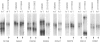Abstract
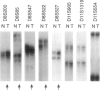
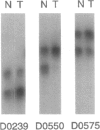
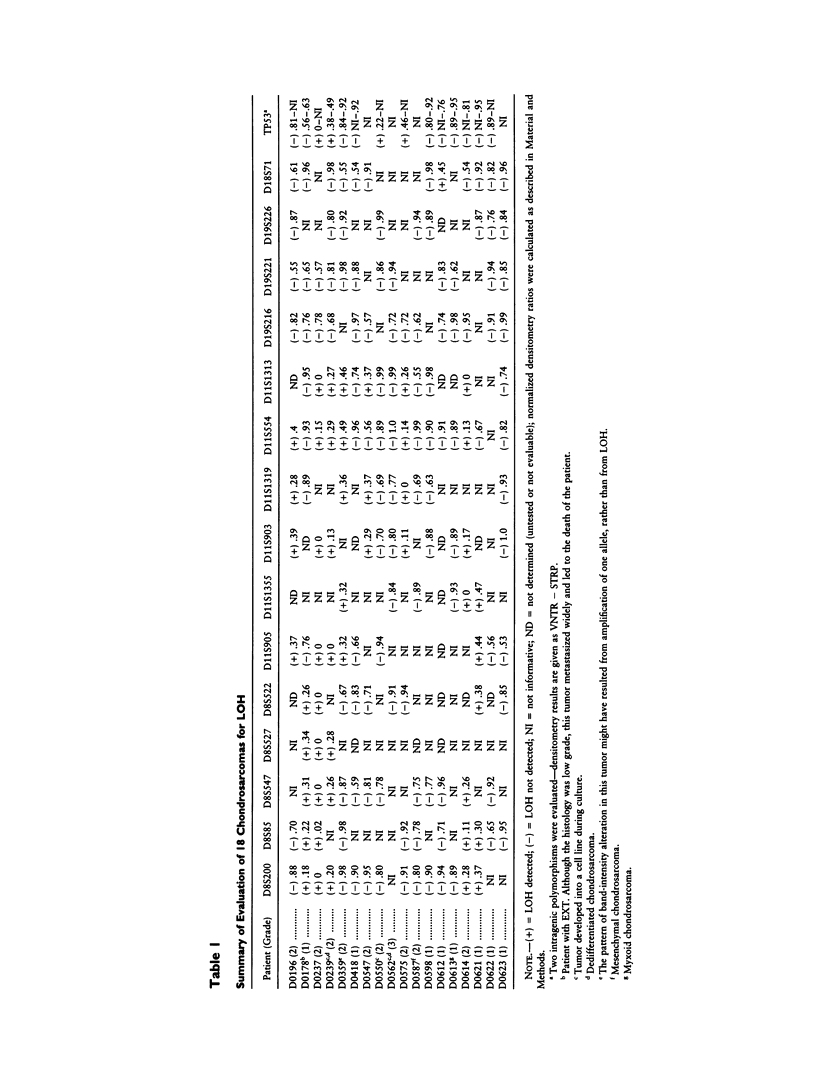
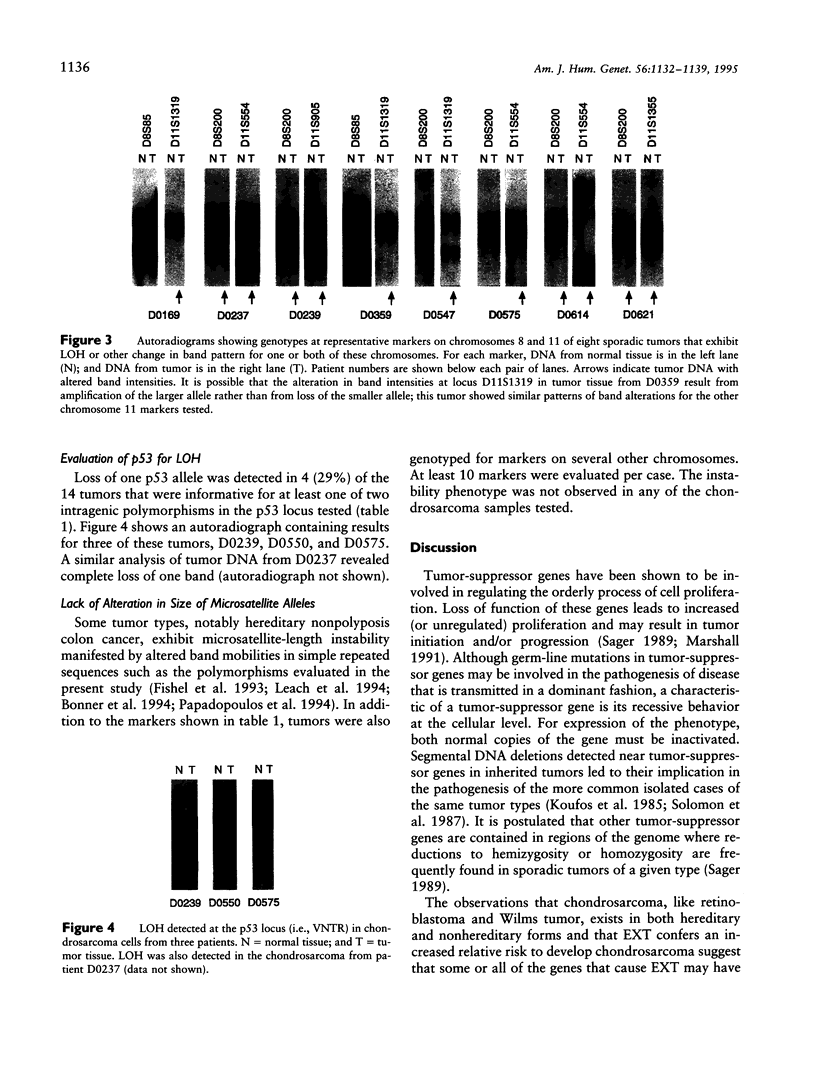
Hereditary multiple exostoses (EXT; MIM 133700) is an autosomal dominant condition characterized by growth of multiple benign cartilage-capped tumors. EXT greatly increases the relative risk to develop chondrosarcoma, although most chondrosarcomas are sporadic. This observation suggests that, like the genes responsible for retinoblastoma and other dominantly inherited cancer susceptibility disorders, the genes that cause EXT may have tumor-suppressor function and may play a role in the pathogenesis of the related sporadic tumors. To investigate this hypothesis, we evaluated chondrosarcomas for loss of constitutional heterozygosity (LOH) at polymorphic loci linked to three recently identified genomic regions containing genes involved in EXT. LOH for markers linked to EXT1 on chromosome 8 was detected in a chondrosarcoma that arose in a man with EXT. Four of 17 sporadic tumors showed LOH for markers linked to EXT1, and 7 showed LOH for markers linked to EXT2 on chromosome 11. In all, LOH was observed for markers linked to EXT1 or EXT2 in 44% of the 18 tumors, whereas heterozygosity was retained for markers on 19p linked to EXT3. These findings support the hypothesis that genes on 8q and the pericentromeric region of 11 have tumor-suppressor function and play a role in the development of chondrosarcomas.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedict W. F., Xu H. J., Hu S. X., Takahashi R. Role of the retinoblastoma gene in the initiation and progression of human cancer. J Clin Invest. 1990 Apr;85(4):988–993. doi: 10.1172/JCI114575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronner C. E., Baker S. M., Morrison P. T., Warren G., Smith L. G., Lescoe M. K., Kane M., Earabino C., Lipford J., Lindblom A. Mutation in the DNA mismatch repair gene homologue hMLH1 is associated with hereditary non-polyposis colon cancer. Nature. 1994 Mar 17;368(6468):258–261. doi: 10.1038/368258a0. [DOI] [PubMed] [Google Scholar]
