Abstract
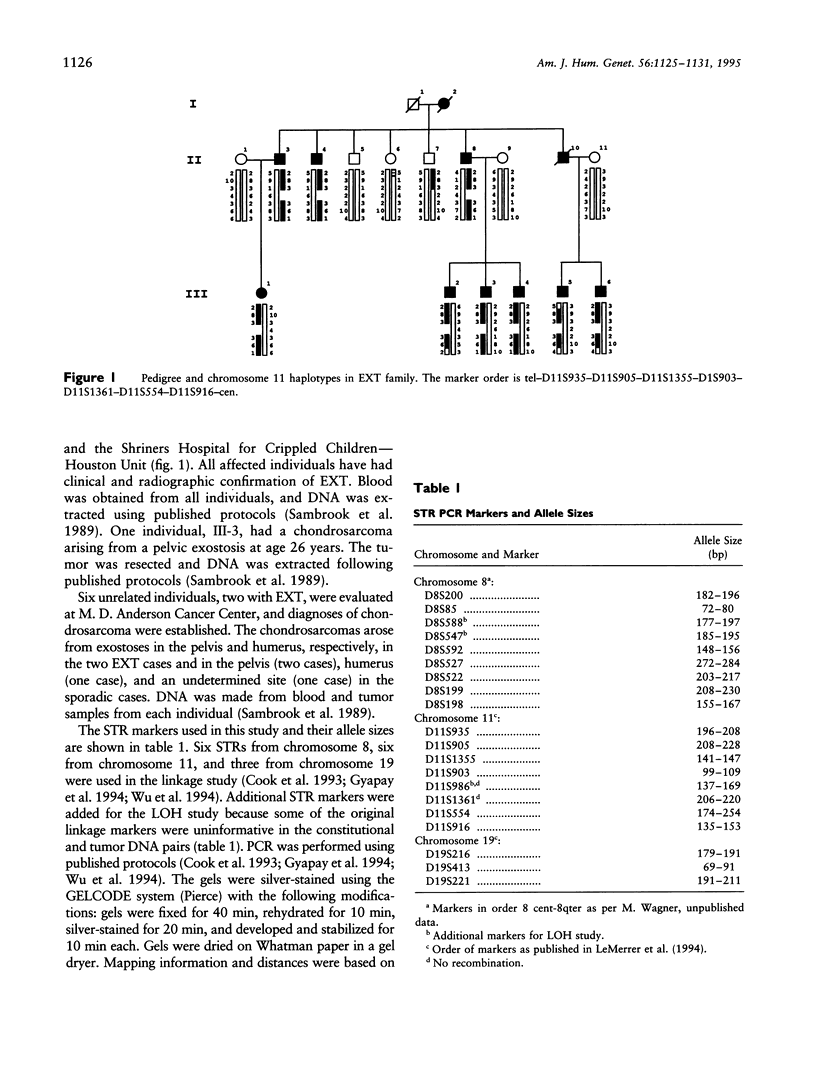
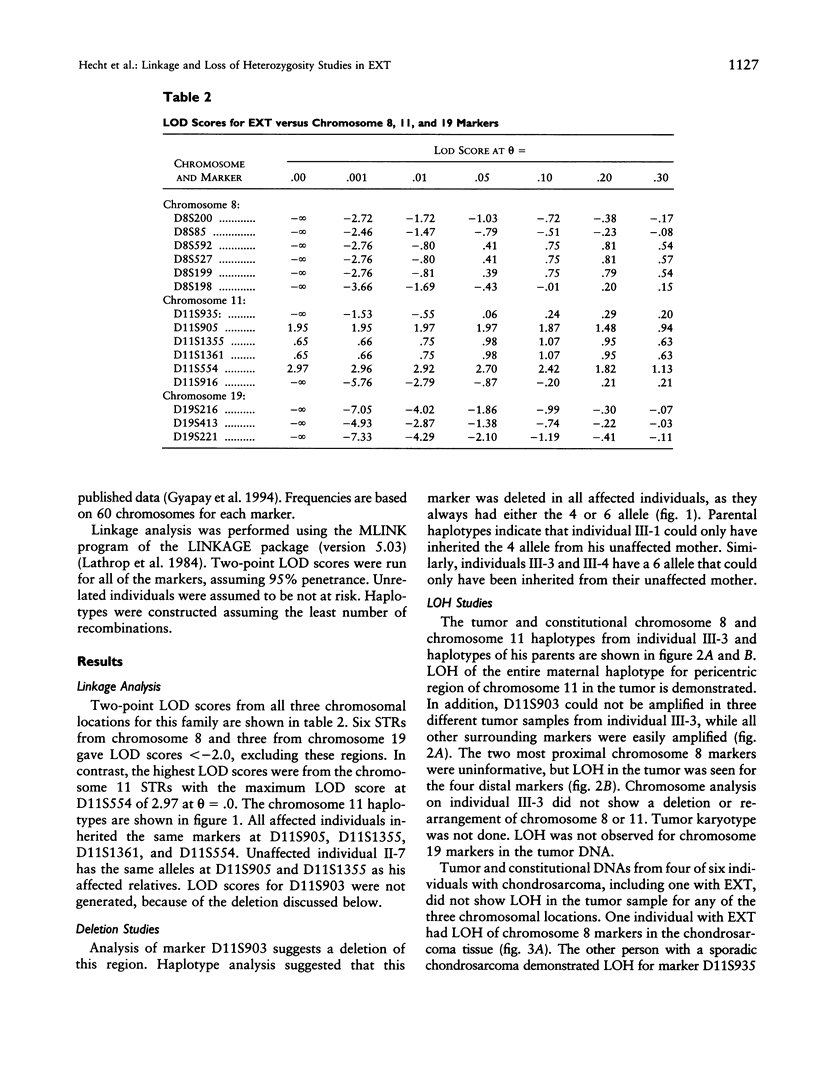
Hereditary multiple exostosis (EXT) is an autosomal dominant disorder characterized by bony exostoses at the ends of the long bones. Linkage studies have recently suggested that there are three chromosomal locations for EXT genes, 8q24.1 (EXT1), the pericentric region of 11 (EXT2), and 19p (EXT3). As part of a larger study to determine the frequencies of the three EXT types in the United States, we have ascertained a large multigenerational family with EXT and one family member with a chondrosarcoma. This family demonstrated linkage of the disease to chromosome 11 markers. The constitutional and tumor DNAs from the affected family member were compared using short-tandem-repeat markers from chromosomes 8, 11, and 19. Loss of heterozygosity (LOH) in the tumor was observed for chromosome 8 and 11 markers, but chromosome 19 markers were intact. An apparent deletion of the marker D11S903 was observed in constitutional DNA from all affected individuals and in the tumor sample. These results indicate that the EXT2 gene maps to the region containing marker D11S903, which is flanked by markers D11S1355 and D11S1361. Additional constitutional and chondrosarcoma DNA pairs from six unrelated individuals, two of whom had EXT, were similarly analyzed. One tumor from an individual with EXT demonstrated LOH for chromosome 8 markers, and a person with a sporadic chondrosarcoma was found to have tumor-specific LOH and a homozygous deletion of chromosome 11 markers. These findings suggest that EXT genes may be tumor-suppressor genes and that the initiation of tumor development may follow a multistep model.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bühler E. M., Malik N. J. The tricho-rhino-phalangeal syndrome(s): chromosome 8 long arm deletion: is there a shortest region of overlap between reported cases? TRP I and TRP II syndromes: are they separate entities? Am J Med Genet. 1984 Sep;19(1):113–119. doi: 10.1002/ajmg.1320190111. [DOI] [PubMed] [Google Scholar]
