Abstract
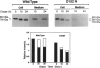
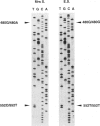
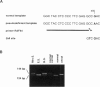
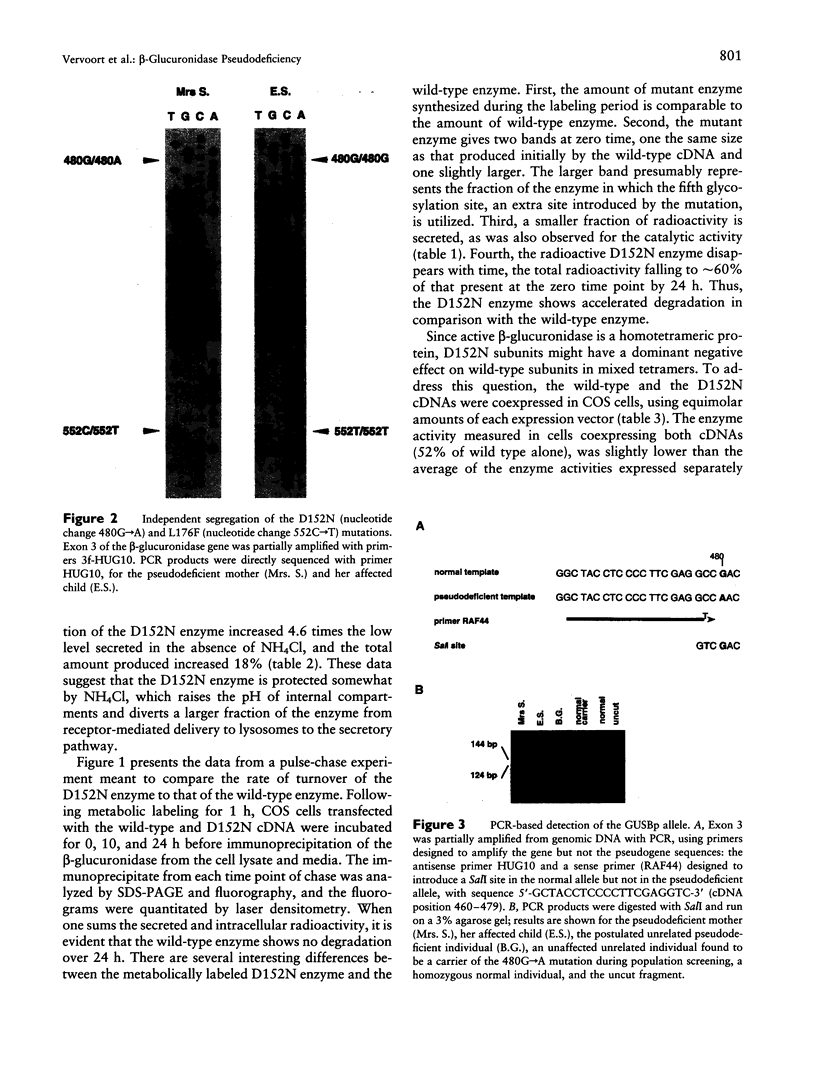
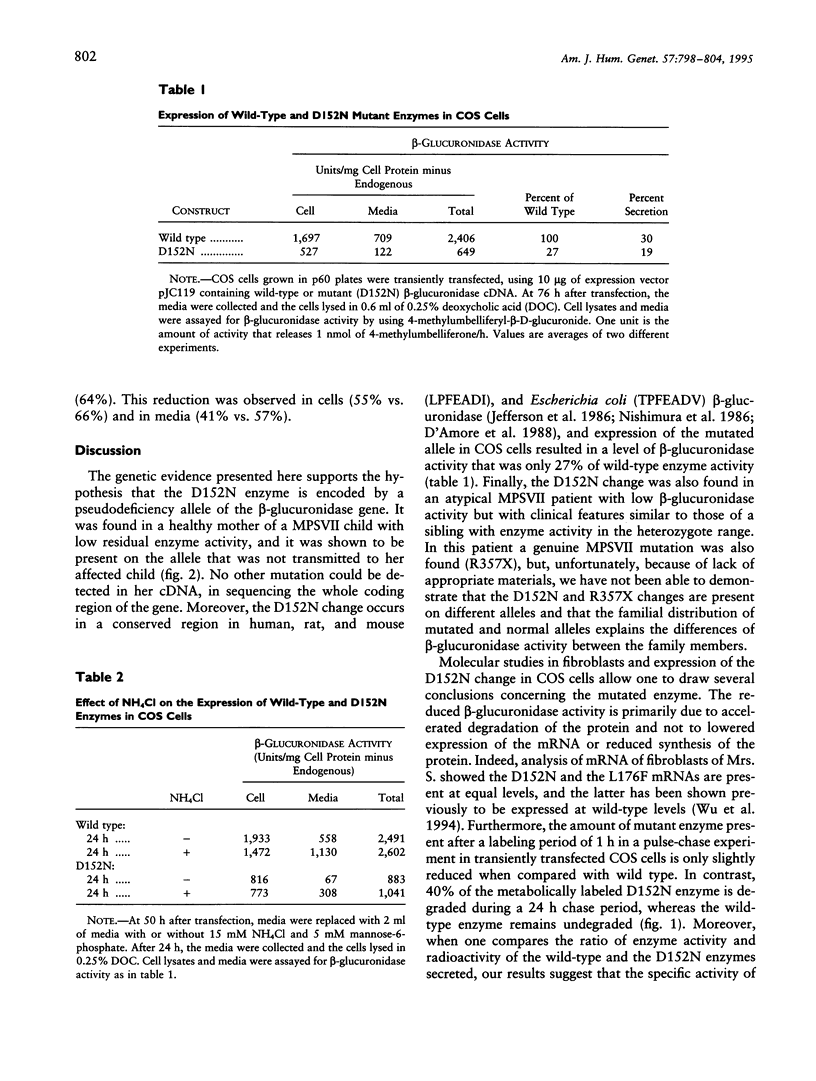
We present evidence that a 480G-->A transition in the coding region of the beta-glucuronidase gene, which results in an aspartic-acid-to-asparagine substitution at amino acid position 152 (D152N), produces a pseudodeficiency allele (GUSBp) that leads to greatly reduced levels of beta-glucuronidase activity without apparent deleterious consequences. The 480G-->A mutation was found initially in the pseudodeficient mother of a child with mucopolysaccharidosis VII (MPSVII), but it was not on her disease-causing allele, which carried the L176F mutation. The 480G-->A change was also present in an unrelated individual with another MPSVII allele who had unusually low beta-glucuronidase activity, but whose clinical symptoms were probably unrelated to beta-glucuronidase deficiency. This individual also had an R357X mutation, probably on his second allele. We screened 100 unrelated normal individuals for the 480G-->A mutation with a PCR method and detected one carrier. Reduced beta-glucuronidase activity following transfection of COS cells with the D152N cDNA supported the causal relationship between the D152N allele and pseudodeficiency. The mutation reduced the fraction of expressed enzyme that was secreted. Pulse-chase experiments indicated that the reduced activity in COS cells was due to accelerated intracellular turnover of the D152N enzyme. They also suggested that a potential glycosylation site created by the mutation is utilized in approximately 50% of the enzyme expressed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernsen P. L., Wevers R. A., Gabreëls F. J., Lamers K. J., Sonnen A. E., Stekhoven J. H. Phenotypic expression in mucopolysaccharidosis VII. J Neurol Neurosurg Psychiatry. 1987 Jun;50(6):699–703. doi: 10.1136/jnnp.50.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao Z., Natowicz M. R., Kaback M. M., Lim-Steele J. S., Prence E. M., Brown D., Chabot T., Triggs-Raine B. L. A second mutation associated with apparent beta-hexosaminidase A pseudodeficiency: identification and frequency estimation. Am J Hum Genet. 1993 Dec;53(6):1198–1205. [PMC free article] [PubMed] [Google Scholar]
- Chabas A., Giros M. L., Guardiola A. Low beta-glucuronidase activity in a healthy member of a family with mucopolysaccharidosis VII. J Inherit Metab Dis. 1991;14(6):908–914. doi: 10.1007/BF01800472. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- D'Amore M. A., Gallagher P. M., Korfhagen T. R., Ganschow R. E. Complete sequence and organization of the murine beta-glucuronidase gene. Biochemistry. 1988 Sep 6;27(18):7131–7140. doi: 10.1021/bi00418a070. [DOI] [PubMed] [Google Scholar]