- Cawkwell L., Bell S. M., Lewis F. A., Dixon M. F., Taylor G. R., Quirke P. Rapid detection of allele loss in colorectal tumours using microsatellites and fluorescent DNA technology. Br J Cancer. 1993 Jun;67(6):1262–1267. doi: 10.1038/bjc.1993.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook A., Raskind W., Blanton S. H., Pauli R. M., Gregg R. G., Francomano C. A., Puffenberger E., Conrad E. U., Schmale G., Schellenberg G. Genetic heterogeneity in families with hereditary multiple exostoses. Am J Hum Genet. 1993 Jul;53(1):71–79. [PMC free article] [PubMed] [Google Scholar]
- Dorfman H. D., Czerniak B. Bone cancers. Cancer. 1995 Jan 1;75(1 Suppl):203–210. doi: 10.1002/1097-0142(19950101)75:1+<203::aid-cncr2820751308>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Evans H. L., Ayala A. G., Romsdahl M. M. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977 Aug;40(2):818–831. doi: 10.1002/1097-0142(197708)40:2<818::aid-cncr2820400234>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Emi M., Ohata H., Kato Y., Nakajima T., Mori T., Nakamura Y. Evidence for the presence of two tumor suppressor genes on chromosome 8p for colorectal carcinoma. Cancer Res. 1993 Mar 1;53(5):1172–1174. [PubMed] [Google Scholar]
- Garrison R. C., Unni K. K., McLeod R. A., Pritchard D. J., Dahlin D. C. Chondrosarcoma arising in osteochondroma. Cancer. 1982 May 1;49(9):1890–1897. doi: 10.1002/1097-0142(19820501)49:9<1890::aid-cncr2820490923>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Gitelis S., Bertoni F., Picci P., Campanacci M. Chondrosarcoma of bone. The experience at the Istituto Ortopedico Rizzoli. J Bone Joint Surg Am. 1981 Oct;63(8):1248–1257. [PubMed] [Google Scholar]
- Gordon S. L., Buchanan J. R., Ladda R. L. Hereditary multiple exostoses: report of a kindred. J Med Genet. 1981 Dec;18(6):428–430. doi: 10.1136/jmg.18.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hampton G. M., Penny L. A., Baergen R. N., Larson A., Brewer C., Liao S., Busby-Earle R. M., Williams A. W., Steel C. M., Bird C. C. Loss of heterozygosity in cervical carcinoma: subchromosomal localization of a putative tumor-suppressor gene to chromosome 11q22-q24. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6953–6957. doi: 10.1073/pnas.91.15.6953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- James M. R., Richard C. W., 3rd, Schott J. J., Yousry C., Clark K., Bell J., Terwilliger J. D., Hazan J., Dubay C., Vignal A. A radiation hybrid map of 506 STS markers spanning human chromosome 11. Nat Genet. 1994 Sep;8(1):70–76. doi: 10.1038/ng0994-70. [DOI] [PubMed] [Google Scholar]
