Abstract
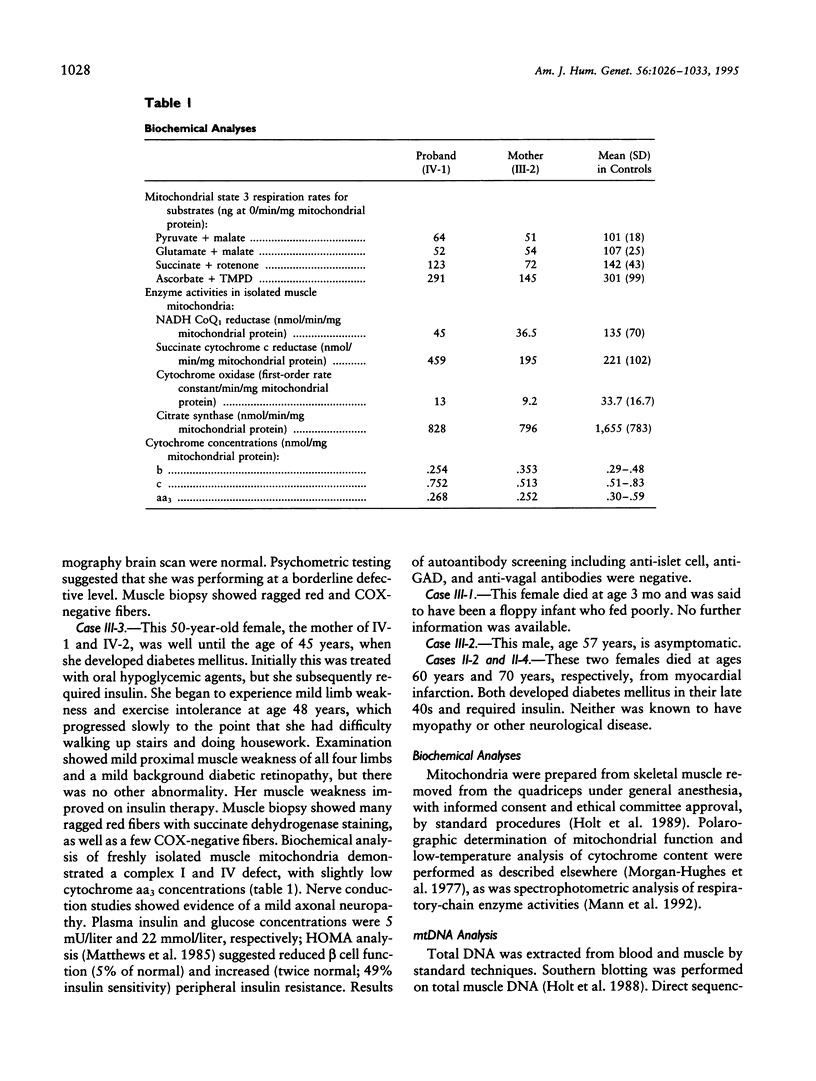
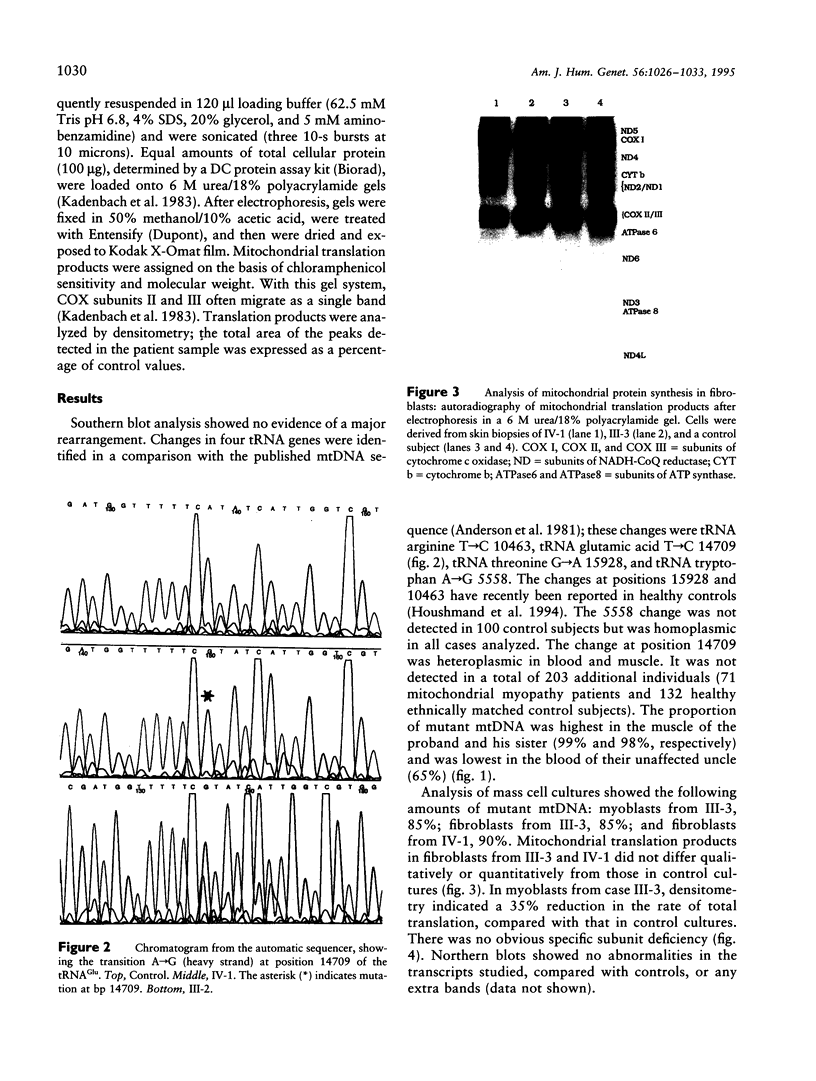
We report the clinical, biochemical, and molecular genetic findings in a family with an unusual mitochondrial disease phenotype harboring a novel mtDNA tRNA glutamic acid mutation at position 14709. The proband and his sister presented with congenital myopathy and mental retardation and subsequently developed cerebellar ataxia. Other family members had either adult-onset diabetes mellitus with muscle weakness or adult-onset diabetes mellitus alone. Ragged-red and cytochrome c oxidase (COX)-negative fibers were present in muscle biopsies. Biochemical studies of muscle mitochondria showed reduced complex I and IV activities. The mtDNA mutation was heteroplasmic in blood and muscle in all matrilineal relatives analyzed. Primary myoblast, but not fibroblast, cultures containing high proportions of mutant mtDNA exhibited impaired mitochondrial translation. These observations indicate that mtDNA tRNA point mutations should be considered in the differential diagnosis of congenital myopathy. In addition they illustrate the diversity of phenotypes associated with this mutation in the same family and further highlight the association between mtDNA mutations and diabetes mellitus.
Full text
PDF




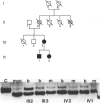
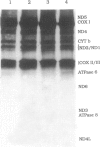
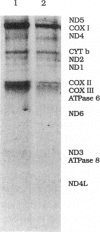
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Bankier A. T., Barrell B. G., de Bruijn M. H., Coulson A. R., Drouin J., Eperon I. C., Nierlich D. P., Roe B. A., Sanger F. Sequence and organization of the human mitochondrial genome. Nature. 1981 Apr 9;290(5806):457–465. doi: 10.1038/290457a0. [DOI] [PubMed] [Google Scholar]
- Attardi G., Ching E. Biogenesis of mitochondrial proteins in HeLa cells. Methods Enzymol. 1979;56:66–79. doi: 10.1016/0076-6879(79)56010-8. [DOI] [PubMed] [Google Scholar]
- Ballinger S. W., Shoffner J. M., Hedaya E. V., Trounce I., Polak M. A., Koontz D. A., Wallace D. C. Maternally transmitted diabetes and deafness associated with a 10.4 kb mitochondrial DNA deletion. Nat Genet. 1992 Apr;1(1):11–15. doi: 10.1038/ng0492-11. [DOI] [PubMed] [Google Scholar]
- Bindoff L. A., Howell N., Poulton J., McCullough D. A., Morten K. J., Lightowlers R. N., Turnbull D. M., Weber K. Abnormal RNA processing associated with a novel tRNA mutation in mitochondrial DNA. A potential disease mechanism. J Biol Chem. 1993 Sep 15;268(26):19559–19564. [PubMed] [Google Scholar]
