI] [PubMed] [Google Scholar]