- Jones M. H., Koi S., Fujimoto I., Hasumi K., Kato K., Nakamura Y. Allelotype of uterine cancer by analysis of RFLP and microsatellite polymorphisms: frequent loss of heterozygosity on chromosome arms 3p, 9q, 10q, and 17p. Genes Chromosomes Cancer. 1994 Feb;9(2):119–123. doi: 10.1002/gcc.2870090207. [DOI] [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hamilton S. R., Hedge P., Markham A. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991 Mar 15;251(4999):1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- Knudson A. G., Jr Mutation and cancer: statistical study of retinoblastoma. Proc Natl Acad Sci U S A. 1971 Apr;68(4):820–823. doi: 10.1073/pnas.68.4.820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koufos A., Hansen M. F., Copeland N. G., Jenkins N. A., Lampkin B. C., Cavenee W. K. Loss of heterozygosity in three embryonal tumours suggests a common pathogenetic mechanism. Nature. 1985 Jul 25;316(6026):330–334. doi: 10.1038/316330a0. [DOI] [PubMed] [Google Scholar]
- Kreicbergs A., Boquist L., Borssén B., Larsson S. E. Prognostic factors in chondrosarcoma: a comparative study of cellular DNA content and clinicopathologic features. Cancer. 1982 Aug 1;50(3):577–583. doi: 10.1002/1097-0142(19820801)50:3<577::aid-cncr2820500332>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Le Merrer M., Legeai-Mallet L., Jeannin P. M., Horsthemke B., Schinzel A., Plauchu H., Toutain A., Achard F., Munnich A., Maroteaux P. A gene for hereditary multiple exostoses maps to chromosome 19p. Hum Mol Genet. 1994 May;3(5):717–722. doi: 10.1093/hmg/3.5.717. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Marshall C. J. Tumor suppressor genes. Cell. 1991 Jan 25;64(2):313–326. doi: 10.1016/0092-8674(91)90641-b. [DOI] [PubMed] [Google Scholar]
- Matise T. C., Perlin M., Chakravarti A. Automated construction of genetic linkage maps using an expert system (MultiMap): a human genome linkage map. Nat Genet. 1994 Apr;6(4):384–390. doi: 10.1038/ng0494-384. [DOI] [PubMed] [Google Scholar]
