Abstract
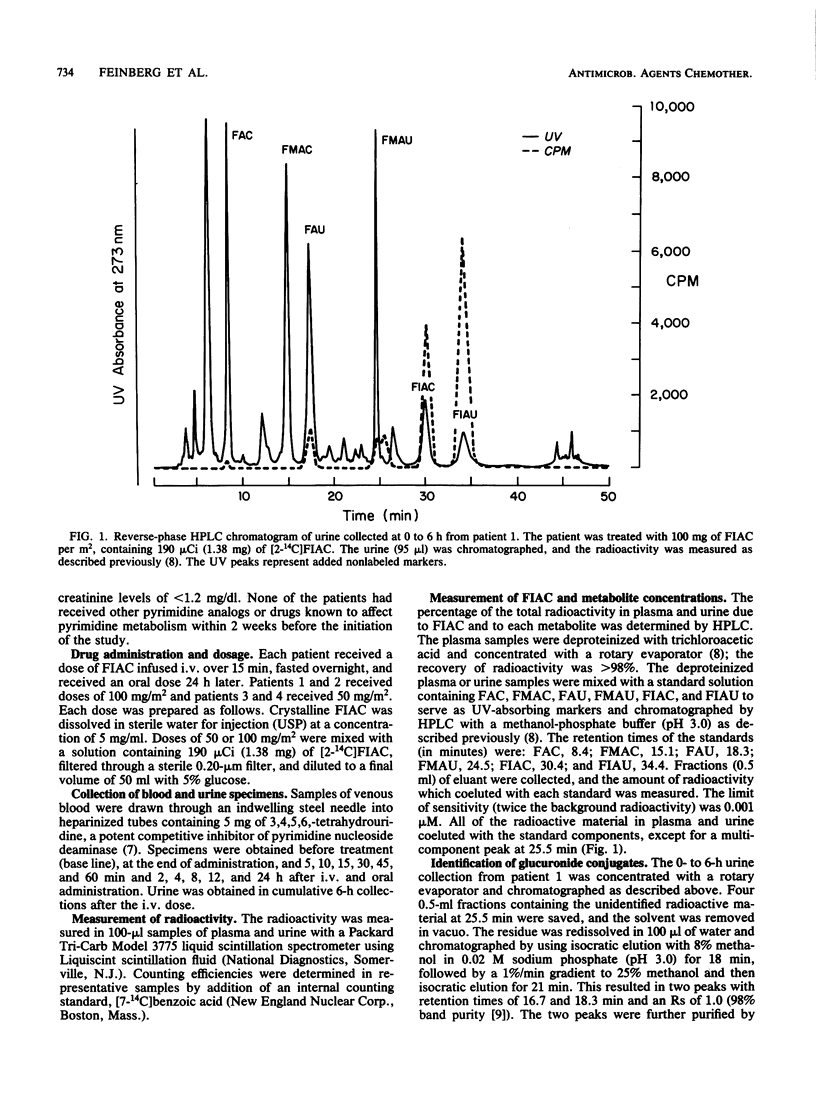
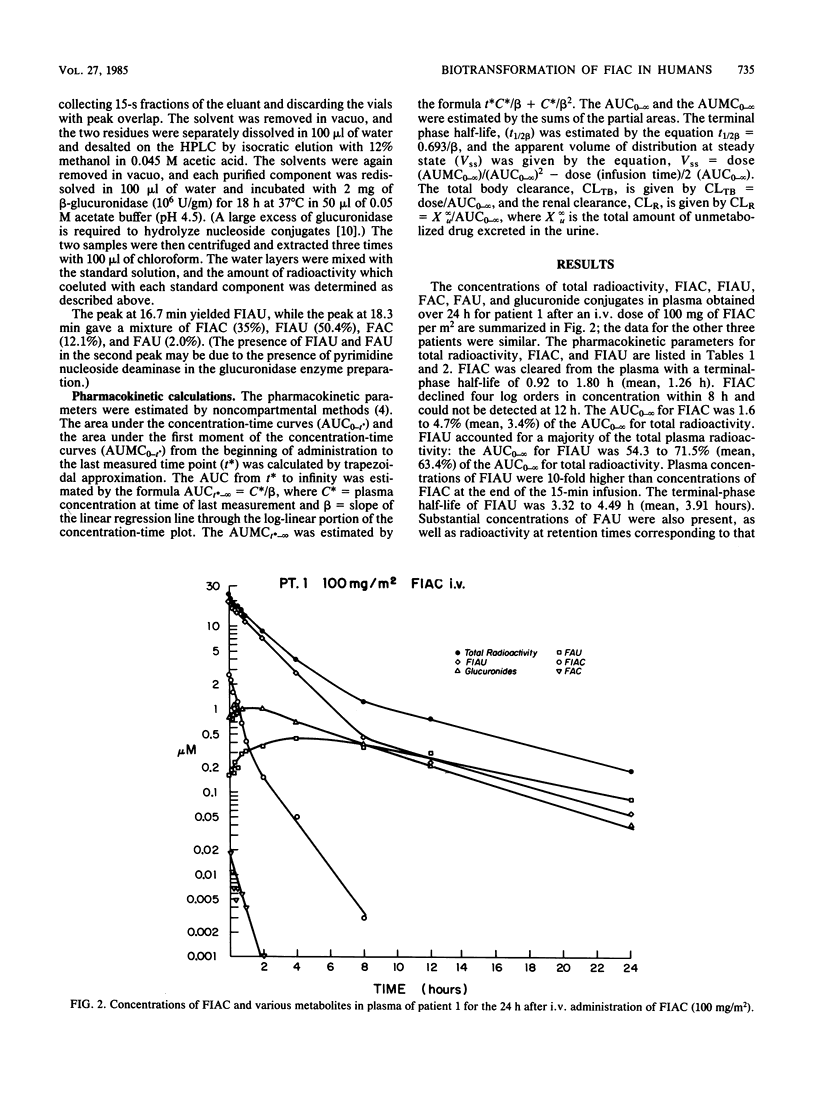
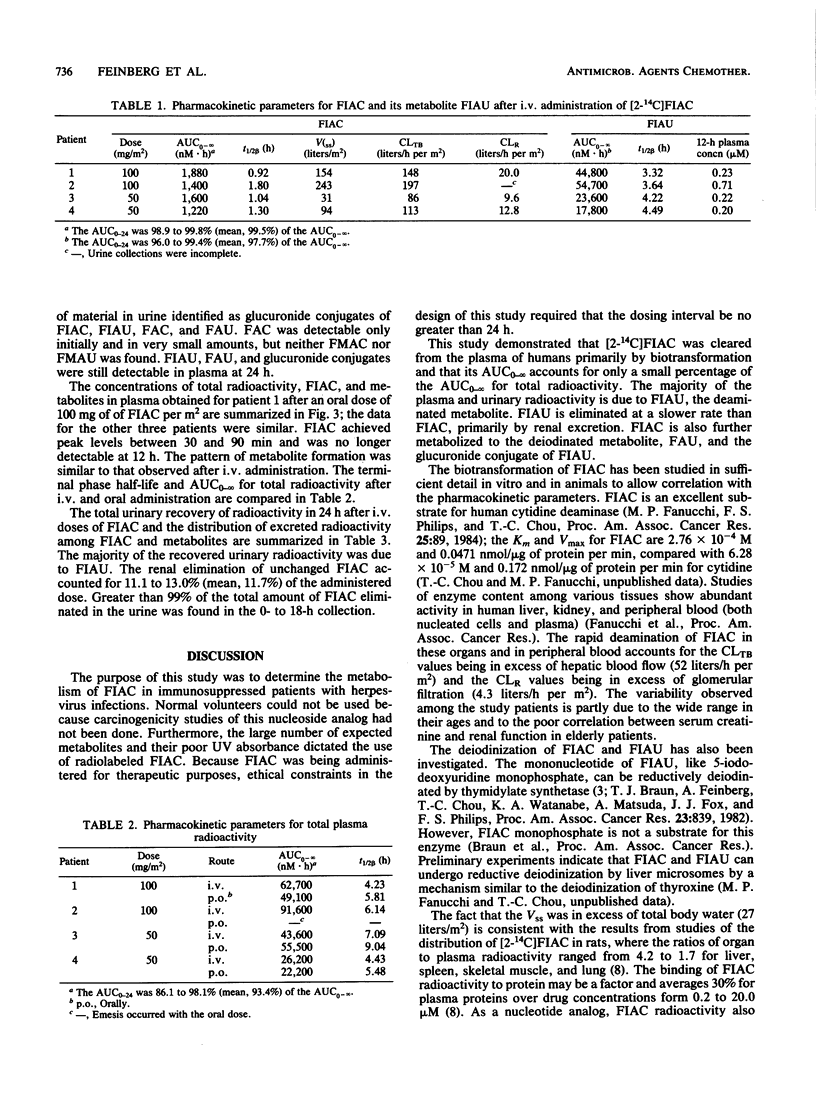
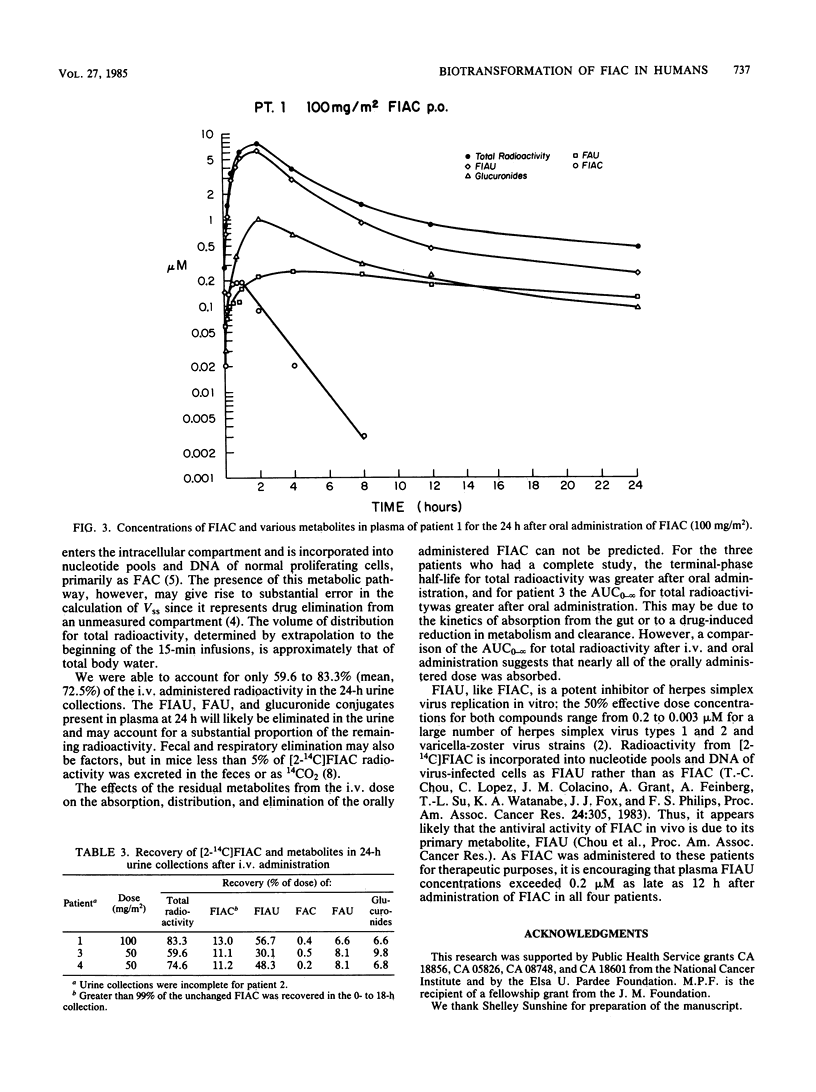
The metabolism of the drug [2-14C]-1-(2'-deoxy-2'-fluoro-beta-D -arabinofuranosyl)-5-iodocytosine (FIAC), a potent inhibitor of herpesvirus replication, was studied in immunosuppressed patients with herpesvirus infections. FIAC was administered intravenously by 15-min infusion and by mouth 24 h later to four patients at doses of 50 or 100 mg/m2. FIAC was cleared from the plasma primarily by biotransformation in liver, kidney, and peripheral blood, with a terminal-phase half-life of 0.92 to 1.80 h (mean, 1.36 h) after intravenous administration. The area under the concentration-time curve from zero to infinity (AUC0-infinity) for FIAC was 1.6 to 4.7% (mean, 3.4%) of the AUC0-infinity for total radioactivity. 1-(2'-Deoxy-2'-fluoro-beta-D-arabinofuranosyl)-5-iodouracil (FIAU) was the major metabolite; the AUC0-infinity for FIAU was 54.3 to 72.5% (mean, 63.4%) of the AUC0-infinity for total radioactivity. The terminal-phase half-life