Abstract
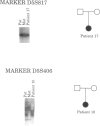
Cri du chat syndrome (CDC) is a segmental aneusomy associated with deletions of chromosome 5p15. In an effort to define regions that produce the phenotypes associated with CDC, we have analyzed deletions from 17 patients. The majority of these patients had atypical CDC features or were asymptomatic. Using these patients, we have mapped several phenotypes associated with deletions of 5p, including speech delay, catlike cry, newborn facial dysmorphism, and adult facial dysmorphism. This phenotypic map should provide a framework with which to begin identification of genes associated with various phenotypic features associated with deletions of distal 5p. We have also analyzed the parental origin of the de novo deletions, to determine if genomic imprinting could be occurring in this region. In addition, we have isolated cosmids that could be useful for both prenatal and postnatal assessments of del5(p) individuals.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baccichetti C., Lenzini E., Artifoni L., Caufin D., Marangoni P. Terminal deletion of the short arm of chromosome 5. Clin Genet. 1988 Oct;34(4):219–223. doi: 10.1111/j.1399-0004.1988.tb02868.x. [DOI] [PubMed] [Google Scholar]
- Bernstein R., Bocian M. E., Cain M. J., Bengtsson U., Wasmuth J. J. Identification of a cryptic t(5;7) reciprocal translocation by fluorescent in situ hybridization. Am J Med Genet. 1993 Apr 1;46(1):77–82. doi: 10.1002/ajmg.1320460113. [DOI] [PubMed] [Google Scholar]
- Brant S. R., Bernstein M., Wasmuth J. J., Taylor E. W., McPherson J. D., Li X., Walker S., Pouyssegur J., Donowitz M., Tse C. M. Physical and genetic mapping of a human apical epithelial Na+/H+ exchanger (NHE3) isoform to chromosome 5p15.3. Genomics. 1993 Mar;15(3):668–672. doi: 10.1006/geno.1993.1122. [DOI] [PubMed] [Google Scholar]
- Breg W. R., Steele M. W., Miller O. J., Warburton D., DeCapoa A., Allderdice P. W. The cri du chat syndrome in adolescents and adults: clinical finding in 13 older patients with partial deletion of the short arm of chromosome No. 5(5p-). J Pediatr. 1970 Nov;77(5):782–791. doi: 10.1016/s0022-3476(70)80236-0. [DOI] [PubMed] [Google Scholar]
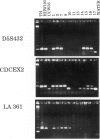
- Dana S., Wasmuth J. J. Linkage of the leuS, emtB, and chr genes on chromosome 5 in humans and expression of human genes encoding protein synthetic components in human--Chinese hamster hybrids. Somatic Cell Genet. 1982 Mar;8(2):245–264. doi: 10.1007/BF01538680. [DOI] [PubMed] [Google Scholar]
- Giros B., el Mestikawy S., Godinot N., Zheng K., Han H., Yang-Feng T., Caron M. G. Cloning, pharmacological characterization, and chromosome assignment of the human dopamine transporter. Mol Pharmacol. 1992 Sep;42(3):383–390. [PubMed] [Google Scholar]
- Haber D. A., Buckler A. J., Glaser T., Call K. M., Pelletier J., Sohn R. L., Douglass E. C., Housman D. E. An internal deletion within an 11p13 zinc finger gene contributes to the development of Wilms' tumor. Cell. 1990 Jun 29;61(7):1257–1269. doi: 10.1016/0092-8674(90)90690-g. [DOI] [PubMed] [Google Scholar]
- Jinno Y., Yun K., Nishiwaki K., Kubota T., Ogawa O., Reeve A. E., Niikawa N. Mosaic and polymorphic imprinting of the WT1 gene in humans. Nat Genet. 1994 Mar;6(3):305–309. doi: 10.1038/ng0394-305. [DOI] [PubMed] [Google Scholar]
- Kushnick T., Rao K. W., Lamb A. N. Familial 5p- syndrome. Clin Genet. 1984 Nov;26(5):472–476. doi: 10.1111/j.1399-0004.1984.tb01091.x. [DOI] [PubMed] [Google Scholar]