- Matsuno T., Ichioka Y., Yagi T., Ishii S. Spindle-cell sarcoma in patients who have osteochondromatosis. A report of two cases. J Bone Joint Surg Am. 1988 Jan;70(1):137–141. [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Goldman D. S., Sallan S. E. Development of homozygosity for chromosome 11p markers in Wilms' tumour. Nature. 1984 May 10;309(5964):172–174. doi: 10.1038/309172a0. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Peterson H. A. Multiple hereditary osteochondromata. Clin Orthop Relat Res. 1989 Feb;(239):222–230. [PubMed] [Google Scholar]
- Sager R. Tumor suppressor genes: the puzzle and the promise. Science. 1989 Dec 15;246(4936):1406–1412. doi: 10.1126/science.2574499. [DOI] [PubMed] [Google Scholar]
- Schmale G. A., Conrad E. U., 3rd, Raskind W. H. The natural history of hereditary multiple exostoses. J Bone Joint Surg Am. 1994 Jul;76(7):986–992. doi: 10.2106/00004623-199407000-00005. [DOI] [PubMed] [Google Scholar]
- Solomon E., Voss R., Hall V., Bodmer W. F., Jass J. R., Jeffreys A. J., Lucibello F. C., Patel I., Rider S. H. Chromosome 5 allele loss in human colorectal carcinomas. Nature. 1987 Aug 13;328(6131):616–619. doi: 10.1038/328616a0. [DOI] [PubMed] [Google Scholar]
- Solomon L. Chondrosarcoma in hereditary multiple exostosis. S Afr Med J. 1974 Apr 6;48(16):671–676. [PubMed] [Google Scholar]
- Srivastava S., Zou Z. Q., Pirollo K., Blattner W., Chang E. H. Germ-line transmission of a mutated p53 gene in a cancer-prone family with Li-Fraumeni syndrome. Nature. 1990 Dec 20;348(6303):747–749. doi: 10.1038/348747a0. [DOI] [PubMed] [Google Scholar]
- Tsuchiya H., Morikawa S., Tomita K. Osteosarcoma arising from a multiple exostoses lesion: case report. Jpn J Clin Oncol. 1990 Sep;20(3):296–298. [PubMed] [Google Scholar]
- Voutsinas S., Wynne-Davies R. The infrequency of malignant disease in diaphyseal aclasis and neurofibromatosis. J Med Genet. 1983 Oct;20(5):345–349. doi: 10.1136/jmg.20.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadayama B., Toguchida J., Yamaguchi T., Sasaki M. S., Yamamuro T. p53 expression and its relationship to DNA alterations in bone and soft tissue sarcomas. Br J Cancer. 1993 Dec;68(6):1134–1139. doi: 10.1038/bjc.1993.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y. Q., Heutink P., de Vries B. B., Sandkuijl L. A., van den Ouweland A. M., Niermeijer M. F., Galjaard H., Reyniers E., Willems P. J., Halley D. J. Assignment of a second locus for multiple exostoses to the pericentromeric region of chromosome 11. Hum Mol Genet. 1994 Jan;3(1):167–171. doi: 10.1093/hmg/3.1.167. [DOI] [PubMed] [Google Scholar]