Abstract
Hereditary nonpolyposis colorectal cancer (HNPCC) is a relatively common autosomal dominant cancer-susceptibility condition. The recent isolation of the DNA mismatch repair genes (hMSH2, hMLH1, hPMS1, and hPMS2) responsible for HNPCC has allowed the search for germ-line mutations in affected individuals. In this study we used denaturing gradient-gel electrophoresis to screen for mutations in the hMSH2 gene. Analysis of all the 16 exons of hMSH2, in 34 unrelated HNPCC kindreds, has revealed seven novel pathogenic germ-line mutations resulting in stop codons either directly or through frameshifts. Additionally, nucleotide substitutions giving rise to one missense, two silent, and one useful polymorphism have been identified. The proportion of families in which hMSH2 mutations were found is 21%. Although the spectrum of mutations spread at the hMSH2 gene among HNPCC patients appears extremely heterogeneous, we were not able to establish any correlation between the site of the individual mutations and the corresponding tumor spectrum. Our results indicate that, given the genomic size and organization of the hMSH2 gene and the heterogeneity of its mutation spectrum, a rapid and efficient mutation detection procedure is necessary for routine molecular diagnosis and presymptomatic detection of the disease in a clinical setup.
Full text
PDF

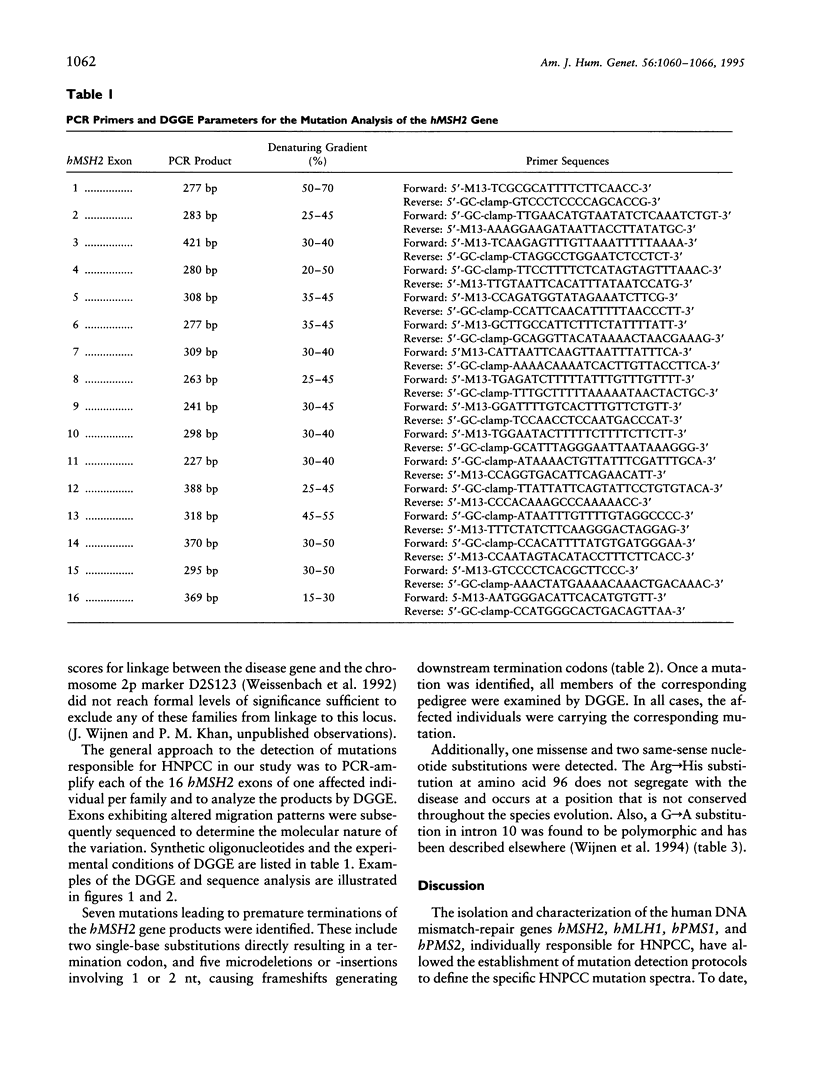
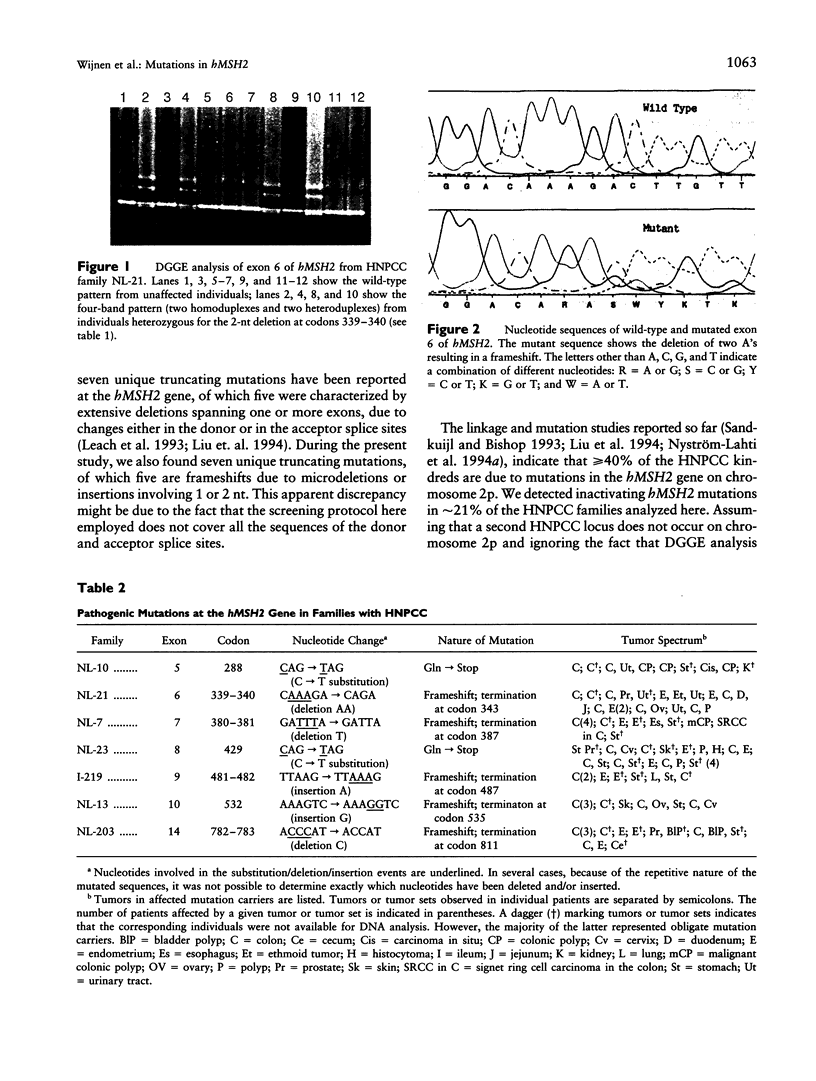
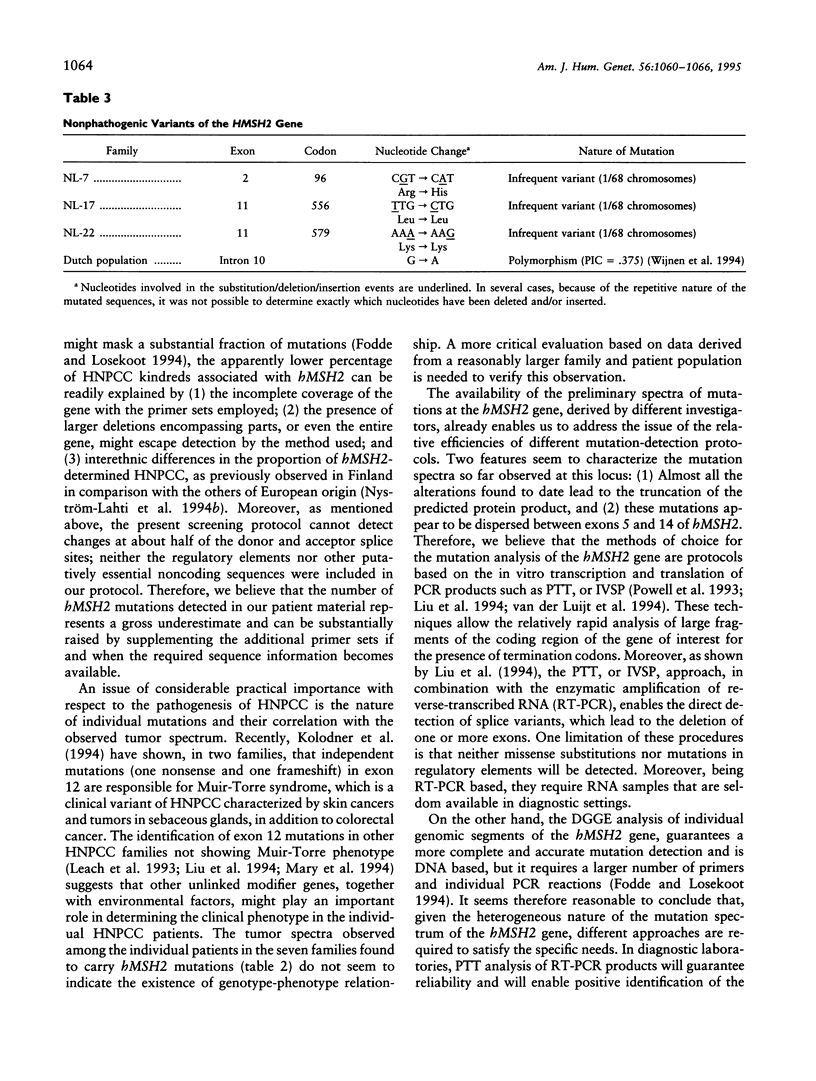


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaltonen L. A., Peltomäki P., Leach F. S., Sistonen P., Pylkkänen L., Mecklin J. P., Järvinen H., Powell S. M., Jen J., Hamilton S. R. Clues to the pathogenesis of familial colorectal cancer. Science. 1993 May 7;260(5109):812–816. doi: 10.1126/science.8484121. [DOI] [PubMed] [Google Scholar]
