Abstract
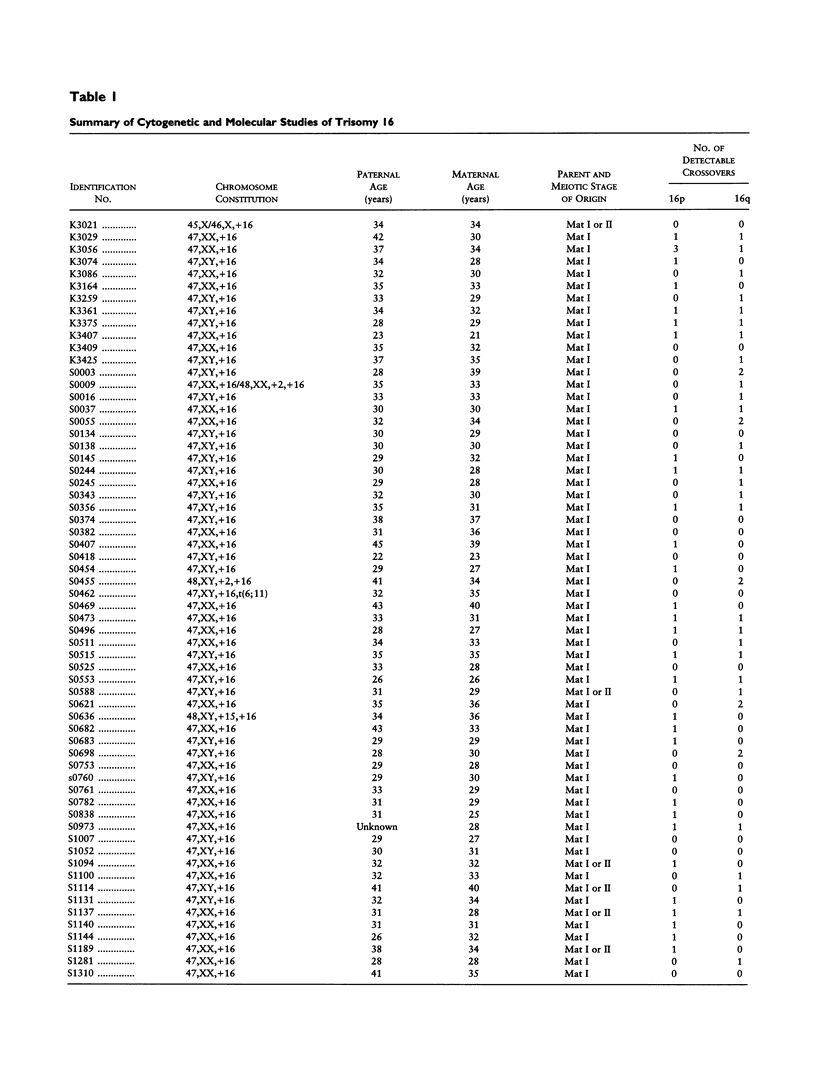
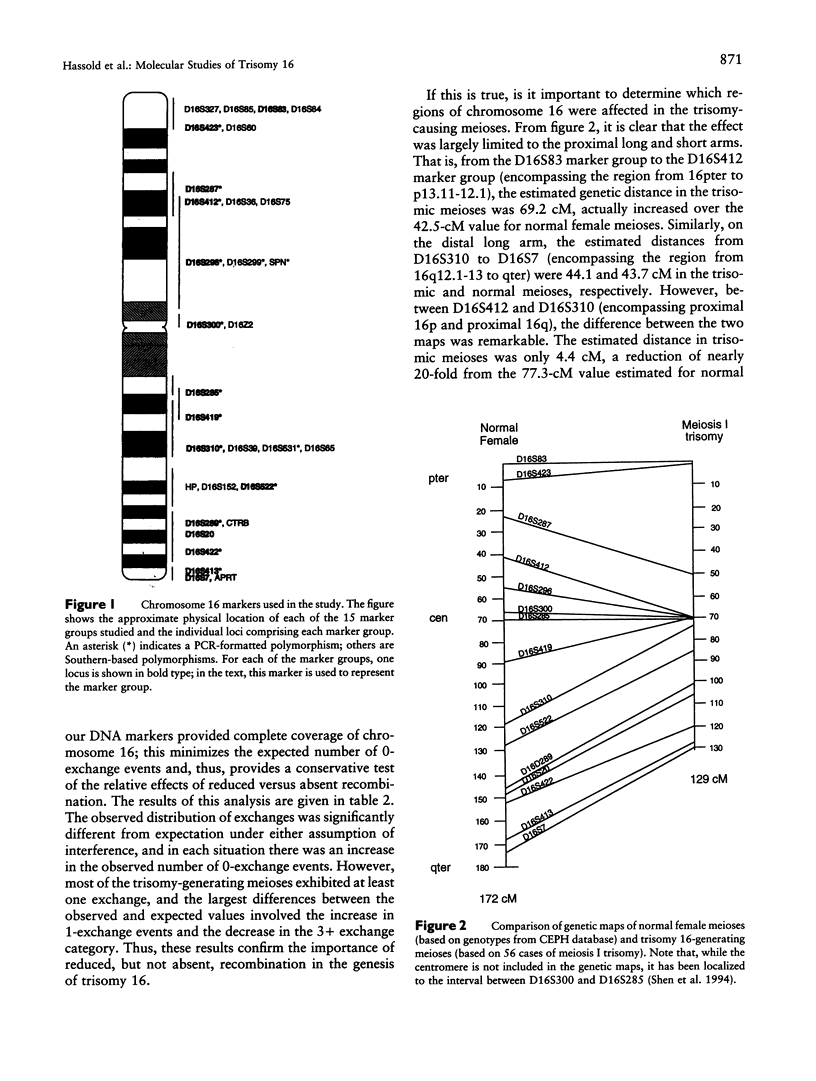
Trisomy 16 is the most common human trisomy, occurring in > or = 1% of all clinically recognized pregnancies. It is thought to be completely dependent on maternal age and thus provides a useful model for studying the association of increasing maternal age and nondisjunction. We have been conducting a study to determine the parent and meiotic stage of origin of trisomy 16 and the possible association of nondisjunction and aberrant recombination. In the present report, we summarize our observations on 62 spontaneous abortions with trisomy 16. All trisomies were maternally derived, and in virtually all the error occurred at meiosis I. In studies of genetic recombination, we observed a highly significant reduction in recombination in the trisomy-generating meioses by comparison with normal female meioses. However, most cases of trisomy 16 had at least one detectable crossover between the nondisjoined chromosomes, indicating that it is reduced--and not absent--recombination that is the important predisposing factor. Additionally, our data indicate an altered distribution of crossing-over in trisomy 16, as we rarely observed crossovers in the proximal long and short arms. Thus, it may be that, at least for trisomy 16, the association between maternal age and trisomy is due to diminished recombination, particularly in the proximal regions of the chromosome.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angell R. R., Xian J., Keith J., Ledger W., Baird D. T. First meiotic division abnormalities in human oocytes: mechanism of trisomy formation. Cytogenet Cell Genet. 1994;65(3):194–202. doi: 10.1159/000133631. [DOI] [PubMed] [Google Scholar]
- Chakravarti A., Majumder P. P., Slaugenhaupt S. A., Deka R., Warren A. C., Surti U., Ferrell R. E., Antonarakis S. E. Gene-centromere mapping and the study of non-disjunction in autosomal trisomies and ovarian teratomas. Prog Clin Biol Res. 1989;311:45–79. [PubMed] [Google Scholar]
- Cheng Y. E., Gartler S. M. A fluorescent in situ hybridization analysis of X chromosome pairing in early human female meiosis. Hum Genet. 1994 Oct;94(4):389–394. doi: 10.1007/BF00201599. [DOI] [PubMed] [Google Scholar]
- Eichenlaub-Ritter U., Boll I. Nocodazole sensitivity, age-related aneuploidy, and alterations in the cell cycle during maturation of mouse oocytes. Cytogenet Cell Genet. 1989;52(3-4):170–176. doi: 10.1159/000132871. [DOI] [PubMed] [Google Scholar]