- Blau H. M., Webster C. Isolation and characterization of human muscle cells. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5623–5627. doi: 10.1073/pnas.78.9.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulet L., Karpati G., Shoubridge E. A. Distribution and threshold expression of the tRNA(Lys) mutation in skeletal muscle of patients with myoclonic epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1992 Dec;51(6):1187–1200. [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chomyn A., Meola G., Bresolin N., Lai S. T., Scarlato G., Attardi G. In vitro genetic transfer of protein synthesis and respiration defects to mitochondrial DNA-less cells with myopathy-patient mitochondria. Mol Cell Biol. 1991 Apr;11(4):2236–2244. doi: 10.1128/mcb.11.4.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMauro S., Moraes C. T. Mitochondrial encephalomyopathies. Arch Neurol. 1993 Nov;50(11):1197–1208. doi: 10.1001/archneur.1993.00540110075008. [DOI] [PubMed] [Google Scholar]
- Goto Y., Nonaka I., Horai S. A mutation in the tRNA(Leu)(UUR) gene associated with the MELAS subgroup of mitochondrial encephalomyopathies. Nature. 1990 Dec 13;348(6302):651–653. doi: 10.1038/348651a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Cooper J. M., Schapira A. H., Toscano A., Clark J. B., Morgan-Hughes J. A. Mitochondrial myopathies: clinical and biochemical features of 30 patients with major deletions of muscle mitochondrial DNA. Ann Neurol. 1989 Dec;26(6):699–708. doi: 10.1002/ana.410260603. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Morgan-Hughes J. A. Deletions of muscle mitochondrial DNA in patients with mitochondrial myopathies. Nature. 1988 Feb 25;331(6158):717–719. doi: 10.1038/331717a0. [DOI] [PubMed] [Google Scholar]
- Holt I. J., Harding A. E., Petty R. K., Morgan-Hughes J. A. A new mitochondrial disease associated with mitochondrial DNA heteroplasmy. Am J Hum Genet. 1990 Mar;46(3):428–433. [PMC free article] [PubMed] [Google Scholar]
- Houshmand M., Larsson N. G., Holme E., Oldfors A., Tulinius M. H., Andersen O. Automatic sequencing of mitochondrial tRNA genes in patients with mitochondrial encephalomyopathy. Biochim Biophys Acta. 1994 Apr 12;1226(1):49–55. doi: 10.1016/0925-4439(94)90058-2. [DOI] [PubMed] [Google Scholar]
- Kadenbach B., Jarausch J., Hartmann R., Merle P. Separation of mammalian cytochrome c oxidase into 13 polypeptides by a sodium dodecyl sulfate-gel electrophoretic procedure. Anal Biochem. 1983 Mar;129(2):517–521. doi: 10.1016/0003-2697(83)90586-9. [DOI] [PubMed] [Google Scholar]
- King M. P., Koga Y., Davidson M., Schon E. A. Defects in mitochondrial protein synthesis and respiratory chain activity segregate with the tRNA(Leu(UUR)) mutation associated with mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes. Mol Cell Biol. 1992 Feb;12(2):480–490. doi: 10.1128/mcb.12.2.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann V. M., Cooper J. M., Krige D., Daniel S. E., Schapira A. H., Marsden C. D. Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson's disease. Brain. 1992 Apr;115(Pt 2):333–342. doi: 10.1093/brain/115.2.333. [DOI] [PubMed] [Google Scholar]
