Abstract
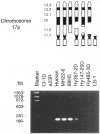
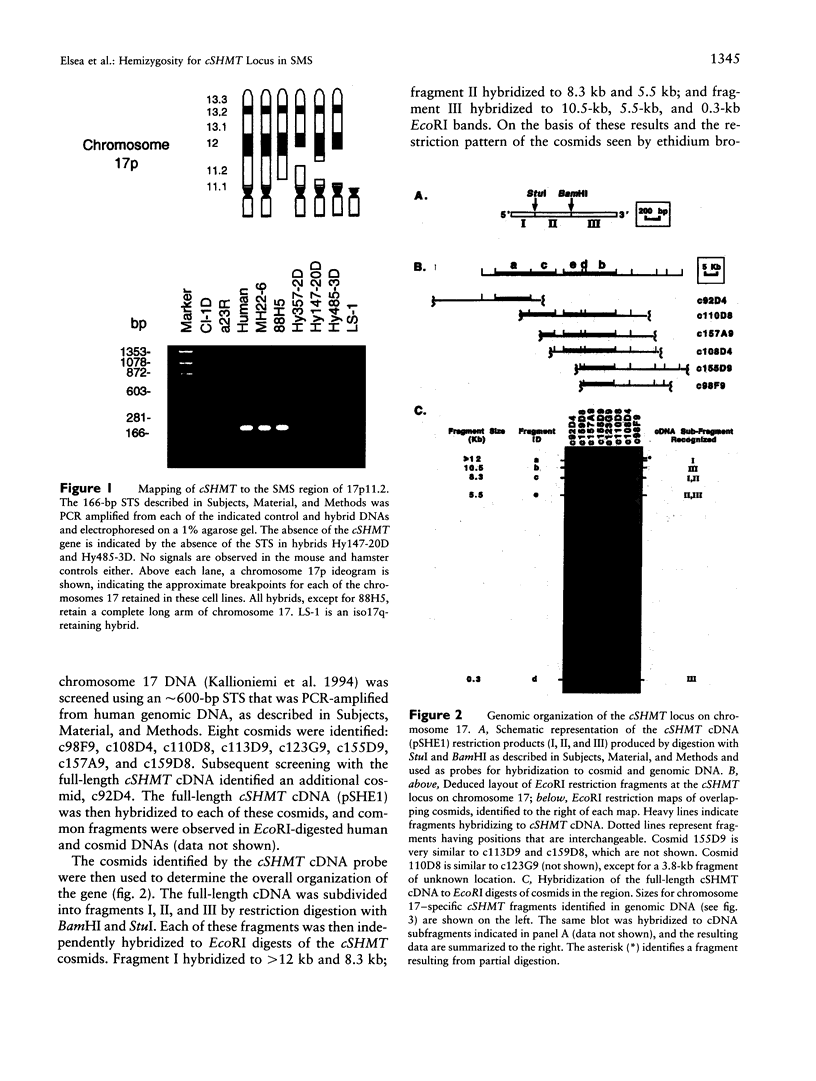
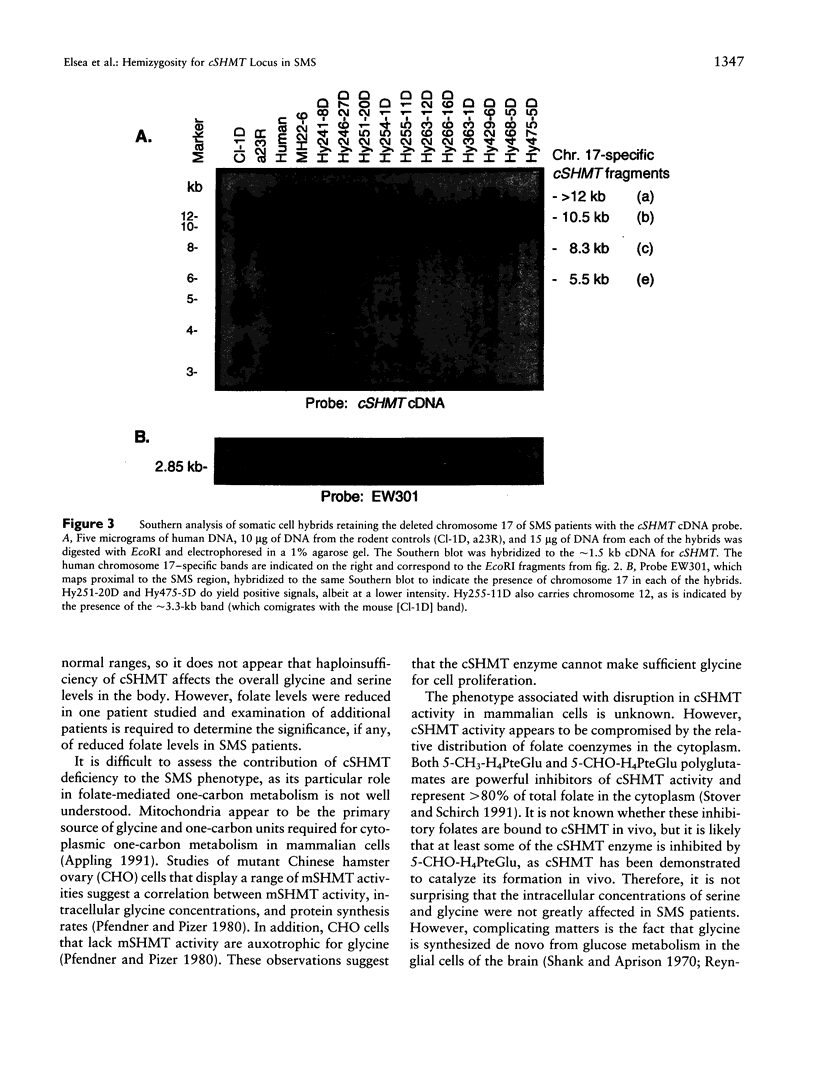
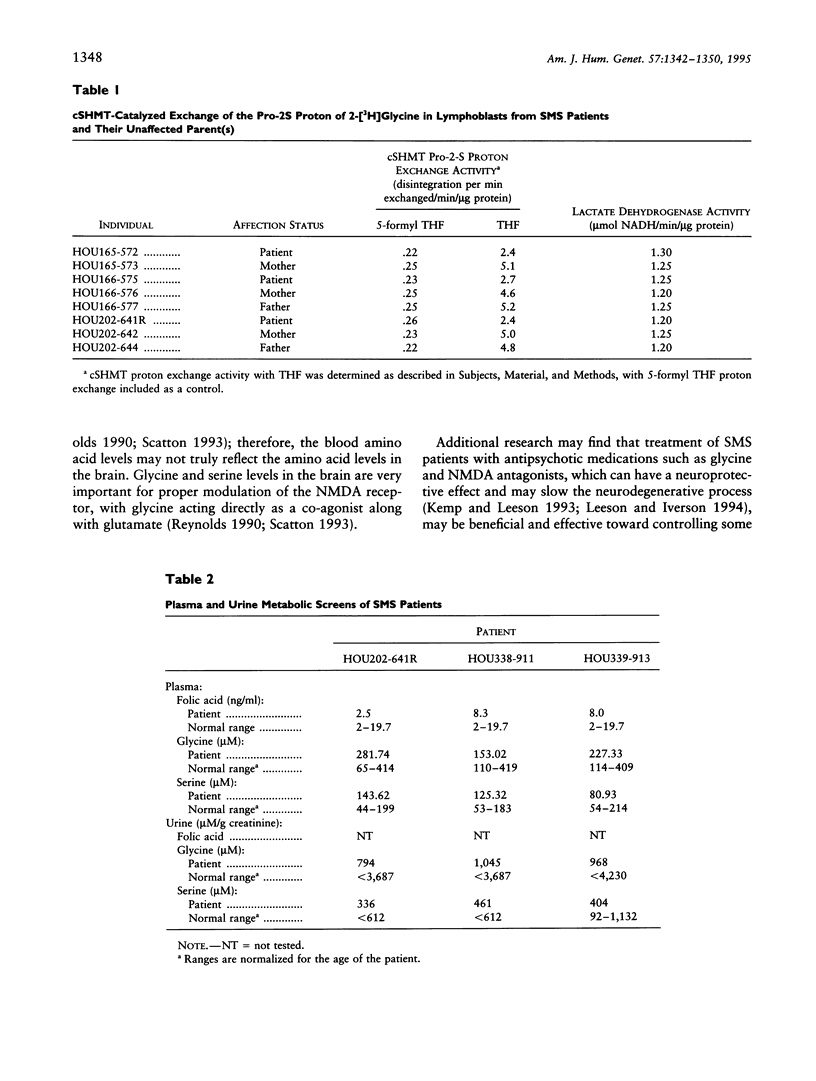
Folate-dependent one-carbon metabolism is critical for the synthesis of numerous cellular constituents required for cell growth, and serine hydroxymethyltransferase (SHMT) is central to this process. Our studies reveal that the gene for cytosolic SHMT (cSHMT) maps to the critical interval for Smith-Magenis syndrome (SMS) on chromosome 17p11.2. The basic organization of the cSHMT locus on chromosome 17 was determined and was found to be deleted in all 26 SMS patients examined by PCR, FISH, and/or Southern analysis. Furthermore, with respect to haploinsufficiency, cSHMT enzyme activity in patient lymphoblasts was determined to be approximately 50% that of unaffected parent lymphoblasts. Serine, glycine, and folate levels were also assessed in three SMS patients and were found to be within normal ranges. The possible effects of cSHMT hemizygosity on the SMS phenotype are discussed.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appling D. R. Compartmentation of folate-mediated one-carbon metabolism in eukaryotes. FASEB J. 1991 Sep;5(12):2645–2651. doi: 10.1096/fasebj.5.12.1916088. [DOI] [PubMed] [Google Scholar]
- Chen K. S., Gunaratne P. H., Hoheisel J. D., Young I. G., Miklos G. L., Greenberg F., Shaffer L. G., Campbell H. D., Lupski J. R. The human homologue of the Drosophila melanogaster flightless-I gene (flil) maps within the Smith-Magenis microdeletion critical region in 17p11.2. Am J Hum Genet. 1995 Jan;56(1):175–182. [PMC free article] [PubMed] [Google Scholar]
- Chevillard C., Le Paslier D., Passage E., Ougen P., Billault A., Boyer S., Mazan S., Bachellerie J. P., Vignal A., Cohen D. Relationship between Charcot-Marie-Tooth 1A and Smith-Magenis regions. snU3 may be a candidate gene for the Smith-Magenis syndrome. Hum Mol Genet. 1993 Aug;2(8):1235–1243. doi: 10.1093/hmg/2.8.1235. [DOI] [PubMed] [Google Scholar]