- Cook A., Raskind W., Blanton S. H., Pauli R. M., Gregg R. G., Francomano C. A., Puffenberger E., Conrad E. U., Schmale G., Schellenberg G. Genetic heterogeneity in families with hereditary multiple exostoses. Am J Hum Genet. 1993 Jul;53(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- Dryja T. P., Mukai S., Petersen R., Rapaport J. M., Walton D., Yandell D. W. Parental origin of mutations of the retinoblastoma gene. Nature. 1989 Jun 15;339(6225):556–558. doi: 10.1038/339556a0. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Langer L. O., Jr, Krassikoff N., Laxova R., Scheer-Williams M., Lutter L. D., Gorlin R. J., Jennings C. G., Day D. W. The tricho-rhino-phalangeal syndrome with exostoses (or Langer-Giedion syndrome): four additional patients without mental retardation and review of the literature. Am J Med Genet. 1984 Sep;19(1):81–112. doi: 10.1002/ajmg.1320190110. [DOI] [PubMed] [Google Scholar]
- Lathrop G. M., Lalouel J. M., Julier C., Ott J. Strategies for multilocus linkage analysis in humans. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3443–3446. doi: 10.1073/pnas.81.11.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Merrer M., Legeai-Mallet L., Jeannin P. M., Horsthemke B., Schinzel A., Plauchu H., Toutain A., Achard F., Munnich A., Maroteaux P. A gene for hereditary multiple exostoses maps to chromosome 19p. Hum Mol Genet. 1994 May;3(5):717–722. doi: 10.1093/hmg/3.5.717. [DOI] [PubMed] [Google Scholar]
- Lemieux N., Milot J., Barsoum-Homsy M., Michaud J., Leung T. K., Richer C. L. First cytogenetic evidence of homozygosity for the retinoblastoma deletion in chromosome 13. Cancer Genet Cytogenet. 1989 Nov;43(1):73–78. doi: 10.1016/0165-4608(89)90129-5. [DOI] [PubMed] [Google Scholar]
- Mertens F., Rydholm A., Kreicbergs A., Willén H., Jonsson K., Heim S., Mitelman F., Mandahl N. Loss of chromosome band 8q24 in sporadic osteocartilaginous exostoses. Genes Chromosomes Cancer. 1994 Jan;9(1):8–12. doi: 10.1002/gcc.2870090103. [DOI] [PubMed] [Google Scholar]
