Abstract
Analysis of 139 mother-to-offspring transmissions of fragile X CGG triplet repeats revealed that the repeat expansion is enhanced in mother-to-son transmissions compared with mother-to-daughter transmissions. Evidence has been based on analysis of mother-offspring differences in the size of repeat (in kb), as well as on comparisons between proportions of male and female offspring with premutations, and full mutations, inherited from mothers carrying a premutation. Mean difference in the repeat size from mother-son transmissions was 1.45 kb, compared with mother-daughter transmissions of 0.76 kb. The difference is due primarily to a greater proportion of male than female offspring with full mutation from the premutation mothers and also to a higher frequency of reduction in repeat size from mothers to daughters than from mothers to sons. Our findings suggest the possibility of an interaction of the normal X homologue in a female zygote with the FMR1 sequence on the fragile X during replication to account for the lower level of expansion in mother-to-daughter transmissions relative to mother-to-son transmissions.
Full text
PDF
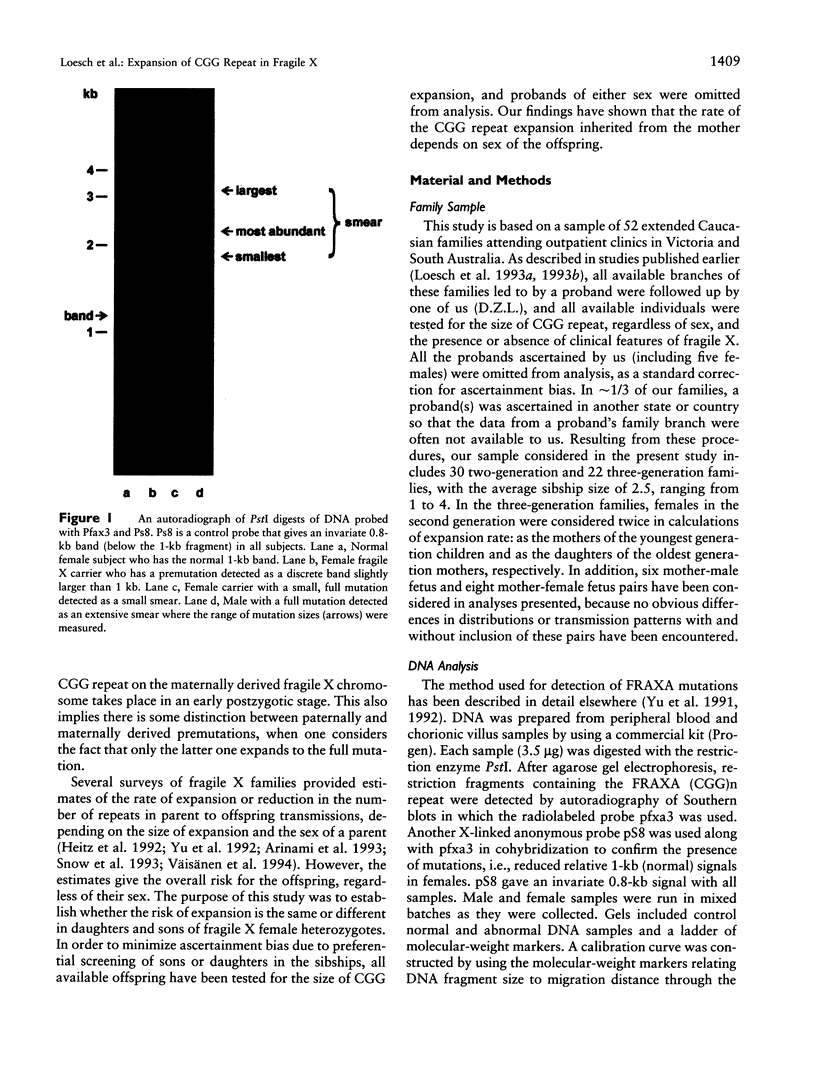
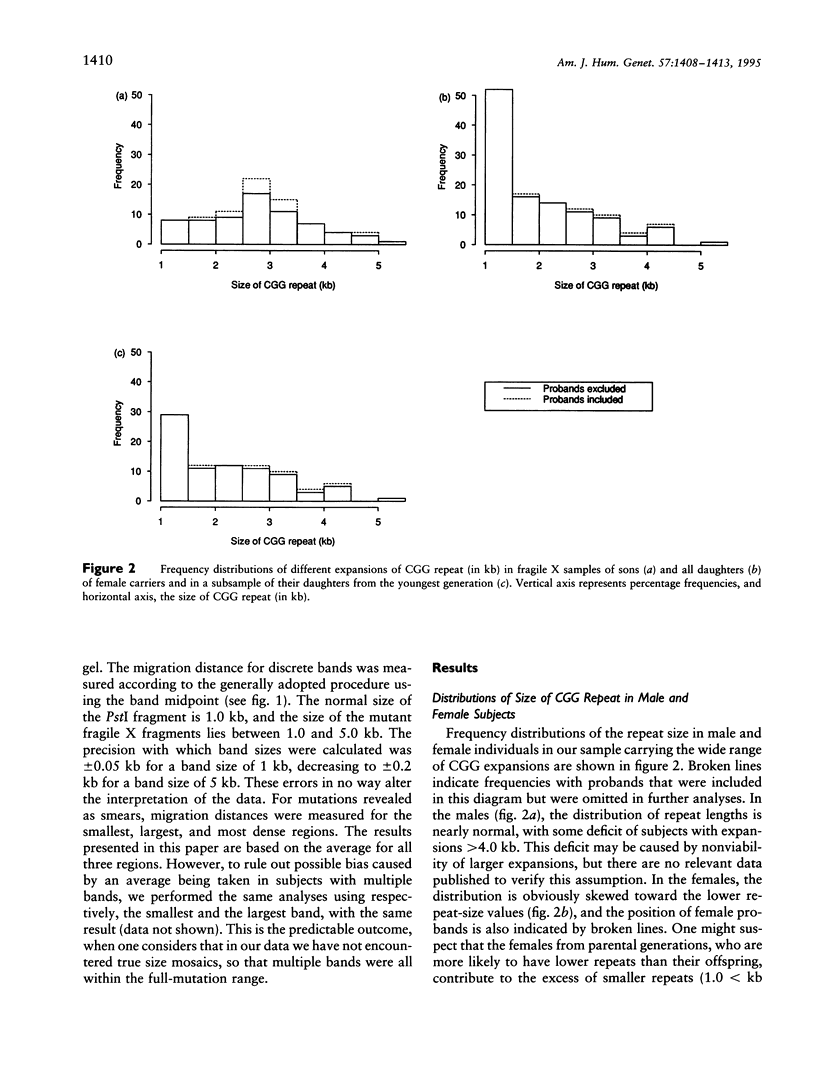
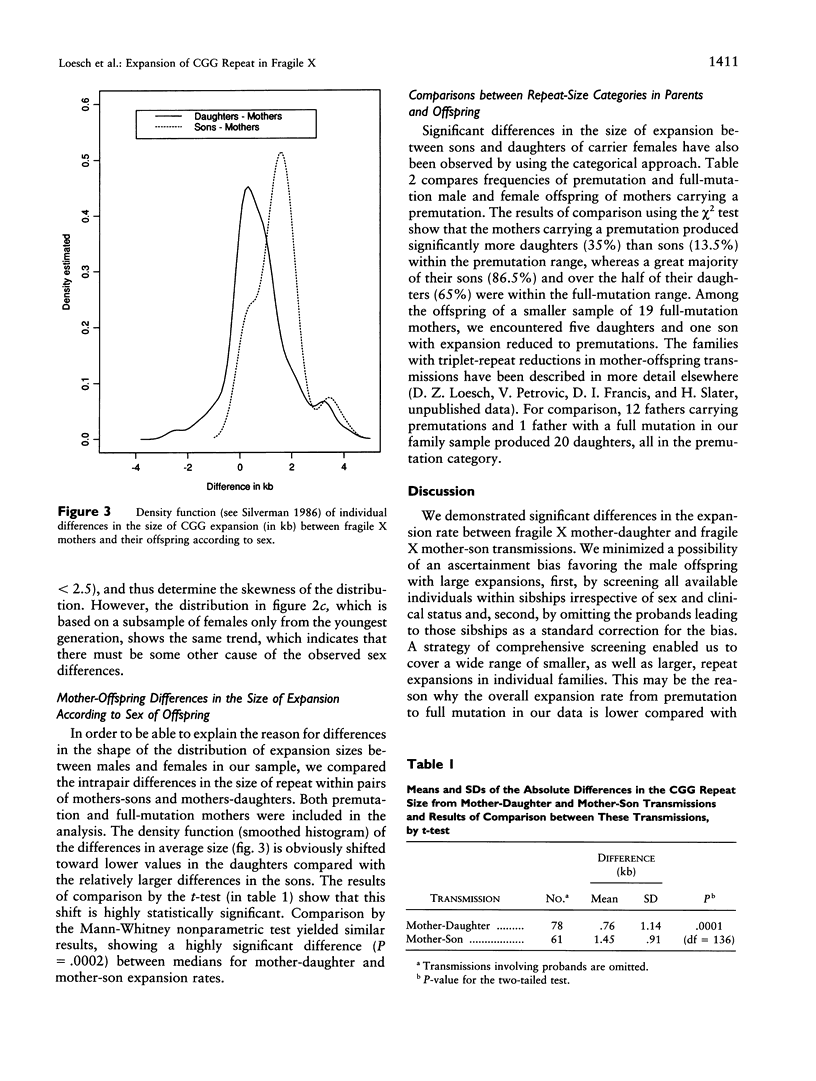


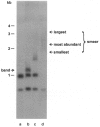
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arinami T., Asano M., Kobayashi K., Yanagi H., Hamaguchi H. Data on the CGG repeat at the fragile X site in the non-retarded Japanese population and family suggest the presence of a subgroup of normal alleles predisposing to mutate. Hum Genet. 1993 Nov;92(5):431–436. doi: 10.1007/BF00216445. [DOI] [PubMed] [Google Scholar]
- Brown W. T., Houck G. E., Jr, Jeziorowska A., Levinson F. N., Ding X., Dobkin C., Zhong N., Henderson J., Brooks S. S., Jenkins E. C. Rapid fragile X carrier screening and prenatal diagnosis using a nonradioactive PCR test. JAMA. 1993 Oct 6;270(13):1569–1575. [PubMed] [Google Scholar]