- Daly E. C., Aprison M. H. Distribution of serine hydroxymethyltransferase and glycine transaminase in several areas of the central nervous system of the rat. J Neurochem. 1974 Jun;22(6):877–885. doi: 10.1111/j.1471-4159.1974.tb04312.x. [DOI] [PubMed] [Google Scholar]
- Deutsch S. I., Mastropaolo J., Schwartz B. L., Rosse R. B., Morihisa J. M. A "glutamatergic hypothesis" of schizophrenia. Rationale for pharmacotherapy with glycine. Clin Neuropharmacol. 1989 Feb;12(1):1–13. [PubMed] [Google Scholar]
- Devor E. J., Waziri R. A familial/genetic study of plasma serine and glycine concentrations. Biol Psychiatry. 1993 Aug 15;34(4):221–225. doi: 10.1016/0006-3223(93)90075-o. [DOI] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Eichler H. G., Hubbard R., Snell K. The role of serine hydroxymethyltransferase in cell proliferation: DNA synthesis from serine following mitogenic stimulation of lymphocytes. Biosci Rep. 1981 Feb;1(2):101–106. doi: 10.1007/BF01117006. [DOI] [PubMed] [Google Scholar]
- Fain P. R., Barker D. F., Goldgar D. E., Wright E., Nguyen K., Carey J., Johnson J., Kivlin J., Willard H., Mathew C. Genetic analysis of NF1: identification of close flanking markers on chromosome 17. Genomics. 1987 Dec;1(4):340–345. doi: 10.1016/0888-7543(87)90034-6. [DOI] [PubMed] [Google Scholar]
- Finucane B. M., Jaeger E. R., Kurtz M. B., Weinstein M., Scott C. I., Jr Eye abnormalities in the Smith-Magenis contiguous gene deletion syndrome. Am J Med Genet. 1993 Feb 15;45(4):443–446. doi: 10.1002/ajmg.1320450409. [DOI] [PubMed] [Google Scholar]
- Finucane B. M., Konar D., Haas-Givler B., Kurtz M. B., Scott C. I., Jr The spasmodic upper-body squeeze: a characteristic behavior in Smith-Magenis syndrome. Dev Med Child Neurol. 1994 Jan;36(1):78–83. doi: 10.1111/j.1469-8749.1994.tb11770.x. [DOI] [PubMed] [Google Scholar]
- Finucane B. M., Kurtz M. B., Babu V. R., Scott C. I., Jr Mosaicism for deletion 17p11.2 in a boy with the Smith-Magenis syndrome. Am J Med Genet. 1993 Feb 15;45(4):447–449. doi: 10.1002/ajmg.1320450410. [DOI] [PubMed] [Google Scholar]
- Garrow T. A., Brenner A. A., Whitehead V. M., Chen X. N., Duncan R. G., Korenberg J. R., Shane B. Cloning of human cDNAs encoding mitochondrial and cytosolic serine hydroxymethyltransferases and chromosomal localization. J Biol Chem. 1993 Jun 5;268(16):11910–11916. [PubMed] [Google Scholar]