- Ogle R. F., Dalzell P., Turner G., Wass D., Yip M. Y. Multiple exostoses in a patient with t(8;11)(q24.11;p15.5). J Med Genet. 1991 Dec;28(12):881–883. doi: 10.1136/jmg.28.12.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponder B. Cancer. Gene losses in human tumours. Nature. 1988 Sep 29;335(6189):400–402. doi: 10.1038/335400a0. [DOI] [PubMed] [Google Scholar]
- Scheffer H., Kruize Y. C., Osinga J., Kuiken G., Oosterhuis J. W., Leeuw J. A., Schraffordt Koops H., Buys C. H. Complete association of loss of heterozygosity of chromosomes 13 and 17 in osteosarcoma. Cancer Genet Cytogenet. 1991 May;53(1):45–55. doi: 10.1016/0165-4608(91)90113-9. [DOI] [PubMed] [Google Scholar]
- Schmale G. A., Conrad E. U., 3rd, Raskind W. H. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994 Jul;76(7):986–992. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- Voutsinas S., Wynne-Davies R. The infrequency of malignant disease in diaphyseal aclasis and neurofibromatosis. J Med Genet. 1983 Oct;20(5):345–349. doi: 10.1136/jmg.20.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicklund C. L., Pauli R. M., Johnston D., Hecht J. T. Natural history study of hereditary multiple exostoses. Am J Med Genet. 1995 Jan 2;55(1):43–46. doi: 10.1002/ajmg.1320550113. [DOI] [PubMed] [Google Scholar]
- Wu Y. Q., Heutink P., de Vries B. B., Sandkuijl L. A., van den Ouweland A. M., Niermeijer M. F., Galjaard H., Reyniers E., Willems P. J., Halley D. J. Assignment of a second locus for multiple exostoses to the pericentromeric region of chromosome 11. Hum Mol Genet. 1994 Jan;3(1):167–171. doi: 10.1093/hmg/3.1.167. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Toguchida J., Yamamuro T., Kotoura Y., Takada N., Kawaguchi N., Kaneko Y., Nakamura Y., Sasaki M. S., Ishizaki K. Allelotype analysis in osteosarcomas: frequent allele loss on 3q, 13q, 17p, and 18q. Cancer Res. 1992 May 1;52(9):2419–2423. [PubMed] [Google Scholar]
- Yamamoto Y., Oguro N., Miyao M., Yanagisawa M. Tricho-rhino-phalangeal syndrome type I with severe mental retardation due to interstitial deletion of 8q23.3-24.13. Am J Med Genet. 1989 Jan;32(1):133–135. doi: 10.1002/ajmg.1320320128. [DOI] [PubMed] [Google Scholar]