- Fisher J. M., Harvey J. F., Morton N. E., Jacobs P. A. Trisomy 18: studies of the parent and cell division of origin and the effect of aberrant recombination on nondisjunction. Am J Hum Genet. 1995 Mar;56(3):669–675. [PMC free article] [PubMed] [Google Scholar]
- German J. Mongolism, delayed fertilization and human sexual behaviour. Nature. 1968 Feb 10;217(5128):516–518. doi: 10.1038/217516a0. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Jacobs P. A. Trisomy in man. Annu Rev Genet. 1984;18:69–97. doi: 10.1146/annurev.ge.18.120184.000441. [DOI] [PubMed] [Google Scholar]
- Hassold T. J., Pettay D., Freeman S. B., Grantham M., Takaesu N. Molecular studies of non-disjunction in trisomy 16. J Med Genet. 1991 Mar;28(3):159–162. doi: 10.1136/jmg.28.3.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassold T., Chen N., Funkhouser J., Jooss T., Manuel B., Matsuura J., Matsuyama A., Wilson C., Yamane J. A., Jacobs P. A. A cytogenetic study of 1000 spontaneous abortions. Ann Hum Genet. 1980 Oct;44(Pt 2):151–178. doi: 10.1111/j.1469-1809.1980.tb00955.x. [DOI] [PubMed] [Google Scholar]
- Hawley R. S., Frazier J. A., Rasooly R. Separation anxiety: the etiology of nondisjunction in flies and people. Hum Mol Genet. 1994 Sep;3(9):1521–1528. doi: 10.1093/hmg/3.9.1521. [DOI] [PubMed] [Google Scholar]
- Henderson S. A., Edwards R. G. Chiasma frequency and maternal age in mammals. Nature. 1968 Apr 6;218(5136):22–28. doi: 10.1038/218022a0. [DOI] [PubMed] [Google Scholar]
- Lander E. S., Green P. Construction of multilocus genetic linkage maps in humans. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2363–2367. doi: 10.1073/pnas.84.8.2363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorda-Sanchez I., Binkert F., Maechler M., Robinson W. P., Schinzel A. A. Reduced recombination and paternal age effect in Klinefelter syndrome. Hum Genet. 1992 Jul;89(5):524–530. doi: 10.1007/BF00219178. [DOI] [PubMed] [Google Scholar]
- MacDonald M., Hassold T., Harvey J., Wang L. H., Morton N. E., Jacobs P. The origin of 47,XXY and 47,XXX aneuploidy: heterogeneous mechanisms and role of aberrant recombination. Hum Mol Genet. 1994 Aug;3(8):1365–1371. doi: 10.1093/hmg/3.8.1365. [DOI] [PubMed] [Google Scholar]
- Martin R. H., Ko E., Rademaker A. Distribution of aneuploidy in human gametes: comparison between human sperm and oocytes. Am J Med Genet. 1991 Jun 1;39(3):321–331. doi: 10.1002/ajmg.1320390315. [DOI] [PubMed] [Google Scholar]
- Matise T. C., Perlin M., Chakravarti A. Automated construction of genetic linkage maps using an expert system (MultiMap): a human genome linkage map. Nat Genet. 1994 Apr;6(4):384–390. doi: 10.1038/ng0494-384. [DOI] [PubMed] [Google Scholar]
