Abstract
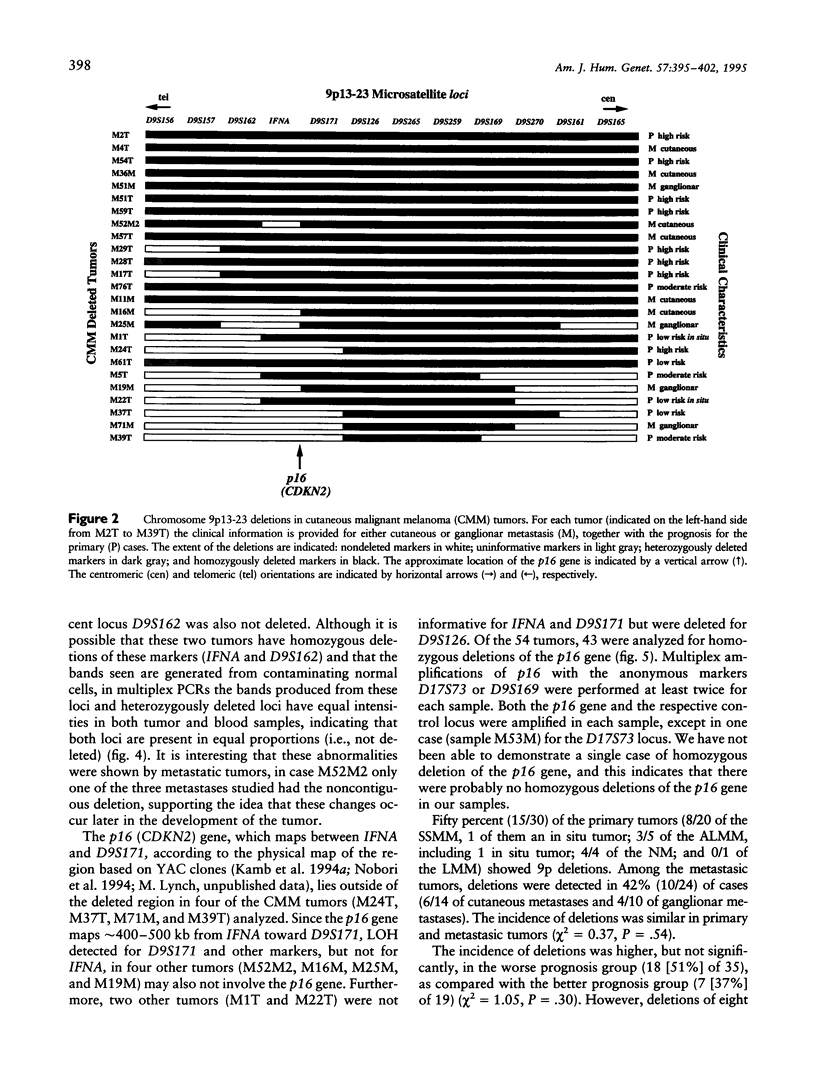
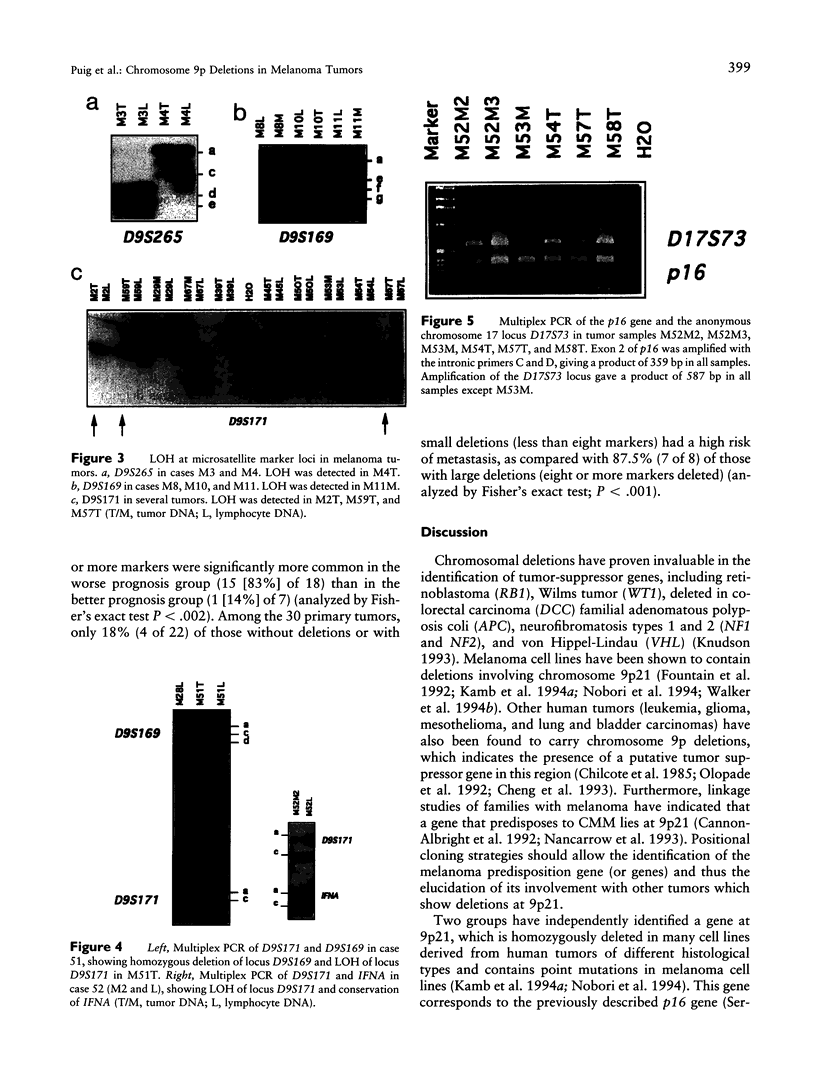
We have analyzed 12 microsatellite markers on chromosome 9p in 54 paired cutaneous malignant melanoma (CMM) tumors and normal tissues. Forty-six percent of the tumors, including two in situ CMMs, showed loss of heterozygosity (LOH) at 9p. Only one tumor was homozygously deleted for 9p markers. The smallest deleted region was defined by five tumors and included markers D9S126 to D9S259. Loss of eight or more markers correlated significantly with worse prognosis (P < .002). Among the primary tumors, 87.5% of those with large deletions have a high risk of metastasis, as compared with only 18% of those without deletions or with loss of fewer than 8 markers (P < .001). It was not possible to demonstrate homozygous deletions of p16 in any of the CMM tumors. In four tumors, the LOH for 9p markers did not involve p16. The reported data suggest the existence of several tumor suppressor genes at 9p that are involved in the predisposition to and/or progression of CMM and exclude p16 from involvement in the early development of some melanoma tumors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bagley F. H., Cady B., Lee A., Legg M. A. Changes in clinical presentation and management of malignant melanoma. Cancer. 1981 May 1;47(9):2126–2134. doi: 10.1002/1097-0142(19810501)47:9<2126::aid-cncr2820470904>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Breslow A. Thickness, cross-sectional areas and depth of invasion in the prognosis of cutaneous melanoma. Ann Surg. 1970 Nov;172(5):902–908. doi: 10.1097/00000658-197011000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Cheng J. Q., Jhanwar S. C., Lu Y. Y., Testa J. R. Homozygous deletions within 9p21-p22 identify a small critical region of chromosomal loss in human malignant mesotheliomas. Cancer Res. 1993 Oct 15;53(20):4761–4763. [PubMed] [Google Scholar]
- Chilcote R. R., Brown E., Rowley J. D. Lymphoblastic leukemia with lymphomatous features associated with abnormalities of the short arm of chromosome 9. N Engl J Med. 1985 Aug 1;313(5):286–291. doi: 10.1056/NEJM198508013130503. [DOI] [PubMed] [Google Scholar]