- Chiurazzi P., Kozak L., Neri G. Unstable triplets and their mutational mechanism: size reduction of the CGG repeat vs. germline mosaicism in the fragile X syndrome. Am J Med Genet. 1994 Jul 15;51(4):517–521. doi: 10.1002/ajmg.1320510446. [DOI] [PubMed] [Google Scholar]
- Devys D., Biancalana V., Rousseau F., Boué J., Mandel J. L., Oberlé I. Analysis of full fragile X mutations in fetal tissues and monozygotic twins indicate that abnormal methylation and somatic heterogeneity are established early in development. 1992 Apr 15-May 1Am J Med Genet. 43(1-2):208–216. doi: 10.1002/ajmg.1320430134. [DOI] [PubMed] [Google Scholar]
- Eichler E. E., Holden J. J., Popovich B. W., Reiss A. L., Snow K., Thibodeau S. N., Richards C. S., Ward P. A., Nelson D. L. Length of uninterrupted CGG repeats determines instability in the FMR1 gene. Nat Genet. 1994 Sep;8(1):88–94. doi: 10.1038/ng0994-88. [DOI] [PubMed] [Google Scholar]
- Fu Y. H., Kuhl D. P., Pizzuti A., Pieretti M., Sutcliffe J. S., Richards S., Verkerk A. J., Holden J. J., Fenwick R. G., Jr, Warren S. T. Variation of the CGG repeat at the fragile X site results in genetic instability: resolution of the Sherman paradox. Cell. 1991 Dec 20;67(6):1047–1058. doi: 10.1016/0092-8674(91)90283-5. [DOI] [PubMed] [Google Scholar]
- Heitz D., Devys D., Imbert G., Kretz C., Mandel J. L. Inheritance of the fragile X syndrome: size of the fragile X premutation is a major determinant of the transition to full mutation. J Med Genet. 1992 Nov;29(11):794–801. doi: 10.1136/jmg.29.11.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch D. Z., Huggins R., Hay D. A., Gedeon A. K., Mulley J. C., Sutherland G. R. Genotype-phenotype relationships in fragile X syndrome: a family study. Am J Hum Genet. 1993 Nov;53(5):1064–1073. [PMC free article] [PubMed] [Google Scholar]
- Loesch D. Z., Sheffield L. J., Hay D. A. Between-generation differences in ascertainment and penetrance: relevance to genetic hypotheses in fragile X. Hum Genet. 1993 Jun;91(5):469–474. doi: 10.1007/BF00217774. [DOI] [PubMed] [Google Scholar]