- Burks R. T., Kessis T. D., Cho K. R., Hedrick L. Microsatellite instability in endometrial carcinoma. Oncogene. 1994 Apr;9(4):1163–1166. [PubMed] [Google Scholar]
- Fishel R., Lescoe M. K., Rao M. R., Copeland N. G., Jenkins N. A., Garber J., Kane M., Kolodner R. The human mutator gene homolog MSH2 and its association with hereditary nonpolyposis colon cancer. Cell. 1993 Dec 3;75(5):1027–1038. doi: 10.1016/0092-8674(93)90546-3. [DOI] [PubMed] [Google Scholar]
- Fodde R., Losekoot M. Mutation detection by denaturing gradient gel electrophoresis (DGGE). Hum Mutat. 1994;3(2):83–94. doi: 10.1002/humu.1380030202. [DOI] [PubMed] [Google Scholar]
- Fodde R., van der Luijt R., Wijnen J., Tops C., van der Klift H., van Leeuwen-Cornelisse I., Griffioen G., Vasen H., Khan P. M. Eight novel inactivating germ line mutations at the APC gene identified by denaturing gradient gel electrophoresis. Genomics. 1992 Aug;13(4):1162–1168. doi: 10.1016/0888-7543(92)90032-n. [DOI] [PubMed] [Google Scholar]
- Han H. J., Yanagisawa A., Kato Y., Park J. G., Nakamura Y. Genetic instability in pancreatic cancer and poorly differentiated type of gastric cancer. Cancer Res. 1993 Nov 1;53(21):5087–5089. [PubMed] [Google Scholar]
- Ionov Y., Peinado M. A., Malkhosyan S., Shibata D., Perucho M. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature. 1993 Jun 10;363(6429):558–561. doi: 10.1038/363558a0. [DOI] [PubMed] [Google Scholar]
- Kolodner R. D., Hall N. R., Lipford J., Kane M. F., Rao M. R., Morrison P., Wirth L., Finan P. J., Burn J., Chapman P. Structure of the human MSH2 locus and analysis of two Muir-Torre kindreds for msh2 mutations. Genomics. 1994 Dec;24(3):516–526. doi: 10.1006/geno.1994.1661. [DOI] [PubMed] [Google Scholar]