- LEJEUNE J., LAFOURCADE J., BERGER R., VIALATTE J., BOESWILLWALD M., SERINGE P., TURPIN R. TROIS CAS DE D'EL'ETION PARTIELLE DU BRAS COURT D'UN CHROMOSOME 5. C R Hebd Seances Acad Sci. 1963 Nov 18;257:3098–3102. [PubMed] [Google Scholar]
- Niebuhr E. The Cri du Chat syndrome: epidemiology, cytogenetics, and clinical features. Hum Genet. 1978 Nov 16;44(3):227–275. doi: 10.1007/BF00394291. [DOI] [PubMed] [Google Scholar]
- Ohlsson R., Nyström A., Pfeifer-Ohlsson S., Töhönen V., Hedborg F., Schofield P., Flam F., Ekström T. J. IGF2 is parentally imprinted during human embryogenesis and in the Beckwith-Wiedemann syndrome. Nat Genet. 1993 May;4(1):94–97. doi: 10.1038/ng0593-94. [DOI] [PubMed] [Google Scholar]
- Overhauser J., Golbus M. S., Schonberg S. A., Wasmuth J. J. Molecular analysis of an unbalanced deletion of the short arm of chromosome 5 that produces no phenotype. Am J Hum Genet. 1986 Jul;39(1):1–10. [PMC free article] [PubMed] [Google Scholar]
- Overhauser J., Huang X., Gersh M., Wilson W., McMahon J., Bengtsson U., Rojas K., Meyer M., Wasmuth J. J. Molecular and phenotypic mapping of the short arm of chromosome 5: sublocalization of the critical region for the cri-du-chat syndrome. Hum Mol Genet. 1994 Feb;3(2):247–252. doi: 10.1093/hmg/3.2.247. [DOI] [PubMed] [Google Scholar]
- Overhauser J., McMahan J., Wasmuth J. J. Identification of 28 DNA fragments that detect RFLPs in 13 distinct physical regions of the short arm of chromosome 5. Nucleic Acids Res. 1987 Jun 11;15(11):4617–4627. doi: 10.1093/nar/15.11.4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overhauser J., McMahon J., Oberlender S., Carlin M. E., Niebuhr E., Wasmuth J. J., Lee-Chen J. Parental origin of chromosome 5 deletions in the cri-du-chat syndrome. Am J Med Genet. 1990 Sep;37(1):83–86. doi: 10.1002/ajmg.1320370119. [DOI] [PubMed] [Google Scholar]
- Rivier D. H., Pillus L. Silencing speaks up. Cell. 1994 Mar 25;76(6):963–966. doi: 10.1016/0092-8674(94)90373-5. [DOI] [PubMed] [Google Scholar]
- Shimada S., Kitayama S., Walther D., Uhl G. Dopamine transporter mRNA: dense expression in ventral midbrain neurons. Brain Res Mol Brain Res. 1992 May;13(4):359–362. doi: 10.1016/0169-328x(92)90220-6. [DOI] [PubMed] [Google Scholar]
- Smith A., Field B., Murray R., Nelson J. Two cases of cri-du-chat syndrome with mild phenotypic effect but with different size of 5p deletion. J Paediatr Child Health. 1990 Jun;26(3):152–154. doi: 10.1111/j.1440-1754.1990.tb02414.x. [DOI] [PubMed] [Google Scholar]
- Vandenbergh D. J., Persico A. M., Hawkins A. L., Griffin C. A., Li X., Jabs E. W., Uhl G. R. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992 Dec;14(4):1104–1106. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Wilkins L. E., Brown J. A., Nance W. E., Wolf B. Clinical heterogeneity in 80 home-reared children with cri du chat syndrome. J Pediatr. 1983 Apr;102(4):528–533. doi: 10.1016/s0022-3476(83)80179-6. [DOI] [PubMed] [Google Scholar]
- Wilkins L. E., Brown J. A., Wolf B. Psychomotor development in 65 home-reared children with cri-du-chat syndrome. J Pediatr. 1980 Sep;97(3):401–405. doi: 10.1016/s0022-3476(80)80189-2. [DOI] [PubMed] [Google Scholar]
- Winokur S. T., Bengtsson U., Feddersen J., Mathews K. D., Weiffenbach B., Bailey H., Markovich R. P., Murray J. C., Wasmuth J. J., Altherr M. R. The DNA rearrangement associated with facioscapulohumeral muscular dystrophy involves a heterochromatin-associated repetitive element: implications for a role of chromatin structure in the pathogenesis of the disease. Chromosome Res. 1994 May;2(3):225–234. doi: 10.1007/BF01553323. [DOI] [PubMed] [Google Scholar]