- Greenberg F., Guzzetta V., Montes de Oca-Luna R., Magenis R. E., Smith A. C., Richter S. F., Kondo I., Dobyns W. B., Patel P. I., Lupski J. R. Molecular analysis of the Smith-Magenis syndrome: a possible contiguous-gene syndrome associated with del(17)(p11.2). Am J Hum Genet. 1991 Dec;49(6):1207–1218. [PMC free article] [PubMed] [Google Scholar]
- Guzzetta V., Franco B., Trask B. J., Zhang H., Saucedo-Cardenas O., Montes de Oca-Luna R., Greenberg F., Chinault A. C., Lupski J. R., Patel P. I. Somatic cell hybrids, sequence-tagged sites, simple repeat polymorphisms, and yeast artificial chromosomes for physical and genetic mapping of proximal 17p. Genomics. 1992 Jul;13(3):551–559. doi: 10.1016/0888-7543(92)90124-b. [DOI] [PubMed] [Google Scholar]
- Ijdo J. W., Lindsay E. A., Wells R. A., Baldini A. Multiple variants in subtelomeric regions of normal karyotypes. Genomics. 1992 Dec;14(4):1019–1025. doi: 10.1016/s0888-7543(05)80125-9. [DOI] [PubMed] [Google Scholar]
- Juyal R. C., Greenberg F., Mengden G. A., Lupski J. R., Trask B. J., van den Engh G., Lindsay E. A., Christy H., Chen K. S., Baldini A. Smith-Magenis syndrome deletion: a case with equivocal cytogenetic findings resolved by fluorescence in situ hybridization. Am J Med Genet. 1995 Sep 11;58(3):286–291. doi: 10.1002/ajmg.1320580317. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P., Kallioniemi A., Mascio L., Sudar D., Pinkel D., Deaven L., Gray J. Physical mapping of chromosome 17 cosmids by fluorescence in situ hybridization and digital image analysis. Genomics. 1994 Mar 1;20(1):125–128. doi: 10.1006/geno.1994.1138. [DOI] [PubMed] [Google Scholar]
- Kemp J. A., Leeson P. D. The glycine site of the NMDA receptor--five years on. Trends Pharmacol Sci. 1993 Jan;14(1):20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- Lin B. F., Huang R. F., Shane B. Regulation of folate and one-carbon metabolism in mammalian cells. III. Role of mitochondrial folylpoly-gamma-glutamate synthetase. J Biol Chem. 1993 Oct 15;268(29):21674–21679. [PubMed] [Google Scholar]