- Leach F. S., Nicolaides N. C., Papadopoulos N., Liu B., Jen J., Parsons R., Peltomäki P., Sistonen P., Aaltonen L. A., Nyström-Lahti M. Mutations of a mutS homolog in hereditary nonpolyposis colorectal cancer. Cell. 1993 Dec 17;75(6):1215–1225. doi: 10.1016/0092-8674(93)90330-s. [DOI] [PubMed] [Google Scholar]
- Lerman L. S., Silverstein K. Computational simulation of DNA melting and its application to denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:482–501. doi: 10.1016/0076-6879(87)55032-7. [DOI] [PubMed] [Google Scholar]
- Lindblom A., Tannergård P., Werelius B., Nordenskjöld M. Genetic mapping of a second locus predisposing to hereditary non-polyposis colon cancer. Nat Genet. 1993 Nov;5(3):279–282. doi: 10.1038/ng1193-279. [DOI] [PubMed] [Google Scholar]
- Liu B., Parsons R. E., Hamilton S. R., Petersen G. M., Lynch H. T., Watson P., Markowitz S., Willson J. K., Green J., de la Chapelle A. hMSH2 mutations in hereditary nonpolyposis colorectal cancer kindreds. Cancer Res. 1994 Sep 1;54(17):4590–4594. [PubMed] [Google Scholar]
- Lynch H. T., Smyrk T. C., Watson P., Lanspa S. J., Lynch J. F., Lynch P. M., Cavalieri R. J., Boland C. R. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993 May;104(5):1535–1549. doi: 10.1016/0016-5085(93)90368-m. [DOI] [PubMed] [Google Scholar]
- Mary J. L., Bishop T., Kolodner R., Lipford J. R., Kane M., Weber W., Torhorst J., Müller H., Spycher M., Scott R. J. Mutational analysis of the hMSH2 gene reveals a three base pair deletion in a family predisposed to colorectal cancer development. Hum Mol Genet. 1994 Nov;3(11):2067–2069. [PubMed] [Google Scholar]
- Myers R. M., Maniatis T., Lerman L. S. Detection and localization of single base changes by denaturing gradient gel electrophoresis. Methods Enzymol. 1987;155:501–527. doi: 10.1016/0076-6879(87)55033-9. [DOI] [PubMed] [Google Scholar]
- Nicolaides N. C., Papadopoulos N., Liu B., Wei Y. F., Carter K. C., Ruben S. M., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M. Mutations of two PMS homologues in hereditary nonpolyposis colon cancer. Nature. 1994 Sep 1;371(6492):75–80. doi: 10.1038/371075a0. [DOI] [PubMed] [Google Scholar]