- Morton N. E., Andrews V. MAP, an expert system for multiple pairwise linkage analysis. Ann Hum Genet. 1989 Jul;53(Pt 3):263–269. doi: 10.1111/j.1469-1809.1989.tb01793.x. [DOI] [PubMed] [Google Scholar]
- Morton N. E., Jacobs P. A., Hassold T., Wu D. Maternal age in trisomy. Ann Hum Genet. 1988 Jul;52(Pt 3):227–235. doi: 10.1111/j.1469-1809.1988.tb01100.x. [DOI] [PubMed] [Google Scholar]
- Morton N. E., MacLean C. J. Multilocus recombination frequencies. Genet Res. 1984 Aug;44(1):99–107. doi: 10.1017/s0016672300026276. [DOI] [PubMed] [Google Scholar]
- Risch N., Stein Z., Kline J., Warburton D. The relationship between maternal age and chromosome size in autosomal trisomy. Am J Hum Genet. 1986 Jul;39(1):68–78. [PMC free article] [PubMed] [Google Scholar]
- Robinson W. P., Bernasconi F., Mutirangura A., Ledbetter D. H., Langlois S., Malcolm S., Morris M. A., Schinzel A. A. Nondisjunction of chromosome 15: origin and recombination. Am J Hum Genet. 1993 Sep;53(3):740–751. [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Kozman H. M., Thompson A., Phillips H. A., Holman K., Nancarrow J., Lane S., Chen L. Z., Apostolou S., Doggett N. A. A PCR-based genetic linkage map of human chromosome 16. Genomics. 1994 Jul 1;22(1):68–76. doi: 10.1006/geno.1994.1346. [DOI] [PubMed] [Google Scholar]
- Sherman S. L., Petersen M. B., Freeman S. B., Hersey J., Pettay D., Taft L., Frantzen M., Mikkelsen M., Hassold T. J. Non-disjunction of chromosome 21 in maternal meiosis I: evidence for a maternal age-dependent mechanism involving reduced recombination. Hum Mol Genet. 1994 Sep;3(9):1529–1535. doi: 10.1093/hmg/3.9.1529. [DOI] [PubMed] [Google Scholar]
- Williams B. J., Ballenger C. A., Malter H. E., Bishop F., Tucker M., Zwingman T. A., Hassold T. J. Non-disjunction in human sperm: results of fluorescence in situ hybridization studies using two and three probes. Hum Mol Genet. 1993 Nov;2(11):1929–1936. doi: 10.1093/hmg/2.11.1929. [DOI] [PubMed] [Google Scholar]
- Zaragoza M. V., Jacobs P. A., James R. S., Rogan P., Sherman S., Hassold T. Nondisjunction of human acrocentric chromosomes: studies of 432 trisomic fetuses and liveborns. Hum Genet. 1994 Oct;94(4):411–417. doi: 10.1007/BF00201603. [DOI] [PubMed] [Google Scholar]
- Zheng C. J., Byers B. Oocyte selection: a new model for the maternal-age dependence of Down syndrome. Hum Genet. 1992 Sep-Oct;90(1-2):1–6. doi: 10.1007/BF00210736. [DOI] [PubMed] [Google Scholar]