- Patel P. I., Franco B., Garcia C., Slaugenhaupt S. A., Nakamura Y., Ledbetter D. H., Chakravarti A., Lupski J. R. Genetic mapping of autosomal dominant Charcot-Marie-Tooth disease in a large French-Acadian kindred: identification of new linked markers on chromosome 17. Am J Hum Genet. 1990 Apr;46(4):801–809. [PMC free article] [PubMed] [Google Scholar]
- Patel P. I., Roa B. B., Welcher A. A., Schoener-Scott R., Trask B. J., Pentao L., Snipes G. J., Garcia C. A., Francke U., Shooter E. M. The gene for the peripheral myelin protein PMP-22 is a candidate for Charcot-Marie-Tooth disease type 1A. Nat Genet. 1992 Jun;1(3):159–165. doi: 10.1038/ng0692-159. [DOI] [PubMed] [Google Scholar]
- Pfendner W., Pizer L. I. The metabolism of serine and glycine in mutant lines of Chinese hamster ovary cells. Arch Biochem Biophys. 1980 Apr 1;200(2):503–512. doi: 10.1016/0003-9861(80)90382-3. [DOI] [PubMed] [Google Scholar]
- Reynolds I. J. Modulation of NMDA receptor responsiveness by neurotransmitters, drugs and chemical modification. Life Sci. 1990;47(20):1785–1792. doi: 10.1016/0024-3205(90)90280-5. [DOI] [PubMed] [Google Scholar]
- Scatton B. The NMDA receptor complex. Fundam Clin Pharmacol. 1993;7(8):389–400. doi: 10.1111/j.1472-8206.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Schirch L. Serine hydroxymethyltransferase. Adv Enzymol Relat Areas Mol Biol. 1982;53:83–112. doi: 10.1002/9780470122983.ch3. [DOI] [PubMed] [Google Scholar]
- Shank R. P., Aprison M. H. The metabolism in vivo of glycine and serine in eight areas of the rat central nervous system. J Neurochem. 1970 Oct;17(10):1461–1475. doi: 10.1111/j.1471-4159.1970.tb00513.x. [DOI] [PubMed] [Google Scholar]
- Smith A. C., McGavran L., Robinson J., Waldstein G., Macfarlane J., Zonona J., Reiss J., Lahr M., Allen L., Magenis E. Interstitial deletion of (17)(p11.2p11.2) in nine patients. Am J Med Genet. 1986 Jul;24(3):393–414. doi: 10.1002/ajmg.1320240303. [DOI] [PubMed] [Google Scholar]
- Snell K. Enzymes of serine metabolism in normal, developing and neoplastic rat tissues. Adv Enzyme Regul. 1984;22:325–400. doi: 10.1016/0065-2571(84)90021-9. [DOI] [PubMed] [Google Scholar]