- Macpherson J. N., Bullman H., Youings S. A., Jacobs P. A. Insert size and flanking haplotype in fragile X and normal populations: possible multiple origins for the fragile X mutation. Hum Mol Genet. 1994 Mar;3(3):399–405. doi: 10.1093/hmg/3.3.399. [DOI] [PubMed] [Google Scholar]
- Mulley J. C., Yu S., Loesch D. Z., Hay D. A., Donnelly A., Gedeon A. K., Carbonell P., López I., Glover G., Gabarrón I. FRAXE and mental retardation. J Med Genet. 1995 Mar;32(3):162–169. doi: 10.1136/jmg.32.3.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieretti M., Zhang F. P., Fu Y. H., Warren S. T., Oostra B. A., Caskey C. T., Nelson D. L. Absence of expression of the FMR-1 gene in fragile X syndrome. Cell. 1991 Aug 23;66(4):817–822. doi: 10.1016/0092-8674(91)90125-i. [DOI] [PubMed] [Google Scholar]
- Reyniers E., Vits L., De Boulle K., Van Roy B., Van Velzen D., de Graaff E., Verkerk A. J., Jorens H. Z., Darby J. K., Oostra B. The full mutation in the FMR-1 gene of male fragile X patients is absent in their sperm. Nat Genet. 1993 Jun;4(2):143–146. doi: 10.1038/ng0693-143. [DOI] [PubMed] [Google Scholar]
- Richards R. I., Holman K., Friend K., Kremer E., Hillen D., Staples A., Brown W. T., Goonewardena P., Tarleton J., Schwartz C. Evidence of founder chromosomes in fragile X syndrome. Nat Genet. 1992 Jul;1(4):257–260. doi: 10.1038/ng0792-257. [DOI] [PubMed] [Google Scholar]
- Rousseau F., Heitz D., Biancalana V., Blumenfeld S., Kretz C., Boué J., Tommerup N., Van Der Hagen C., DeLozier-Blanchet C., Croquette M. F. Direct diagnosis by DNA analysis of the fragile X syndrome of mental retardation. N Engl J Med. 1991 Dec 12;325(24):1673–1681. doi: 10.1056/NEJM199112123252401. [DOI] [PubMed] [Google Scholar]
- Schlötterer C., Tautz D. Slippage synthesis of simple sequence DNA. Nucleic Acids Res. 1992 Jan 25;20(2):211–215. doi: 10.1093/nar/20.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snow K., Doud L. K., Hagerman R., Pergolizzi R. G., Erster S. H., Thibodeau S. N. Analysis of a CGG sequence at the FMR-1 locus in fragile X families and in the general population. Am J Hum Genet. 1993 Dec;53(6):1217–1228. [PMC free article] [PubMed] [Google Scholar]