for FIAU was 3.32 to 4.49 h (mean, 3.91 h); FIAU was cleared from plasma by renal elimination and further biotransformation. lesser amounts of 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)uracil, 1-(2'-deoxy-2'-fluoro-beta-D-arabinofuranosyl)cytosine, the glucuronide conjugates of these metabolites, and the glucuronide conjugates of FIAC and FIAU were also formed. A comparison of the AUC0-infinity for total radioactivity after intravenous and oral administration suggested that nearly all of the oral dose was absorbed. Plasma levels of FIAU, also a potent inhibitor of herpesvirus replication in vitro, exceeded the 50% effective dose for herpes simplex virus and varicella-zoster virus as late as 12 h after administration of FIAC.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chou T. C., Feinberg A., Grant A. J., Vidal P., Reichman U., Watanabe K. A., Fox J. J., Philips F. S. Pharmacological disposition and metabolic fate of 2'-fluoro-5-iodo-1-beta-D-arabinofuranosylcytosine in mice and rats. Cancer Res. 1981 Sep;41(9 Pt 1):3336–3342. [PubMed] [Google Scholar]
- Garrett C., Wataya Y., Santi D. V. Thymidylate synthetase. Catalysis of dehalogenation of 5-bromo- and 5-iodo-2'-deoxyuridylate. Biochemistry. 1979 Jun 26;18(13):2798–2804. doi: 10.1021/bi00580a017. [DOI] [PubMed] [Google Scholar]
- Grant A. J., Feinberg A., Chou T. C., Watanabe K. A., Fox J. J., Philips F. S. Incorporation of metabolites of 2'-fluoro-5-iodo-1-beta-D-arabinofuranosylcytosine into deoxyribonucleic acid of neoplastic and normal mammalian tissues. Biochem Pharmacol. 1982 Mar 15;31(6):1103–1108. doi: 10.1016/0006-2952(82)90349-5. [DOI] [PubMed] [Google Scholar]
- Lopez C., Watanabe K. A., Fox J. J. 2'-fluoro-5-iodo-aracytosine, a potent and selective anti-herpesvirus agent. Antimicrob Agents Chemother. 1980 May;17(5):803–806. doi: 10.1128/aac.17.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maley F., Maley G. F. Tetrahydrodeoxyuridylate: a potent inhibitor of deoxycytidylate deaminase. Arch Biochem Biophys. 1971 Jun;144(2):723–729. doi: 10.1016/0003-9861(71)90379-1. [DOI] [PubMed] [Google Scholar]