- Nyström-Lahti M., Parsons R., Sistonen P., Pylkkänen L., Aaltonen L. A., Leach F. S., Hamilton S. R., Watson P., Bronson E., Fusaro R. Mismatch repair genes on chromosomes 2p and 3p account for a major share of hereditary nonpolyposis colorectal cancer families evaluable by linkage. Am J Hum Genet. 1994 Oct;55(4):659–665. [PMC free article] [PubMed] [Google Scholar]
- Nyström-Lahti M., Sistonen P., Mecklin J. P., Pylkkänen L., Aaltonen L. A., Järvinen H., Weissenbach J., de la Chapelle A., Peltomäki P. Close linkage to chromosome 3p and conservation of ancestral founding haplotype in hereditary nonpolyposis colorectal cancer families. Proc Natl Acad Sci U S A. 1994 Jun 21;91(13):6054–6058. doi: 10.1073/pnas.91.13.6054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Suzuki Y., Sekiya T., Hayashi K. Rapid and sensitive detection of point mutations and DNA polymorphisms using the polymerase chain reaction. Genomics. 1989 Nov;5(4):874–879. doi: 10.1016/0888-7543(89)90129-8. [DOI] [PubMed] [Google Scholar]
- Papadopoulos N., Nicolaides N. C., Wei Y. F., Ruben S. M., Carter K. C., Rosen C. A., Haseltine W. A., Fleischmann R. D., Fraser C. M., Adams M. D. Mutation of a mutL homolog in hereditary colon cancer. Science. 1994 Mar 18;263(5153):1625–1629. doi: 10.1126/science.8128251. [DOI] [PubMed] [Google Scholar]
- Powell S. M., Petersen G. M., Krush A. J., Booker S., Jen J., Giardiello F. M., Hamilton S. R., Vogelstein B., Kinzler K. W. Molecular diagnosis of familial adenomatous polyposis. N Engl J Med. 1993 Dec 30;329(27):1982–1987. doi: 10.1056/NEJM199312303292702. [DOI] [PubMed] [Google Scholar]