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985 Jul;28(7):412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Merante F., Tein I., Benson L., Robinson B. H. Maternally inherited hypertrophic cardiomyopathy due to a novel T-to-C transition at nucleotide 9997 in the mitochondrial tRNA(glycine) gene. Am J Hum Genet. 1994 Sep;55(3):437–446. [PMC free article] [PubMed] [Google Scholar]
- Moraes C. T., Ciacci F., Bonilla E., Ionasescu V., Schon E. A., DiMauro S. A mitochondrial tRNA anticodon swap associated with a muscle disease. Nat Genet. 1993 Jul;4(3):284–288. doi: 10.1038/ng0793-284. [DOI] [PubMed] [Google Scholar]
- Moraes C. T., DiMauro S., Zeviani M., Lombes A., Shanske S., Miranda A. F., Nakase H., Bonilla E., Werneck L. C., Servidei S. Mitochondrial DNA deletions in progressive external ophthalmoplegia and Kearns-Sayre syndrome. N Engl J Med. 1989 May 18;320(20):1293–1299. doi: 10.1056/NEJM198905183202001. [DOI] [PubMed] [Google Scholar]
- Morgan-Hughes J. A., Darveniza P., Kahn S. N., Landon D. N., Sherratt R. M., Land J. M., Clark J. B. A mitochondrial myopathy characterized by a deficiency in reducible cytochrome b. Brain. 1977 Dec;100(4):617–640. doi: 10.1093/brain/100.4.617. [DOI] [PubMed] [Google Scholar]
- Morten K. J., Cooper J. M., Brown G. K., Lake B. D., Pike D., Poulton J. A new point mutation associated with mitochondrial encephalomyopathy. Hum Mol Genet. 1993 Dec;2(12):2081–2087. doi: 10.1093/hmg/2.12.2081. [DOI] [PubMed] [Google Scholar]
- Petty R. K., Harding A. E., Morgan-Hughes J. A. The clinical features of mitochondrial myopathy. Brain. 1986 Oct;109(Pt 5):915–938. doi: 10.1093/brain/109.5.915. [DOI] [PubMed] [Google Scholar]
- Poulton J., Deadman M. E., Gardiner R. M. Duplications of mitochondrial DNA in mitochondrial myopathy. Lancet. 1989 Feb 4;1(8632):236–240. doi: 10.1016/s0140-6736(89)91256-7. [DOI] [PubMed] [Google Scholar]
- Reardon W., Ross R. J., Sweeney M. G., Luxon L. M., Pembrey M. E., Harding A. E., Trembath R. C. Diabetes mellitus associated with a pathogenic point mutation in mitochondrial DNA. Lancet. 1992 Dec 5;340(8832):1376–1379. doi: 10.1016/0140-6736(92)92560-3. [DOI] [PubMed] [Google Scholar]
- Shoffner J. M., Lott M. T., Lezza A. M., Seibel P., Ballinger S. W., Wallace D. C. Myoclonic epilepsy and ragged-red fiber disease (MERRF) is associated with a mitochondrial DNA tRNA(Lys) mutation. Cell. 1990 Jun 15;61(6):931–937. doi: 10.1016/0092-8674(90)90059-n. [DOI] [PubMed] [Google Scholar]