- Snell K., Natsumeda Y., Weber G. The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem J. 1987 Jul 15;245(2):609–612. doi: 10.1042/bj2450609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stover P., Schirch V. 5-Formyltetrahydrofolate polyglutamates are slow tight binding inhibitors of serine hydroxymethyltransferase. J Biol Chem. 1991 Jan 25;266(3):1543–1550. [PubMed] [Google Scholar]
- Usha R., Savithri H. S., Rao N. A. The primary structure of sheep liver cytosolic serine hydroxymethyltransferase and an analysis of the evolutionary relationships among serine hydroxymethyltransferases. Biochim Biophys Acta. 1994 Jan 11;1204(1):75–83. doi: 10.1016/0167-4838(94)90035-3. [DOI] [PubMed] [Google Scholar]
- Waziri R., Baruah S., Hegwood T. S., Sherman A. D. Abnormal serine hydroxymethyl transferase activity in the temporal lobes of schizophrenics. Neurosci Lett. 1990 Dec 11;120(2):237–240. doi: 10.1016/0304-3940(90)90048-e. [DOI] [PubMed] [Google Scholar]
- Waziri R., Baruah S., Sherman A. D. Abnormal serine-glycine metabolism in the brains of schizophrenics. Schizophr Res. 1993 Jan;8(3):233–243. doi: 10.1016/0920-9964(93)90021-a. [DOI] [PubMed] [Google Scholar]
- Wright E. C., Goldgar D. E., Fain P. R., Barker D. F., Skolnick M. H. A genetic map of human chromosome 17p. Genomics. 1990 May;7(1):103–109. doi: 10.1016/0888-7543(90)90524-x. [DOI] [PubMed] [Google Scholar]
- Zhao Z., Lee C. C., Jiralerspong S., Juyal R. C., Lu F., Baldini A., Greenberg F., Caskey C. T., Patel P. I. The gene for a human microfibril-associated glycoprotein is commonly deleted in Smith-Magenis syndrome patients. Hum Mol Genet. 1995 Apr;4(4):589–597. doi: 10.1093/hmg/4.4.589. [DOI] [PubMed] [Google Scholar]
- Zori R. T., Lupski J. R., Heju Z., Greenberg F., Killian J. M., Gray B. A., Driscoll D. J., Patel P. I., Zackowski J. L. Clinical, cytogenetic, and molecular evidence for an infant with Smith-Magenis syndrome born from a mother having a mosaic 17p11.2p12 deletion. Am J Med Genet. 1993 Sep 15;47(4):504–511. doi: 10.1002/ajmg.1320470414. [DOI] [PubMed] [Google Scholar]