- Risinger J. I., Berchuck A., Kohler M. F., Watson P., Lynch H. T., Boyd J. Genetic instability of microsatellites in endometrial carcinoma. Cancer Res. 1993 Nov 1;53(21):5100–5103. [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheffield V. C., Cox D. R., Lerman L. S., Myers R. M. Attachment of a 40-base-pair G + C-rich sequence (GC-clamp) to genomic DNA fragments by the polymerase chain reaction results in improved detection of single-base changes. Proc Natl Acad Sci U S A. 1989 Jan;86(1):232–236. doi: 10.1073/pnas.86.1.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibodeau S. N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993 May 7;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- Vasen H. F., Mecklin J. P., Khan P. M., Lynch H. T. The International Collaborative Group on Hereditary Non-Polyposis Colorectal Cancer (ICG-HNPCC). Dis Colon Rectum. 1991 May;34(5):424–425. doi: 10.1007/BF02053699. [DOI] [PubMed] [Google Scholar]
- Watson P., Lynch H. T. Extracolonic cancer in hereditary nonpolyposis colorectal cancer. Cancer. 1993 Feb 1;71(3):677–685. doi: 10.1002/1097-0142(19930201)71:3<677::aid-cncr2820710305>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Weissenbach J., Gyapay G., Dib C., Vignal A., Morissette J., Millasseau P., Vaysseix G., Lathrop M. A second-generation linkage map of the human genome. Nature. 1992 Oct 29;359(6398):794–801. doi: 10.1038/359794a0. [DOI] [PubMed] [Google Scholar]
- Wijnen J., Fodde R., Khan P. M. DGGE polymorphism in intron 10 of MSH2, the HNPCC gene. Hum Mol Genet. 1994 Dec;3(12):2268–2268. doi: 10.1093/hmg/3.12.2268-a. [DOI] [PubMed] [Google Scholar]
- Wooster R., Cleton-Jansen A. M., Collins N., Mangion J., Cornelis R. S., Cooper C. S., Gusterson B. A., Ponder B. A., von Deimling A., Wiestler O. D. Instability of short tandem repeats (microsatellites) in human cancers. Nat Genet. 1994 Feb;6(2):152–156. doi: 10.1038/ng0294-152. [DOI] [PubMed] [Google Scholar]