- Snow K., Tester D. J., Kruckeberg K. E., Schaid D. J., Thibodeau S. N. Sequence analysis of the fragile X trinucleotide repeat: implications for the origin of the fragile X mutation. Hum Mol Genet. 1994 Sep;3(9):1543–1551. doi: 10.1093/hmg/3.9.1543. [DOI] [PubMed] [Google Scholar]
- Verheij C., Bakker C. E., de Graaff E., Keulemans J., Willemsen R., Verkerk A. J., Galjaard H., Reuser A. J., Hoogeveen A. T., Oostra B. A. Characterization and localization of the FMR-1 gene product associated with fragile X syndrome. Nature. 1993 Jun 24;363(6431):722–724. doi: 10.1038/363722a0. [DOI] [PubMed] [Google Scholar]
- Verkerk A. J., Pieretti M., Sutcliffe J. S., Fu Y. H., Kuhl D. P., Pizzuti A., Reiner O., Richards S., Victoria M. F., Zhang F. P. Identification of a gene (FMR-1) containing a CGG repeat coincident with a breakpoint cluster region exhibiting length variation in fragile X syndrome. Cell. 1991 May 31;65(5):905–914. doi: 10.1016/0092-8674(91)90397-h. [DOI] [PubMed] [Google Scholar]
- Väisänen M. L., Kähkönen M., Leisti J. Diagnosis of fragile X syndrome by direct mutation analysis. Hum Genet. 1994 Feb;93(2):143–147. doi: 10.1007/BF00210599. [DOI] [PubMed] [Google Scholar]
- Wöhrle D., Hennig I., Vogel W., Steinbach P. Mitotic stability of fragile X mutations in differentiated cells indicates early post-conceptional trinucleotide repeat expansion. Nat Genet. 1993 Jun;4(2):140–142. doi: 10.1038/ng0693-140. [DOI] [PubMed] [Google Scholar]
- Yu S., Mulley J., Loesch D., Turner G., Donnelly A., Gedeon A., Hillen D., Kremer E., Lynch M., Pritchard M. Fragile-X syndrome: unique genetics of the heritable unstable element. Am J Hum Genet. 1992 May;50(5):968–980. [PMC free article] [PubMed] [Google Scholar]
- Zhong N., Liu X., Gou S., Houck G. E., Jr, Li S., Dobkin C., Brown W. T. Distribution of FMR-1 and associated microsatellite alleles in a normal Chinese population. Am J Med Genet. 1994 Jul 15;51(4):417–422. doi: 10.1002/ajmg.1320510423. [DOI] [PubMed] [Google Scholar]