- Sweeney M. G., Hammans S. R., Duchen L. W., Cooper J. M., Schapira A. H., Kennedy C. R., Jacobs J. M., Youl B. D., Morgan-Hughes J. A., Harding A. E. Mitochondrial DNA mutation underlying Leigh's syndrome: clinical, pathological, biochemical, and genetic studies of a patient presenting with progressive myoclonic epilepsy. J Neurol Sci. 1994 Jan;121(1):57–65. doi: 10.1016/0022-510x(94)90157-0. [DOI] [PubMed] [Google Scholar]
- Tanaka M., Ino H., Ohno K., Hattori K., Sato W., Ozawa T., Tanaka T., Itoyama S. Mitochondrial mutation in fatal infantile cardiomyopathy. Lancet. 1990 Dec 8;336(8728):1452–1452. doi: 10.1016/0140-6736(90)93162-i. [DOI] [PubMed] [Google Scholar]
- Taniike M., Fukushima H., Yanagihara I., Tsukamoto H., Tanaka J., Fujimura H., Nagai T., Sano T., Yamaoka K., Inui K. Mitochondrial tRNA(Ile) mutation in fatal cardiomyopathy. Biochem Biophys Res Commun. 1992 Jul 15;186(1):47–53. doi: 10.1016/s0006-291x(05)80773-9. [DOI] [PubMed] [Google Scholar]
- Wallace D. C., Singh G., Lott M. T., Hodge J. A., Schurr T. G., Lezza A. M., Elsas L. J., 2nd, Nikoskelainen E. K. Mitochondrial DNA mutation associated with Leber's hereditary optic neuropathy. Science. 1988 Dec 9;242(4884):1427–1430. doi: 10.1126/science.3201231. [DOI] [PubMed] [Google Scholar]
- Yasin R., Van Beers G., Nurse K. C., Al-Ani S., Landon D. N., Thompson E. J. A quantitative technique for growing human adult skeletal muscle in culture starting from mononucleated cells. J Neurol Sci. 1977 Jul;32(3):347–360. doi: 10.1016/0022-510x(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Yoon K. L., Ernst S. G., Rasmussen C., Dooling E. C., Aprille J. R. Mitochondrial disorder associated with newborn cardiopulmonary arrest. Pediatr Res. 1993 May;33(5):433–440. doi: 10.1203/00006450-199305000-00002. [DOI] [PubMed] [Google Scholar]
- Zeviani M., Amati P., Bresolin N., Antozzi C., Piccolo G., Toscano A., DiDonato S. Rapid detection of the A----G(8344) mutation of mtDNA in Italian families with myoclonus epilepsy and ragged-red fibers (MERRF). Am J Hum Genet. 1991 Feb;48(2):203–211. [PMC free article] [PubMed] [Google Scholar]
- Zeviani M., Gellera C., Antozzi C., Rimoldi M., Morandi L., Villani F., Tiranti V., DiDonato S. Maternally inherited myopathy and cardiomyopathy: association with mutation in mitochondrial DNA tRNA(Leu)(UUR). Lancet. 1991 Jul 20;338(8760):143–147. doi: 10.1016/0140-6736(91)90136-d. [DOI] [PubMed] [Google Scholar]
- van den Ouweland J. M., Lemkes H. H., Ruitenbeek W., Sandkuijl L. A., de Vijlder M. F., Struyvenberg P. A., van de Kamp J. J., Maassen J. A. Mutation in mitochondrial tRNA(Leu)(UUR) gene in a large pedigree with maternally transmitted type II diabetes mellitus and deafness. Nat Genet. 1992 Aug;1(5):368–371. doi: 10.1038/ng0892-368. [DOI] [PubMed] [Google Scholar]