- Clark W. H., Jr, From L., Bernardino E. A., Mihm M. C. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. 1969 Mar;29(3):705–727. [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry D., 4th, Synnestvedt M., Elder D. E., Schultz D. Lessons from tumor progression: the invasive radial growth phase of melanoma is common, incapable of metastasis, and indolent. J Invest Dermatol. 1993 Mar;100(3):342S–345S. doi: 10.1111/1523-1747.ep12470248. [DOI] [PubMed] [Google Scholar]
- Gyapay G., Morissette J., Vignal A., Dib C., Fizames C., Millasseau P., Marc S., Bernardi G., Lathrop M., Weissenbach J. The 1993-94 Généthon human genetic linkage map. Nat Genet. 1994 Jun;7(2 Spec No):246–339. doi: 10.1038/ng0694supp-246. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Isshiki K., Seng B. A., Elder D. E., Guerry D., Linnenbach A. J. Chromosome 9 deletion in sporadic and familial melanomas in vivo. Oncogene. 1994 Jun;9(6):1649–1653. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kamb A., Shattuck-Eidens D., Eeles R., Liu Q., Gruis N. A., Ding W., Hussey C., Tran T., Miki Y., Weaver-Feldhaus J. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994 Sep;8(1):23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- Knudson A. G. Antioncogenes and human cancer. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10914–10921. doi: 10.1073/pnas.90.23.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh H. K. Cutaneous melanoma. N Engl J Med. 1991 Jul 18;325(3):171–182. doi: 10.1056/NEJM199107183250306. [DOI] [PubMed] [Google Scholar]
- MacGeoch C., Bishop J. A., Bataille V., Bishop D. T., Frischauf A. M., Meloni R., Cuzick J., Pinney E., Spurr N. K. Genetic heterogeneity in familial malignant melanoma. Hum Mol Genet. 1994 Dec;3(12):2195–2200. doi: 10.1093/hmg/3.12.2195. [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D., Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res. 1988 Feb 11;16(3):1215–1215. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morral N., Estivill X. Multiplex PCR amplification of three microsatellites within the CFTR gene. Genomics. 1992 Aug;13(4):1362–1364. doi: 10.1016/0888-7543(92)90071-y. [DOI] [PubMed] [Google Scholar]