- Dlott B., d'Azzo A., Quon D. V., Neufeld E. F. Two mutations produce intron insertion in mRNA and elongated beta-subunit of human beta-hexosaminidase. J Biol Chem. 1990 Oct 15;265(29):17921–17927. [PubMed] [Google Scholar]
- Gieselmann V., Polten A., Kreysing J., von Figura K. Arylsulfatase A pseudodeficiency: loss of a polyadenylylation signal and N-glycosylation site. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9436–9440. doi: 10.1073/pnas.86.23.9436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Noriega A., Grubb J. H., Talkad V., Sly W. S. Chloroquine inhibits lysosomal enzyme pinocytosis and enhances lysosomal enzyme secretion by impairing receptor recycling. J Cell Biol. 1980 Jun;85(3):839–852. doi: 10.1083/jcb.85.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam M. R., Grubb J. H., Sly W. S. C-terminal processing of human beta-glucuronidase. The propeptide is required for full expression of catalytic activity, intracellular retention, and proper phosphorylation. J Biol Chem. 1993 Oct 25;268(30):22627–22633. [PubMed] [Google Scholar]
- Jefferson R. A., Burgess S. M., Hirsh D. beta-Glucuronidase from Escherichia coli as a gene-fusion marker. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8447–8451. doi: 10.1073/pnas.83.22.8447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel L. M., Smith K. D., Boyer S. H., Borgaonkar D. S., Wachtel S. S., Miller O. J., Breg W. R., Jones H. W., Jr, Rary J. M. Analysis of human Y-chromosome-specific reiterated DNA in chromosome variants. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1245–1249. doi: 10.1073/pnas.74.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. D., Hoffmann J. W., Powell P. P., Kyle J. W., Shipley J. M., Bachinsky D. R., Sly W. S. Cloning and characterization of the human beta-glucuronidase gene. Genomics. 1990 Jun;7(2):280–283. doi: 10.1016/0888-7543(90)90552-6. [DOI] [PubMed] [Google Scholar]
- Nelson A., Peterson L., Frampton B., Sly W. S. Mucopolysaccharidosis VII (beta-glucuronidase deficiency) presenting as nonimmune hydrops fetalis. J Pediatr. 1982 Oct;101(4):574–576. doi: 10.1016/s0022-3476(82)80707-5. [DOI] [PubMed] [Google Scholar]
- Nishimura Y., Rosenfeld M. G., Kreibich G., Gubler U., Sabatini D. D., Adesnik M., Andy R. Nucleotide sequence of rat preputial gland beta-glucuronidase cDNA and in vitro insertion of its encoded polypeptide into microsomal membranes. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7292–7296. doi: 10.1073/pnas.83.19.7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orita M., Iwahana H., Kanazawa H., Hayashi K., Sekiya T. Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2766–2770. doi: 10.1073/pnas.86.8.2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshima A., Kyle J. W., Miller R. D., Hoffmann J. W., Powell P. P., Grubb J. H., Sly W. S., Tropak M., Guise K. S., Gravel R. A. Cloning, sequencing, and expression of cDNA for human beta-glucuronidase. Proc Natl Acad Sci U S A. 1987 Feb;84(3):685–689. doi: 10.1073/pnas.84.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sewell A. C., Gehler J., Mittermaier G., Meyer E. Mucopolysaccharidosis type VII (beta-glucuronidase deficiency): a report of a new case and a survey of those in the literature. Clin Genet. 1982 Jun;21(6):366–373. doi: 10.1111/j.1399-0004.1982.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Shipley J. M., Grubb J. H., Sly W. S. The role of glycosylation and phosphorylation in the expression of active human beta-glucuronidase. J Biol Chem. 1993 Jun 5;268(16):12193–12198. [PubMed] [Google Scholar]
- Shipley J. M., Klinkenberg M., Wu B. M., Bachinsky D. R., Grubb J. H., Sly W. S. Mutational analysis of a patient with mucopolysaccharidosis type VII, and identification of pseudogenes. Am J Hum Genet. 1993 Mar;52(3):517–526. [PMC free article] [PubMed] [Google Scholar]
- Sly W. S., Quinton B. A., McAlister W. H., Rimoin D. L. Beta glucuronidase deficiency: report of clinical, radiologic, and biochemical features of a new mucopolysaccharidosis. J Pediatr. 1973 Feb;82(2):249–257. doi: 10.1016/s0022-3476(73)80162-3. [DOI] [PubMed] [Google Scholar]
- Thomas G. H. "Pseudodeficiencies" of lysosomal hydrolases. Am J Hum Genet. 1994 Jun;54(6):934–940. [PMC free article] [PubMed] [Google Scholar]
- Triggs-Raine B. L., Mules E. H., Kaback M. M., Lim-Steele J. S., Dowling C. E., Akerman B. R., Natowicz M. R., Grebner E. E., Navon R., Welch J. P. A pseudodeficiency allele common in non-Jewish Tay-Sachs carriers: implications for carrier screening. Am J Hum Genet. 1992 Oct;51(4):793–801. [PMC free article] [PubMed] [Google Scholar]
- Vervoort R., Lissens W., Liebaers I. Molecular analysis of a patient with hydrops fetalis caused by beta-glucuronidase deficiency, and evidence for additional pseudogenes. Hum Mutat. 1993;2(6):443–445. doi: 10.1002/humu.1380020604. [DOI] [PubMed] [Google Scholar]
- Wu B. M., Tomatsu S., Fukuda S., Sukegawa K., Orii T., Sly W. S. Overexpression rescues the mutant phenotype of L176F mutation causing beta-glucuronidase deficiency mucopolysaccharidosis in two Mennonite siblings. J Biol Chem. 1994 Sep 23;269(38):23681–23688. [PubMed] [Google Scholar]
- Zlotogora J., Bach G. Deficiency of lysosomal hydrolases in apparently healthy individuals. Am J Med Genet. 1983 Jan;14(1):73–80. doi: 10.1002/ajmg.1320140112. [DOI] [PubMed] [Google Scholar]