- Philips F. S., Feinberg A., Chou T. C., Vidal P. M., Su T. L., Watanabe K. A., Fox J. J. Distribution, metabolism, and excretion of 1-(2-fluoro-2-deoxy-beta-D- arabinofuranosyl)thymine and 1-(2-fluoro-2-deoxy-beta-D-arabinofuranosyl)-5- iodocytosine. Cancer Res. 1983 Aug;43(8):3619–3627. [PubMed] [Google Scholar]
- Watanabe K. A., Matsuda A., Halat M. J., Hollenberg D. H., Nisselbaum J. S., Fox J. J. Nucleosides. 114. 5'-O-Glucuronides of 5-fluorouridine and 5-fluorocytidine. Masked precursors of anticancer nucleosides. J Med Chem. 1981 Jul;24(7):893–897. doi: 10.1021/jm00139a026. [DOI] [PubMed] [Google Scholar]
- Watanabe K. A., Reichman U., Hirota K., Lopez C., Fox J. J. Nucleosides. 110. Synthesis and antiherpes virus activity of some 2'-fluoro-2'-deoxyarabinofuranosylpyrimidine nucleosides. J Med Chem. 1979 Jan;22(1):21–24. doi: 10.1021/jm00187a005. [DOI] [PubMed] [Google Scholar]
- Young C. W., Schneider R., Leyland-Jones B., Armstrong D., Tan C. T., Lopez C., Watanabe K. A., Fox J. J., Philips F. S. Phase I evaluation of 2'-fluoro-5-iodo-1-beta-D-arabinofuranosylcytosine in immunosuppressed patients with herpesvirus infection. Cancer Res. 1983 Oct;43(10):5006–5009. [PubMed] [Google Scholar]