- Nancarrow D. J., Mann G. J., Holland E. A., Walker G. J., Beaton S. C., Walters M. K., Luxford C., Palmer J. M., Donald J. A., Weber J. L. Confirmation of chromosome 9p linkage in familial melanoma. Am J Hum Genet. 1993 Oct;53(4):936–942. [PMC free article] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Ohta M., Nagai H., Shimizu M., Rasio D., Berd D., Mastrangelo M., Singh A. D., Shields J. A., Shields C. L., Croce C. M. Rarity of somatic and germline mutations of the cyclin-dependent kinase 4 inhibitor gene, CDK4I, in melanoma. Cancer Res. 1994 Oct 15;54(20):5269–5272. [PubMed] [Google Scholar]
- Olopade O. I., Bohlander S. K., Pomykala H., Maltepe E., Van Melle E., Le Beau M. M., Diaz M. O. Mapping of the shortest region of overlap of deletions of the short arm of chromosome 9 associated with human neoplasia. Genomics. 1992 Oct;14(2):437–443. doi: 10.1016/s0888-7543(05)80238-1. [DOI] [PubMed] [Google Scholar]
- Parmiter A. H., Nowell P. C. Cytogenetics of melanocytic tumors. J Invest Dermatol. 1993 Mar;100(3):254S–258S. [PubMed] [Google Scholar]
- Petty E. M., Gibson L. H., Fountain J. W., Bolognia J. L., Yang-Feng T. L., Housman D. E., Bale A. E. Molecular definition of a chromosome 9p21 germ-line deletion in a woman with multiple melanomas and a plexiform neurofibroma: implications for 9p tumor-suppressor gene(s). Am J Hum Genet. 1993 Jul;53(1):96–104. [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Ueki K., Rubio M. P., Ramesh V., Correa K. M., Rutter J. L., von Deimling A., Buckler A. J., Gusella J. F., Louis D. N. MTS1/CDKN2 gene mutations are rare in primary human astrocytomas with allelic loss of chromosome 9p. Hum Mol Genet. 1994 Oct;3(10):1841–1845. doi: 10.1093/hmg/3.10.1841. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Palmer J. M., Walters M. K., Nancarrow D. J., Parsons P. G., Hayward N. K. Refined localization of the melanoma (MLM) gene on chromosome 9p by analysis of allelic deletions. Oncogene. 1994 Mar;9(3):819–824. [PubMed] [Google Scholar]
- Weaver-Feldhaus J., Gruis N. A., Neuhausen S., Le Paslier D., Stockert E., Skolnick M. H., Kamb A. Localization of a putative tumor suppressor gene by using homozygous deletions in melanomas. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7563–7567. doi: 10.1073/pnas.91.16.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]